Published online Oct 28, 2014. doi: 10.3748/wjg.v20.i40.15001
Revised: May 7, 2014
Accepted: June 26, 2014
Published online: October 28, 2014
Processing time: 221 Days and 5.7 Hours
We describe the computed tomography (CT) imaging findings in six cases (five males and one female; age range 61-78 years; mean age 67.3 years) with histologically proven hepatoid adenocarcinoma of the stomach (HAS). Five of the six patients had elevated serum alpha-fetoprotein levels. The most common type of gross appearance HAS on CT is a polypoid mass (83%, 5/6). The most common contrast enhancement pattern was heterogeneous. All six patients had a regional lymphadenopathy larger than 6 mm in its short axis. Liver metastases (n = 3) were noted. Venous tumor thrombosis was identified in the portal vein (n = 2) of the regions near primary gastric tumors or metastatic masses. Our findings suggest in an elderly, male patients with a large heterogeneous enhancement tumor, the presence of distant metastases, regional lymphadenopathy and characteristically increased serum alpha-fetoprotein levels indicates a high likelihood of HAS.
Core tip: Hepatoid adenocarcinoma of the stomach (HAS) has different clinicopathological features and prognosis compared with common stomach cancer without the hepatoid differentiation areas (non-HAS). This case report evaluated the computed tomography (CT) findings of patients with pathologically proved hepatoid adenocarcinoma of the stomach. The results will aid the recognition of HAS and demonstrate that CT is useful in the differentiation of HAS from non-HAS.
- Citation: Ren A, Cai F, Shang YN, Ma ES, Huang ZG, Wang W, Luo J. Gastric hepatoid adenocarcinoma: A computed tomography report of six cases. World J Gastroenterol 2014; 20(40): 15001-15006
- URL: https://www.wjgnet.com/1007-9327/full/v20/i40/15001.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i40.15001
Hepatoma is a rare variant of adenocarcinoma that can occur in the stomach. Ishikura et al[1] proposed the term hepatoid adenocarcinoma of the stomach (HAS) in 1985. Since that report, there have been subsequent reports of its clinical manifestation and pathological features[2-4]; however, few of them concerned computed tomography (CT) findings of HAS[5,6]. This tumor type has poor prognosis; therefore, accurate diagnosis of hepatoid adenocarcinoma is very important. In this report, we present the CT findings of patients with pathologically proved HAS.
A computer search of the pathology and medical records of two institutions between January 2010 and December 2012 revealed six patients with pathologically proven hepatoid adenocarcinoma of the stomach. Four patients had epigastric pain (cases 1-4), and two patients had fatigue and weight loss (cases 5-6).
All patients underwent endoscopy. For all six patients, histopathological examination of the surgical or biopsy specimens revealed typical features of hepatoid adenocarcinoma. Five patients had elevated serum alpha-fetoprotein (AFP) levels. Five tumors had available pathological specimens, all of which showed positive AFP staining of tumor tissue. Four patients underwent surgery (total gastrectomy, cases 2-4), except for case 5, who underwent a palliative operation. The principal clinical findings, histopathology and laboratory findings are summarized in Table 1. Tumor enhancement patterns were predominantly heterogeneous (5/6). Mild enhancement was observed in four cases; and moderate enhancement was observed in two cases. All six patients had evidence of lymph node metastasis on CT scans, involving perigastric, paraaortic and hepatoduodenal ligaments. For the four surgical patients, surgery revealed lymphadenopathy. All patients had multiple enlarged lymph nodes.
| Case/age/sex | Location | Tumor thickness (cm) | AFP staining | Serum AFP (ng/mL) | Follow-Status |
| 1 66 F | body | 8 | Positive | 1210 | Died of at 13 mo |
| 2 63 M | body | 5 | Positive | 92.3 | Alive 35 mo |
| 3 61 M | antrum | 3.5 | NA | 2.89 | Alive 13 mo |
| 4 78 M | antrum | 7 | Positive | 513 | NA |
| 5 61 M | body | 7 | Positive | 60500 | Alive 3 mo |
| 6 75 M | diffuse | 3.2 | Positive | 281.7 | Died of at 3 mo |
Liver metastases were observed in three cases. Venous tumor thrombosis was identified in the left portal veins around metastatic hepatic masses in two patients (Table 2).
| Case Gross | Enhancement arterial portal | Delay | Lymphadenopathy metastases appearance | Pattern phase | Phase | Phase |
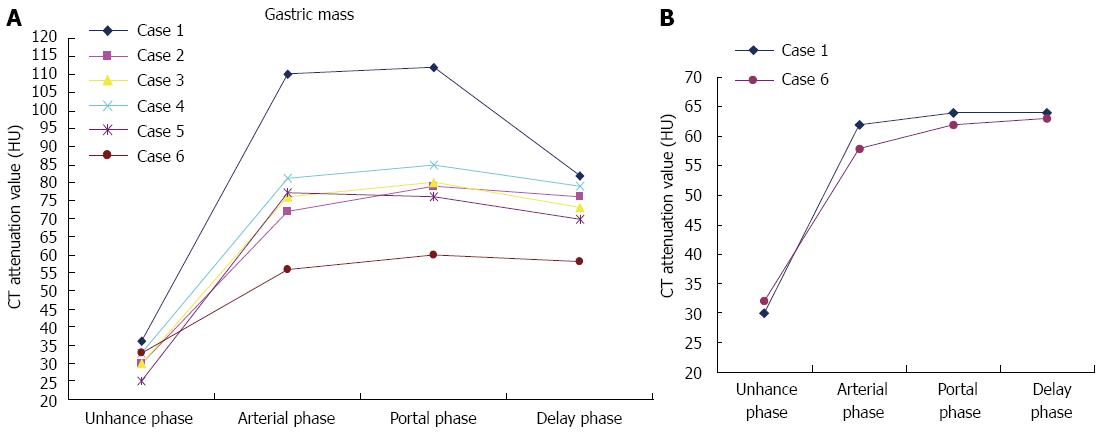
| 1 Polypoid | Heterogeneous | 110 HU | 112 HU | 82 HU | + | Liver (thrombus) |
| 2 Polypoid | Heterogeneous | 72 HU | 79 HU | 76 HU | + | - |
| 3 Polypoid | Heterogeneous | 72 HU | 80 HU | 73 HU | + | - |
| 4 Polypoid | Heterogeneous | 81 HU | 85 HU | 79 HU | + | - |
| 5 Polypoid | Heterogeneous | 77 HU | 76 HU | 70 HU | + | Liver |
| 6 Diffuse | Layered | 56 HU | 60 HU | 58 HU | + | Liver (thrombus) |
Ishikura et al[1] proposed the term “hepatoid adenocarcinoma of the stomach” for primary gastric carcinomas characterized histologically by hepatoid differentiation and the production of large amounts of AFP. AFP producing gastric cancer has a higher incidence of liver metastases and poorer prognosis than non-AFP producing gastric cancer. The incidence of HAS was reported to be 0.3%[7].
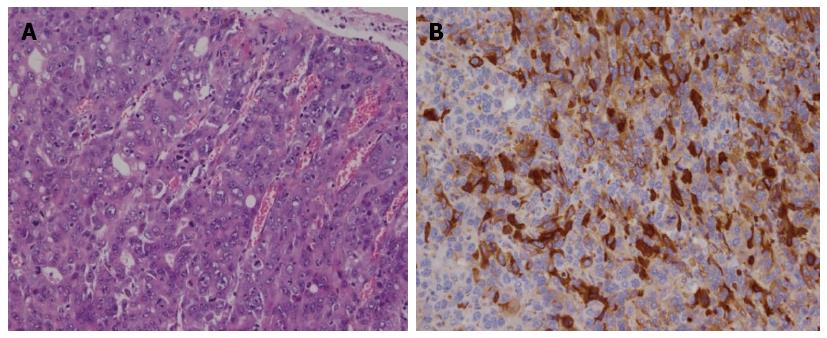
The pathological appearance of HAS is characterized by the presence of both adenocarcinomatous and hepatoid components (Figure 1). Areas of necrosis and hemorrhage are often found in the tumor[8]. Even at the early stage, hepatoid adenocarcinoma has an extremely poor prognosis, because of the frequent occurrence of liver and/or lymph node metastases. The survival rate for HAS is extremely low. The 5-year survival rate in HAS is 11.9% compared with 38.2% in non-HAS[9].
The reasons for this poor prognosis are not clearly understood. One possibility is that hepatoid adenocarcinoma produces AAT and/or ACT (alpha antitrypsin;/alpha antichymotrypsin) as well as AFP. AAT and ACT have immunosuppressive and protease-inhibitory properties that enhance invasiveness[10]. Also, AFP has a suppressive effect on lymphocyte transformation[1].
Most patient with HAS show an elevated serum AFP level, and the serum AFP level decreases rapidly after gastrectomy. However, normal serum levels have also been reported[11]. In our series, one of the six patients with available serum AFP showed a normal range. Therefore, definitive diagnosis is made on the basis of histopathological features of the tumor, including immunohistochemical analysis and is not dependent on whether AFP is produced. HAS shows different clinicopathological features and prognosis from non-HAS; there is an urgent necessity to distinguish them in clinical practice.
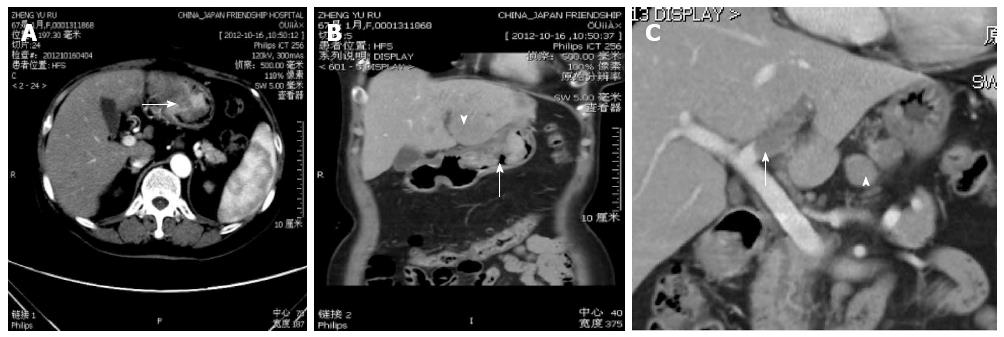
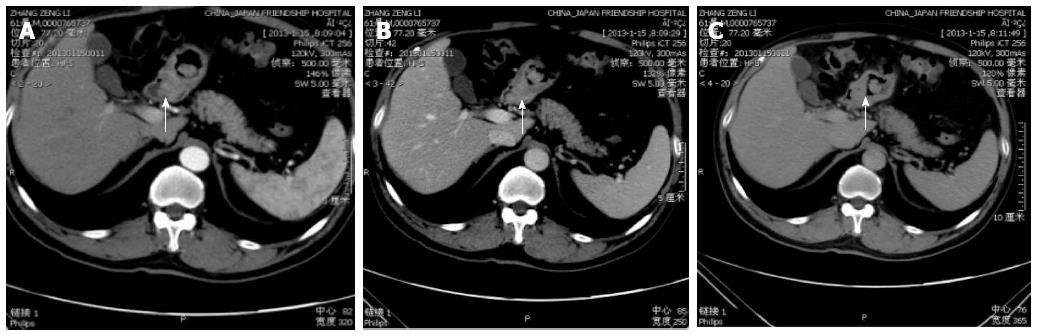
HAS usually presents in elderly patients. Although there were no major symptoms sufficient to allow diagnosis of this type of cancer, epigastric pain, weight loss and general fatigue were the most common symptoms. A strong male predominance has been reported, with a male-female ratio of 11:4[12]. In our series of HAS, the mean age was 67 years and five of the six (83%) patients were men. HAS tends to form a giant tumor. In our series, tumors showed a large asymmetrical gastric wall mass. None of the patients in the present series had a tumor that was less than 3 cm in size. The result agreed with that of a previous report, where the average maximal tumor diameter of HAS was 6.5 cm (range, 1.6-14.0 cm) in 85 HAS cases reported in the literature since 1985 to 1998[3]. The unenhanced CT images for all of our patients showed that the tumor was isodense compared with the surrounding gastric wall. The most common appearance at CT in our series was a polypoid type (5/6), which manifested as an intraluminal growing mass. The use of multiplanar reformations (MPRs) allowed us to choose an optimal imaging plane to evaluate accurately the morphological features (Figure 2B and C). The polypoid appearance is rare among the other histological types of non-HAS advanced gastric carcinoma. Park et al[12] reported that in 62 patients with mucinous or nonmucinous gastric carcinomas (Figure 3), the most common type of gross appearance in both carcinomas was the fungating type, which was confirmed by Lee et al[13].
In our series, tumor enhancement patterns were predominantly heterogeneous (5/6, 83%) vs adjacent non-tumorous gastric walls.
The results of previous studies[12] suggest that the most common constrast enhancement pattern was homogeneous in non-mucinous carcinomas and layered in mucinous carcinomas.
Nevertheless, heterogeneous contrast enhancement of the asymmetrical thickening was more commonly seen in HAS than in non-HAS. This may be explained by HAS having a larger tumor size with high likelihood of coagulation necrosis and hemorrhage[3]. The area of low attenuation on CT images was thought to represent necrosis and internal hemorrhage.
Liver metastasis is the hallmark of both HAS and gastric cancer. In previous studies[2,14], the incidence of liver metastasis in the HAS group was significantly higher than that for non-HAS group (75.6% vs 8.9%). HAS with liver metastasis may closely mimic, and be indistinguishable from hepatocellular carcinoma (HCC), because HAS and HCC share numerous clinicopathological features such as the elevated AFP serum level and their morphological changes.
The following contributed to excluding the type of primary HCC on CT images: (1) the presence of a hepatic tumor in the absence of the risk factors of hepatocellular carcinoma, such as cirrhosis in the liver. There is a close association between liver cirrhosis and HCC; and (2) typical HCC usually demonstrates intense non-homogeneous enhancement seen in the hepatic arterial -dominant phase and contrast washout in the portal venous phase. Liver metastasis usually showed slightly peripheral enhancement of low attenuation masses with a bull’s eye-like appearance and there was washout of the contrast in the delayed phase.
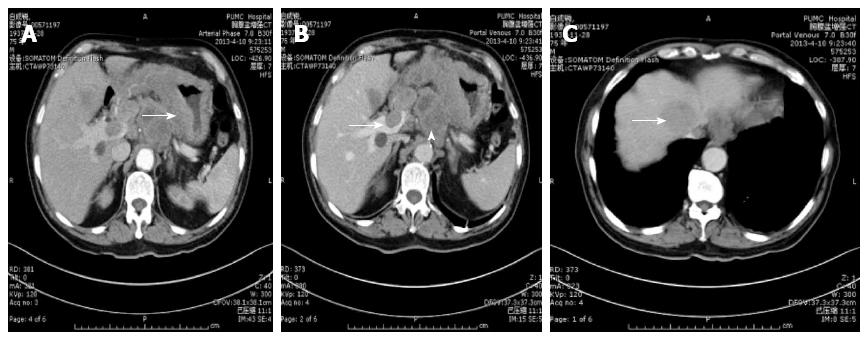
In our series, liver metastasis was noted in three patients; two of them were complicated by portal venous tumor thrombosis (Figure 2C and Figure 4B). Tumor thrombi were judged to be present if there were low-attenuation structures in the portal vein system around the metastatic hepatic masses after administration of contrast material. Contrast enhancement is necessary to differentiate between neoplastic and non-neoplastic thrombosis.
Tumor thrombosis has been reported to be associated with primary HCC. Although rare, portal venous thrombosis may be the result of an invasive gastric carcinoma. Aruga et al first reported in 1986 that they had found three cases of portal venous tumor thrombosis caused by gastric carcinoma. Lee et al[5] reported one case of HAS complicated by portal venous tumor thrombosis without hepatic metastasis. The tumor thrombosis was presumed to have arisen from vascular primary gastric carcinoma, followed by invasions of portal veins without liver parenchymal invasion. In our series, the enhancement of the filling defect in the portal vein excluded the likelihood of non-tumorous venous thrombosis (Figure 2C), and the shapes of the time-density curves were similar for both the tumor thrombosis and the gastric mass after constrast enhancement, reflecting indirectly that the intraluminal tumor thrombus originated from hepatoid adenocarcinoma of the stomach (Figure 5B).
Araki et al[15] concluded that gastric carcinoma should be considered a possibility in the diagnosis of portal venous neoplastic thromboses, even if the serum AFP level is elevated and a liver tumor is identified.
HAS presented a higher incidence of lymphadenopathy than non-NAS advanced gastric carcinoma. In our series, the incidence of regional lymphadenopathy (6/6) on CT was 100%; four of six were confirmed by histological examination. This result agrees with a previous CT report on HAS[2], whereas in the literatures[16,17], the incidence of non-HAS gastric cancer was 12.8%. In addition, CT imaging showed that all patients had multiple enlarged lymph nodes in our study.
In conclusion, the morphological appearance of the enhancement pattern on CT imaging may assist in distinguishing HAS from other types of advanced gastric carcinoma.
According to our results, HAS should be considered in elderly, male patients with presence of CT findings such as a large polypoid appearance, heterogeneous contrast enhancement of the asymmetrical gastric wall thickening, liver metastasis, regional lymphadenopathy and characteristic increased serum AFP level. In this setting, a gastric endoscopy should then be performed to rule out HAS.
Six patients (five males and one female; age range 61-78 years; mean age 67.3 years) presented with epigastric pain and general fatigue.
Suspected gastric cancer.
The serum level of alpha-fetoprotein (AFP) was elevated in four of five patients (92.3-60500 ng/mL). Five tumors for which pathological specimens were available showed positive AFP staining of the tumor tissue.
In the six cases of thickened gastric wall at computed tomography (CT), the mean wall thickness was 5.6 cm (range, 3.2-8 cm). In their series, the most common appearance of the tumor at CT was the polypoid type mass (5/6), which manifested as an intraluminal growing mass. Heterogeneity was identified in all cases. Liver metastases were observed in three cases. Venous tumor thrombosis was identified in the left portal veins around metastatic hepatic masses in all six patients. All patients had evidence of lymph node metastasis on CT scans, involving perigastric, paraaortic and hepatoduodenal ligaments. For four of the six patients, surgery revealed lymphadenopathy. All liver patients had multiple enlarged lymph nodes.
In all six patients, histopathological examination of the surgical or biopsy specimens revealed typical features of hepatoid adenocarcinoma. Five tumors for which pathological specimens were available showed positive AFP staining of the tumor tissue.
Four patients underwent surgery (total gastrectomy, n = 3; subtotal gastrectomy, n = 1). Their serum AFP levels decreased rapidly after gastrectomy.
The CT findings were similar in both hepatoid adenocarcinoma of the stomach (HAS) and no-HAS gastric cancer. However, HAS shows a preference for the liver, a tendency toward lymph node metastasis and venous invasion around primary gastric tumors or metastatic hepatic masses.
AFP is a fetal serum protein produced by fetal and yolk sac cells, and by some fetal gastrointestinal cells. After birth, the level of the protein in serum rapidly decreases. However, its level is elevated in patients with hepatocellular carcinoma. Recently, AFP-producing tumors have been reported in several different organs, with gastric adenocarcinoma being one of the most common of these tumors.
HAS has poor prognosis; therefore, accurate diagnosis of HAS is important. HAS displays some CT findings that are similar to non-HAS advanced gastric cancer and HAS tends to invade veins and metastasize to the liver. In this it resembles HCC, which often invades the portal and hepatic veins.
This article describes the CT findings of these rare cases of hepatoid adenocarcinoma of the stomach, and reviews the literature concerning the clinicopathological aspects.
P- Reviewer: Ao R, Casciaro S, Sureka B, Yew DT, Yazdi HR S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Ishikura H, Fukasawa Y, Ogasawara K, Natori T, Tsukada Y, Aizawa M. An AFP-producing gastric carcinoma with features of hepatic differentiation. A case report. Cancer. 1985;56:840-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 140] [Reference Citation Analysis (0)] |
| 2. | Liu X, Cheng Y, Sheng W, Lu H, Xu X, Xu Y, Long Z, Zhu H, Wang Y. Analysis of clinicopathologic features and prognostic factors in hepatoid adenocarcinoma of the stomach. Am J Surg Pathol. 2010;34:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Inagawa S, Shimazaki J, Hori M, Yoshimi F, Adachi S, Kawamoto T, Fukao K, Itabashi M. Hepatoid adenocarcinoma of the stomach. Gastric Cancer. 2001;4:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Ye MF, Tao F, Liu F, Sun AJ. Hepatoid adenocarcinoma of the stomach: a report of three cases. World J Gastroenterol. 2013;19:4437-4442. [PubMed] |
| 5. | Lee MW, Lee JY, Kim YJ, Park EA, Choi JY, Kim SH, Lee JM, Han JK, Choi BI. Gastric hepatoid adenocarcinoma: CT findings. Abdom Imaging. 2007;32:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Wu Z, Upadhyaya M, Zhu H, Qiao Z, Chen K, Miao F. Hepatoid adenocarcinoma: computed tomographic imaging findings with histopathologic correlation in 6 cases. J Comput Assist Tomogr. 2007;31:846-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Kumashiro Y, Yao T, Aishima S, Hirahashi M, Nishiyama K, Yamada T, Takayanagi R, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach: histogenesis and progression in association with intestinal phenotype. Hum Pathol. 2007;38:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Plaza JA, Vitellas K, Frankel WL. Hepatoid adenocarcinoma of the stomach. Ann Diagn Pathol. 2004;8:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Nagai E, Ueyama T, Yao T, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach. A clinicopathologic and immunohistochemical analysis. Cancer. 1993;72:1827-1835. [PubMed] |
| 10. | Syneok LL. [Peculiarities of acid-base reserves in aging]. Ukr Biokhim Zh. 1976;48:72-77. [PubMed] |
| 11. | deLorimier A, Park F, Aranha GV, Reyes C. Hepatoid carcinoma of the stomach. Cancer. 1993;71:293-296. [PubMed] |
| 12. | Park MS, Yu JS, Kim MJ, Yoon SW, Kim SH, Noh TW, Lee KH, Lee JT, Yoo HS, Kim KW. Mucinous versus nonmucinous gastric carcinoma: differentiation with helical CT. Radiology. 2002;223:540-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 13. | Lee JH, Park MS, Kim KW, Yu JS, Kim MJ, Yang SW, Lee YC. Advanced gastric carcinoma with signet ring cell carcinoma versus non-signet ring cell carcinoma: differentiation with multidetector CT. J Comput Assist Tomogr. 2006;30:880-884. [PubMed] |
| 14. | Chang YC, Nagasue N, Abe S, Kohno H, Kumar DD, Nakamura T. alpha Fetoprotein producing early gastric cancer with liver metastasis: report of three cases. Gut. 1991;32:542-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Araki T, Suda K, Sekikawa T, Ishii Y, Hihara T, Kachi K. Portal venous tumor thrombosis associated with gastric adenocarcinoma. Radiology. 1990;174:811-814. [PubMed] |
| 16. | Etoh T, Sasako M, Ishikawa K, Katai H, Sano T, Shimoda T. Extranodal metastasis is an indicator of poor prognosis in patients with gastric carcinoma. Br J Surg. 2006;93:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |