Published online Oct 28, 2014. doi: 10.3748/wjg.v20.i40.14992
Revised: May 19, 2014
Accepted: July 16, 2014
Published online: October 28, 2014
Processing time: 295 Days and 18.4 Hours
For patients with extensive bilobar colorectal liver metastases (CRLM), initial surgery may not be feasible and a multimodal approach including microwave ablation (MWA) provides the only chance for prolonged survival. Intraoperative navigation systems may improve the accuracy of ablation and surgical resection of so-called “vanishing lesions”, ultimately improving patient outcome. Clinical application of intraoperative navigated liver surgery is illustrated in a patient undergoing combined resection/MWA for multiple, synchronous, bilobar CRLM. Regular follow-up with computed tomography (CT) allowed for temporal development of the ablation zones. Of the ten lesions detected in a preoperative CT scan, the largest lesion was resected and the others were ablated using an intraoperative navigation system. Twelve months post-surgery a new lesion (Seg IVa) was detected and treated by trans-arterial embolization. Nineteen months post-surgery new liver and lung metastases were detected and a palliative chemotherapy started. The patient passed away four years after initial diagnosis. For patients with extensive CRLM not treatable by standard surgery, navigated MWA/resection may provide excellent tumor control, improving longer-term survival. Intraoperative navigation systems provide precise, real-time information to the surgeon, aiding the decision-making process and substantially improving the accuracy of both ablation and resection. Regular follow-ups including 3D modeling allow for early discrimination between ablation zones and recurrent tumor lesions.
Core tip: For patients with extensive bilobar colorectal liver metastases (CRLM), surgery may not be an option and a multimodal approach including computer navigated intervention may be the only chance for prolonged survival. Here we report on a 59-year-old patient undergoing combined resection/microwave ablation for multiple, synchronous bilobar CRLM using an intraoperative navigation system. Of the ten lesions detected in a preoperative computed tomography scan, the largest was resected and the remaining nine ablated. Navigated interventions may provide excellent tumor control, possibly improve longer-term survival, provide real-time information to the surgeon, thus aiding the decision-making process and substantially improving ablation and resection accuracy.
- Citation: Banz VM, Baechtold M, Weber S, Peterhans M, Inderbitzin D, Candinas D. Computer planned, image-guided combined resection and ablation for bilobar colorectal liver metastases. World J Gastroenterol 2014; 20(40): 14992-14996
- URL: https://www.wjgnet.com/1007-9327/full/v20/i40/14992.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i40.14992
Improved surgical and interventional techniques combined with increasingly efficient chemotherapy yield 5-year survival rates of up to 50% in patients with colorectal cancer[1,2]. Unfortunately, only a minority of patients qualify for surgery and local hepatic recurrence affects up to three quarters of all radically operated patients[3,4]. Limitations of classical surgery include achieving complete (R0) tumor removal while leaving behind sufficient liver volume post-resection, a problem often encountered in patients with multiple, bilobar colorectal cancer liver metastases (CRLM). Although such patients (i.e., those deemed non-resectable) may profit from ablation techniques, until now this has been associated with worse long-term survival compared to resection[5]. Whether this difference is due to confounding factors, with ablation generally offered to patients with an a priori poorer prognosis[6], or results from a lack of ablative precision[7], is not yet fully understood.
A further problem concerns the so-called “vanishing lesions”, where lack of an intraoperative-tumor-site identification renders adequate treatment difficult.
A 59-year old patient with multiple, synchronous, bilobar CRLM deemed inoperable with regard to tumor extent and location underwent computer-navigated, combined resection and microwave ablation (MWA). This paper focuses on the challenges faced in such complex clinical settings and possible new technological advantages available with intraoperative navigation systems for soft tissue surgery.
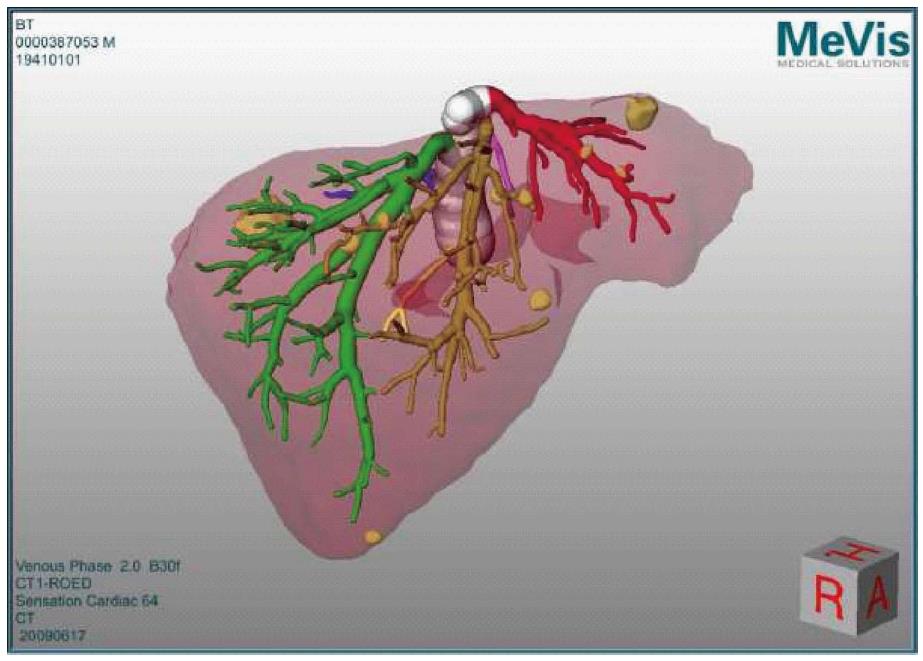
Our patient underwent a preoperative abdominal tri-phasic MeVis computed tomography (CT) (MeVis Research, Bremen, Germany) scan, depicting detailed liver anatomy with identification of vascular territories (portal, arterial and venous) as well as the exact location of the metastases.
The patient received a low anterior rectum resection in January 2008 for a T3 N2 (4/33) M1 G2 R0 rectal adenocarcinoma. Ten liver lesions were distributed in all lobes, except segment V. Neoadjuvant chemotherapy included eight cycles of FOLFOX-Avastin. Eighteen months post-surgery, follow-up MeVis CT and fludeoxyglucose (FDG)-positron emission tomography (PET)/CT excluded extra-hepatic tumor deposits. While most liver metastases remained stable, one (segment VII) showed progression. Following interdisciplinary discussions, the decision was taken to combine local resection (largest lesion, segment VII) with intraoperative MWA to improve local-tumor control and long-term outcome; especially as chemotherapy was not supported well and partial metastatic progression was noted.
Preparation: The planning data (MeVis) were loaded directly into the navigation system as a 3D-surface model, providing an optimal connection between preoperative surgical planning and intraoperative task execution (Figure 1).
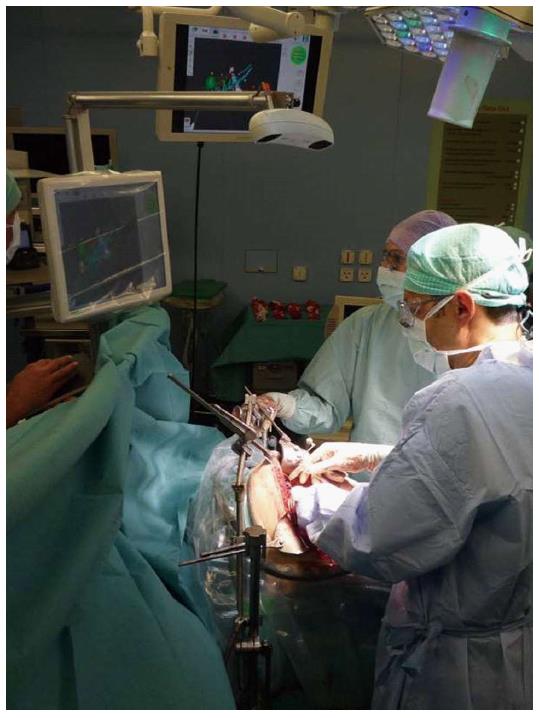
Set-up: The CAScination navigation system is placed at the head end of the patient with sterile-covered touch screens facing the surgical team (Figure 2).
Sterile markers are attached to the instruments of choice (CUSA/ultrasound probe/MWA hand piece) and patient registration carried out using a combination of external (surface) landmarks on the liver as well as internal structures (e.g., bifurcation main portal veins/hepatic veins, tumor sites). Details of instrument calibration/guidance as well as patient-to-CT registration are provided in a previous study by Peterhans et al[8].
Instrument guidance: Upon completion of the registration process, the registered tools in use are displayed/superimposed onto the patient’s 3D liver model on the touch screen. This then allows, for example, the correct placement of the MWA needle into the lesions-avoiding injury to vessels or biliary structures.
Most of the smaller lesions could not be identified by bimanual palpation and intraoperative ultrasound (IOUS). Nine lesions were ablated using navigated MWA (Accu5i applicator, Microsulis, United Kingdom), with an energy of 100 W for 90-120 s, while the lesion in segment VII was removed by an atypical resection. There were no intra-/peri-operative complications. The patient was discharged after eight days.
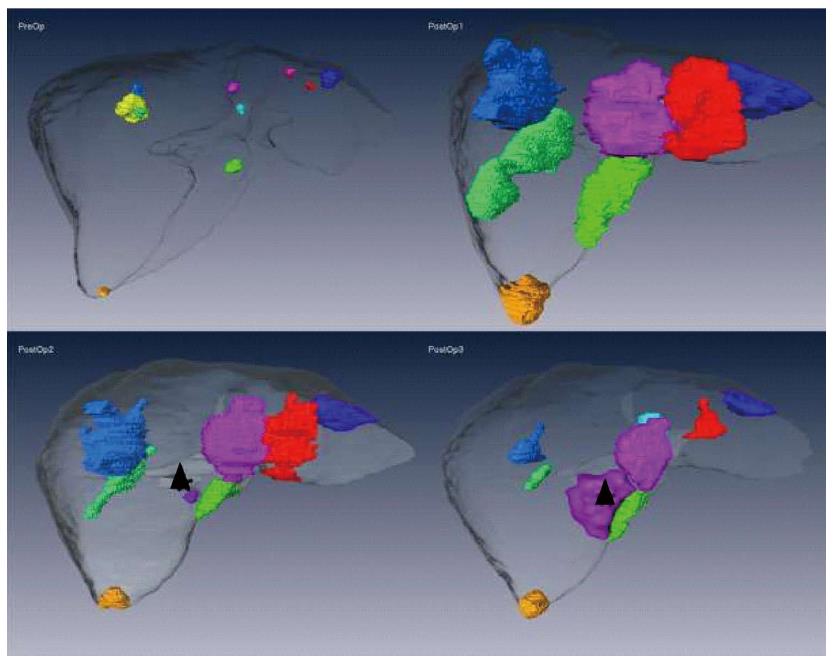
Figure 3 shows MeVis CT data sets preoperatively and at three, nine and twelve months postoperatively. A tiny lesion of initially uncertain dignity was detected nine months postoperatively. At one year post-intervention, recurrent disease (Seg IVa) was treated by trans-arterial chemo-embolization. Nineteen months post liver-surgery, new hepatic and pulmonary metastases were detected and a palliative chemotherapy started. The patient succumbed to the disease three years and ten months after initial diagnosis.
Low-risk loco-regional therapy options, such as radiofrequency ablation or MWA, may be a valid option for patients with CRLM[9,10]. Owing to very low morbidity and mortality rates, ablation techniques have gained wide acceptance from patients and clinicians alike. However, high local recurrence rates of up to 39%[11], particularly in lesions greater than 3 cm[12], remain problematic.
Additionally, when lesions are not radiologically visible (“vanishing lesions”) anymore, oncologists, interventional radiologists and surgeons are left with a difficult task. It is often the case that extended hepatic resections, aiming to remove all affected liver segments, are neither feasible (insufficient liver mass/chemotherapy-associated liver injury) nor desired.
As was the case in our patient with extensive, bilobar CRLM, a multimodal approach was required to determine the best treatment strategy. The decision to use intraoperative computer-navigated resection and MWA arises mainly because of the following (three) issues: the complexity of the tumor distribution, the need to preserve hepatic tissue (chemotherapy induced liver injury) and tumor dynamics, which suggest an aggressive, disseminated disease.
Tools for the preoperative virtual planning of resections and intraoperative ablations have been developed several years ago and are used in clinical routine[13-15]. The difficulty remains in bringing this preoperative information back into the operating theater. Intraoperative navigation systems allow the surgeon to integrate the previously gained virtual environments into real time[16]. One of the main challenges when applying navigated surgery onto moving soft tissues, such as the liver, lies in the accurate, intraoperative patient registration[8]. As of today, only very few studies are available in which the use of navigation systems has reached clinical levels[8,17,18].
Here we present a 59-year old patient who, despite having an extensive tumor load and surgically un-resectable CRLM, was treated by combined, navigated local resection and multiple MWA, which resulted in favorable short-term disease control at minimal patient burden. Intraoperative navigation systems may not only provide local tumor control but also improve longer-term survival in carefully selected patients, initially deemed inoperable or not amenable for local ablative therapies.
A 59-year old patient with multiple, hypodense liver lesions.
Multiple, synchronous bilobar colorectal liver metastases.
Other malignancies with multiple liver metastases such as neuroendocrine tumors.
Laboratory findings: Carcinoembryonic antigen 3.5 ng/mL (normal range < 5 ng/mL), normal white blood cell and hemoglobin.
The patient underwent a preoperative abdominal tri-phasic MeVis computed tomography scan depicting detailed liver anatomy with identification of vascular territories (portal, arterial and venous) as well as the exact location of the metastases.
Pathology revealed moderately differentiated adenocarcinoma of colorectal origin located within the hepatic parenchyma, compatible with a metastasis from the original T3N2M1 (liver) G2 rectal cancer.
Of the ten lesions detected in a preoperative computed tomography scan, the largest lesion in segment VII was resected and the others were ablated with an energy of 100 W for 90-120 s using an intraoperative navigation system.
Computer assisted liver surgery refers to a surgical concept and set of methods, that rely on computer technology for presurgical planning, and for guiding and performing surgical interventions.
Intraoperative navigation systems may not only provide local tumor control but also improve longer-term survival in carefully selected patients, initially deemed inoperable or not amenable for local ablative therapies.
Multimodal, individualized therapies increasingly result in long-term tumor control in patients with advanced colorectal liver metastases. While navigated ablation +/- resection is feasible and applicable in a clinical setting, its role in future treatment strategies remains to be defined.
P- Reviewer: Greco A, Grassetto G S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Bentrem DJ, Dematteo RP, Blumgart LH. Surgical therapy for metastatic disease to the liver. Annu Rev Med. 2005;56:139-156. [PubMed] |
| 2. | Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, Lillemoe KD, Yeo CJ, Cameron JL. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759-766. [PubMed] |
| 3. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 1444] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 4. | Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982-999. [PubMed] |
| 5. | Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, Hess K, Curley SA. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818-25; discussion 825-7. [PubMed] |
| 6. | Smith MD, McCall JL. Systematic review of tumour number and outcome after radical treatment of colorectal liver metastases. Br J Surg. 2009;96:1101-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Jolesz FA. MRI-guided focused ultrasound surgery. Annu Rev Med. 2009;60:417-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 8. | Peterhans M, vom Berg A, Dagon B, Inderbitzin D, Baur C, Candinas D, Weber S. A navigation system for open liver surgery: design, workflow and first clinical applications. Int J Med Robot. 2011;7:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441-451. [PubMed] |
| 10. | de Baère T, Risse O, Kuoch V, Dromain C, Sengel C, Smayra T, Gamal El Din M, Letoublon C, Elias D. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol. 2003;181:695-700. [PubMed] |
| 11. | White TJ, Roy-Choudhury SH, Breen DJ, Cast J, Maraveyas A, Smyth EF, Hartley JE, Monson JR. Percutaneous radiofrequency ablation of colorectal hepatic metastases - initial experience. An adjunct technique to systemic chemotherapy for those with inoperable colorectal hepatic metastases. Dig Surg. 2004;21:314-320. [PubMed] |
| 12. | Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, Cova L, Halpern EF, Gazelle GS. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159-166. [PubMed] |
| 13. | Selle D, Preim B, Schenk A, Peitgen HO. Analysis of vasculature for liver surgical planning. IEEE Trans Med Imaging. 2002;21:1344-1357. [PubMed] |
| 14. | Lange T, Wenckebach TH, Lamecker H, Seebass M, Hünerbein M, Eulenstein S, Gebauer B, Schlag PM. Registration of different phases of contrast-enhanced CT/MRI data for computer-assisted liver surgery planning: evaluation of state-of-the-art methods. Int J Med Robot. 2005;1:6-20. [PubMed] |
| 15. | Radtke A, Sotiropoulos GC, Molmenti EP, Schroeder T, Peitgen HO, Frilling A, Broering DC, Broelsch CE, Malago’ M. Computer-assisted surgery planning for complex liver resections: when is it helpful? A single-center experience over an 8-year period. Ann Surg. 2010;252:876-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Chopra SS, Hünerbein M, Eulenstein S, Lange T, Schlag PM, Beller S. Development and validation of a three dimensional ultrasound based navigation system for tumor resection. Eur J Surg Oncol. 2008;34:456-461. [PubMed] |
| 17. | Bale R, Widmann G, Stoffner DI. Stereotaxy: breaking the limits of current radiofrequency ablation techniques. Eur J Radiol. 2010;75:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Buchs NC, Volonte F, Pugin F, Toso C, Fusaglia M, Gavaghan K, Majno PE, Peterhans M, Weber S, Morel P. Augmented environments for the targeting of hepatic lesions during image-guided robotic liver surgery. J Surg Res. 2013;184:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |