Published online Oct 28, 2014. doi: 10.3748/wjg.v20.i40.14973
Revised: May 8, 2014
Accepted: May 25, 2014
Published online: October 28, 2014
Processing time: 243 Days and 5.3 Hours
AIM: To assess the efficacy and safety of standard triple therapy compared with other pre-existing and new therapies in China.
METHODS: Literature searches were conducted in the following databases: PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, the VIP database, the China National Knowledge Infrastructure database, and the Chinese Biomedical Database. A meta-analysis of all randomized controlled trials (RCTs) comparing standard triple therapy for the eradication of Helicobacter pylori with pre-existing and new therapies in China was performed using Comprehensive Meta-Analysis 2.0. There were 49 studies that met our criteria and the qualities of these studies were assessed using the Jadad scale. The Mantel-Haenszel method was used for pooling dichotomous data. We also conducted subgroup analyses according to age, duration of treatment and drug type. Sensitivity analyses and a cumulative meta-analysis were also performed with CMA 2.0. Publication bias was evaluated using Egger’s test, Begg’s test or a funnel plot.
RESULTS: A total of 49 RCTs including 8332 patients were assessed. This meta-analysis showed that standard triple therapy with proton pump inhibitors (PPIs), amoxicillin (AMO) and clarithromycin (CLA) was inferior to sequential therapy [relative risk (RR) = 0.863; 95% confidence interval (CI): 0.824-0.904], but was not superior to quadruple therapy (RR = 1.073; 95%CI: 0.849-1.357) or other triple therapies (RR = 1.01; 95%CI: 0.936-1.089). The meta-analysis also suggested that standard triple therapy is slightly more effective than dual therapy (RR = 1.14; 95%CI: 0.99-1.31). However, the differences were not statistically significant. We removed the only trial with a regimen lasting 14 d by sensitivity analysis and found that 7-d standard triple therapy was superior to 7-d dual therapy (RR = 1.222; 95%CI: 1.021-1.461). Moreover, a sub-analysis based on the duration of quadruple therapy indicated that the 7-d and 10-d standard triple therapies were inferior to sequential therapy (RR = 0.790; 95%CI: 0.718-0.868; RR = 0.917; 95%CI: 0.839-1.002, respectively). Additionally, there were no significant differences in cure rate or adverse events among standard triple therapy, quadruple therapy, and other triple therapies (RR = 0.940; 95%CI: 0.825-1.072; RR = 1.081; 95%CI: 0.848-1.378, respectively). Standard triple therapy had a higher occurrence of side effects than sequential therapy (RR = 1.283; 95%CI: 1.066-1.544).
CONCLUSION: The eradication rates with a standard triple therapy consisting of PPI, AMO, and CLA are suboptimal in China, and new treatment agents need to be developed.
Core tip: This study compared the efficiency of standard triple therapy with other pre-existing and new therapies on the Chinese mainland and examined the eradication rates for Helicobacter pylori in China. The results showed that the standard triple therapy including proton pump inhibitors, amoxicillin and clarithromycin might not be suitable for first-line therapy.
-
Citation: Wang B, Lv ZF, Wang YH, Wang H, Liu XQ, Xie Y, Zhou XJ. Standard triple therapy for
Helicobacter pylori infection in China: A meta-analysis. World J Gastroenterol 2014; 20(40): 14973-14985 - URL: https://www.wjgnet.com/1007-9327/full/v20/i40/14973.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i40.14973
Helicobacter pylori (H. pylori) infection is currently very common[1], and infection plays an important role in some gastrointestinal diseases, such as peptic ulcers, chronic gastritis, gastric cancer, and gastric malignant disease[2]. Eliminating H. pylori can reduce the recurrence of peptic ulcers and prevent gastric cancer[2]. Standard triple therapy with a proton pump inhibitor (PPI), amoxicillin (AMO) and clarithromycin (CLA) has been extensively employed around the world, and contributed to an eradication success rate of over 90% in the 1990s[3]. However, because of the wide application in H. pylori infection, eradication rates have declined to less than 60%[4,5]. The primary reason for this decline is the resistance of H. pylori to antibiotics, particularly CLA and metronidazole[5].
The standard triple therapy with PPI, AMO and CLA is still recommended as one of the first line anti-H. pylori treatments when the resistance rate of H. pylori to CLA is less than 15%-20%[6-8]. This therapy has encountered some challenges, and although the eradication rate is decreasing[4], the resistance of H. pylori to CLA has increased[9]. One recent study suggested that the therapy could not achieve an acceptable eradication rate[10].
The efficacy of triple therapy consisting of PPI, AMO and CLA has been demonstrated by several studies during the past decade in China. However, the use of antibiotics for other diseases without rigorous supervision has caused a rapid increase in the prevalence of antibiotic-resistant H. pylori in China. A recent study showed that the resistance of H. pylori to CLA is more than 80% and another study suggested that the primary resistance of H. pylori to CLA is 17.2%[11,12]. These data indicate that the current effectiveness of standard triple therapy consisting of PPI, AMO and CLA may be diminished in some areas[13-15]. Furthermore, one study indicated that standard triple therapy was not superior to sequential therapy[16]. Despite the development of resistance, experts consider standard triple therapy effective in areas where H. pylori resistance rates to CLA are less than 15%[7].
Whether the standard triple therapy is suitable as a first-line therapy for H. pylori infection in China remains controversial. The changes in eradication rates of standard triple therapy containing PPI, AMO and CLA with time also remains uncertain in China. To further assess the efficacy and safety of standard triple therapy compared with other eradication treatments (other triple therapies, quadruple treatments and sequential treatments), we conducted this systemic review and meta-analysis. Furthermore, we also performed a cumulative meta-analysis to investigate the changes in the eradication rate of the standard triple regime over time in China.
We searched PubMed (to November 2013), EMBASE (to November 2013), the Cochrane Central Register of Controlled Trials (Issue 11, 2013), the VIP database, the China National Knowledge Infrastructure database (CNKI), and the Chinese Biomedical Database (CBM). The specific search algorithm used for each database was the following: PubMed - [(amoxicillin AND clarithromycin) AND triple] AND “Helicobacter pylori”[Mesh], Filters: Randomized Controlled Trial); Embase - “Helicobacter pylori”/expand “amoxicillin”/exp and “clarithromycin”/exp and triple and “human”/de and “randomized controlled trial”/de; the Cochrane Central Register of Controlled Trials - amoxicillin AND clarithromycin AND triple AND helicobacter pylori; the VIP database, CNKI database and CBM database - were searched with the following keywords: “Helicobacter pylori”, “amoxicillin”, “clarithromycin” and “triple”.
The articles eligible for inclusion in the meta-analysis met the following inclusion criteria: (1) randomized controlled trial; (2) inclusion of at least 2 branches of treatment, including: (1) standard triple therapy (PPI, AMO, CLA), (2) dual therapy (PPI, a type of antibiotic), quadruple therapy, sequential therapy or other triple therapy; (3) an H. pylori diagnosis by urea breath test (UBT), rapid urease test, histology, and/or fecal antigen testing; (4) eradication testing with UBT and/or histology at least 4 wk after completion of therapy; (5) an available eradication rate; (6) no restrictions in age or sex; (7) a study population composed of subjects who had never been treated for H. pylori; (8) inclusion of mainland Chinese residents; and (9) studies published in Chinese must have been published in core journals (Peking University Library Chinese Core Periodical Catalog, 2012).
The following were exclusion criteria: (1) articles and/or abstracts not reporting tests used to diagnose infection and/or follow-up infection; (2) articles and/or abstracts not conducted on the Chinese mainland; and (3) articles with inappropriate treatments in the control group or standard triple group, including the use of traditional Chinese medicine or probiotics.
The primary study outcomes for the meta-analysis included the following: (1) the efficacy of standard triple therapy compared with established and new therapies in eradicating H. pylori infection; and (2) the incidence of adverse events in standard triple therapy vs other therapies.
Two independent reviewers (Wang B and Lv ZF) extracted the data from the selected studies using standardized data extraction forms. Any disagreement was resolved by consensus.
The following data were extracted: study design, number of patients in each treatment arm, testing used to confirm persistent infection prior to study enrollment and eradication after the completion of treatment, drug regimen, duration of treatment, eradication rates by intention to treat (ITT) analysis, percentage of adverse effects and severe adverse effects. The study quality was assessed using the Jadad scale.
The data analysis was performed using the Mantel-Haenszel method by meta-analysis software Comprehensive Meta-Analysis 2.0 (Biostat, Englewood, NJ, United States). For each trial, we calculated the relative risk (RR) for the primary measure. The RRs were presented with 95% confidence intervals (CIs), with a P-value < 0.05 considered significant. The study endpoints were calculated by ITT. We estimated the degree of heterogeneity among the trial results using the χ2 statistic (with a P-value < 0.10 considered significant) and the I2 test (25%, 50%, and 75% represent low, moderate and high heterogeneity, respectively). Whenever significant heterogeneity (P < 0.1 or I2 > 50%) was achieved, we used the random effect model to combine the effect sizes of the included studies. If no significant heterogeneity was found, we selected a fixed effect to pool the data. The subgroup or sensitivity analyses were performed where appropriate. We assessed the presence of publication bias with Egger’s test and Begg’s test or a funnel plot if necessary.
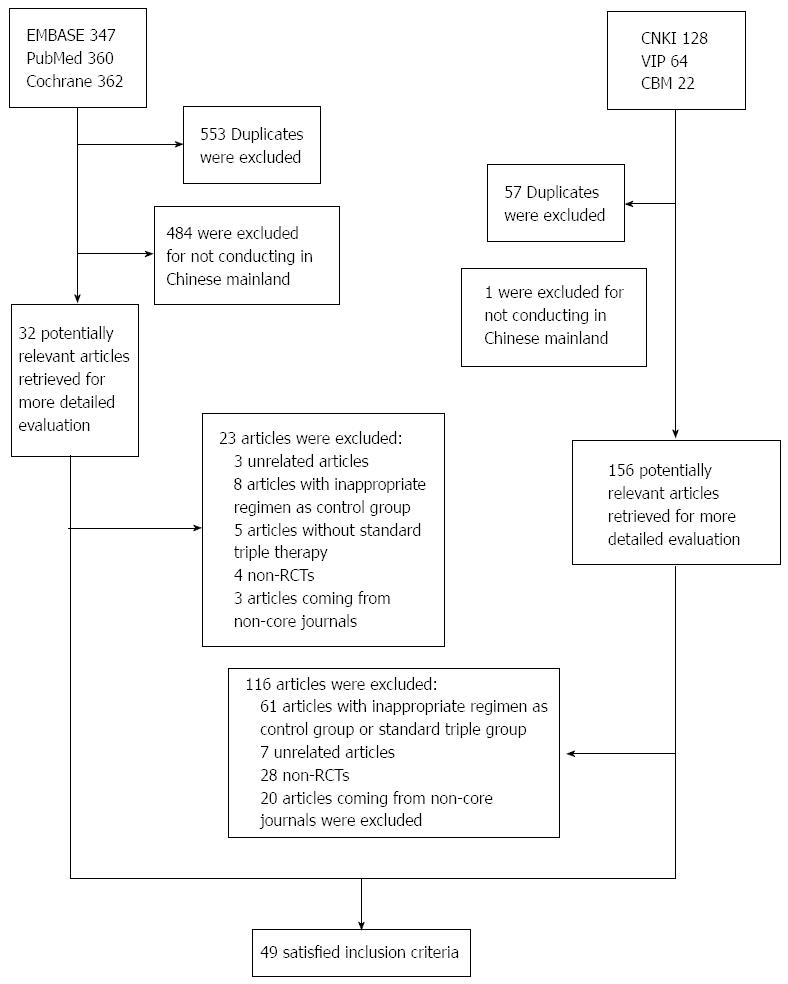
The bibliographical search yielded a total of 1283 studies. Of these studies, 1069 studies were from PubMed, Cochrane and EMBASE, while the other 214 studies published in Chinese were from CBM, VIP and CNKI. Among the studies that were found in PubMed, Cochrane and EMBASE, we excluded 553 duplicate studies and an additional 484 studies that were not conducted on the Chinese mainland. Therefore, we retrieved 32 potentially relevant articles for a more detailed assessment. After examining the titles and abstracts, we excluded 3 unrelated articles. After reviewing the full-text articles we excluded 13 articles with inappropriate treatments in the control group or standard triple group, 4 non-randomized controlled trials (RCTs) and 3 articles that were published in non-core journals. Finally, 9 English language RCTs met the inclusion criteria. For the Chinese articles, we excluded 57 duplicates and 1 study that was not conducted on the Chinese mainland. We also excluded 7 unrelated articles, 61 articles with inappropriate treatments in the control group or standard triple group, 28 non-RCTs and 20 articles that were published in non-core journals after examining all titles, abstracts and full texts. Finally, we identified 40 Chinese RCTs that met the inclusion criteria. In conclusion, 49 RCTs[14-62] met the inclusion criteria. The flowchart of reviews showed the detailed process of selection (Figure 1). The characteristics and quality score of the 49 trials included in the meta-analysis are summarized in Table 1.
| Ref. | Age | Standard triple therapy | Control group therapy | H. pylori infection initial diagnosis ⁄ re-checking | Eradication rate by ITT (standard triple therapy/control group therapy) | Side effects | Jadad scores |
| Geng et al[17], 2003 | Adult | P A C | O A | RUT/RUT | 86.6% (71/82)/71.3% (57/80) | 4 /7 | 1 |
| Wu et al[18], 2004 | Child | O A C | O C | H or C/UBT | 93.3% (56/60)/76.0% (38/50) | - | 1 |
| Gao et al[19], 2006 | Adult | R A C | R A | RUT UBT/RUT UBT | 93.8% (45/48)/91.5% (43/47) | 4/5 | 1 |
| Huang et al[15], 2013 | Child | O A C | ST-10 (O A/O C M) | H, RUT ST/ H, RUT ST | 64.87% (157/242)/81.4% (96/118) | 61/32 | 2 |
| Yan et al[16], 2011 | Adult | E A C | ST-10 (E A/E C T) | H RUT/UBT H | 75.10% (220/341)/75.20% (185/281) | - | 2 |
| Liu et al[22], 2011 | Child | O A C | ST-10 (O A/O C M) | UBT/UBT | 60.61% (20/33)/91.18% (31/34) | 3 | |
| OAM group: O A M | 69.70% (23/33) | ||||||
| Jia et al[41], 2012 | Adult | E A C | ST-10 (E A/E C L) | RUT UBT/RUT UBT | 76.0% (38/50)/94.0% (47/50) | 3/4 | 1 |
| Zhang et al[42], 2012 | Adult | R A C | ST-9 (RA/RCOrn) | UBT RUT/UBT | 80.2% (89/111)/90.2% (101/112) | 10/6 | 2 |
| R A C B | 91.1% (102/112) | 7 | |||||
| Li et al[43], 2012 | Child | O A C | ST-10 (O A/O C F) | UBT RUT/UBT | 69.7% (23/33)/91.2% (31/34) | 5/4 | 2 |
| Zhou et al[44], 2011 | Adult | O A C | ST-10 (O A/O C L ) | RUT H/RUT H | 79.60% (35/44)/88.9% (40/45) | - | 2 |
| Wu et al[45], 2011 | Adult | E A C | ST-10 (E A/E C T) | UBT H RUT/UBT H RUT | 90.20% (46/51)/90.40% (47/52) | 18/12 | 2 |
| Zhu et al[46], 2010 | Child | O A C | ST-10 (R A/R C T) | UBT S/UBT S | 70.73% (29/41)/92.68% (76/82) | 7/5 | 1 |
| Zhang et al[47], 2010 | Adult | P A C | ST-10 (P A/P C T) | UBT/UBT | 73.0% (61/74)/92.3% (36/39) | 12/6 | 3 |
| Lu et al[48], 2010 | Child | O A C | ST-10 (O A/O C T) | S UBT RUT/UBT | 82.43% (26/36)/90% (36/40) | 6/7 | 2 |
| Hu et al[49], 2009 | Adult | E A C | ST-10 (E A/E C T) | RUT H/UBT | 77.50% (31/40)/94.87% (37/39) | 8/7 | 2 |
| Pang et al[50], 2009 | Adult | O A C | ST-10 (O A/O C T) | RUT H UBT/RUT UBT | 89.60% (60/67)/91.90% (63/69) | 19/10 | 2 |
| Wang et al[51], 2009 | Adult | E A C | ST-10 (E A/E C T) | RUT H/UBT | 76.92% (40/52)/92.00% (46/50) | 12/10 | 1 |
| Zhao et al[52], 2009 | Adult | P A C | ST-10 (P A/P C ) | H/UBT | 67.24% (39/58)/83.87% (52/62) | 6/5 | 2 |
| Huang et al[53], 2009 | Adult | O A C | ST-10 (O A/O C T) | UBT/UBT | 69.2% (36/52)/92.5% (49/53) | - | 1 |
| Ma et al[54], 2008 | Adult | O A C | ST-10 (O A/O C T) | RUT UBT/RUT UBT | 65.1% (41/63)/83.6% (56/67) | 12/11 | 3 |
| Huang et al[55], 2012 | Adult | E A C | ST-10 (E A/E C M) | RUT H/RUT H | 78.4% (40/51)/80.0% (40/50) | 9/10 | 3 |
| Gao et al[56], 2010 | Adult | O A C | RAB L; | RUT H/UBT | 80.56% (58/71)/83.33% (60/72) | 11/6 | 2 |
| ST-10 (O A/O C T) | 88.89% (64/72) | 14 | |||||
| Huang et al[61], 2012 | Child | O A C | ST-10 (O A/O C M) | B C/UBT | 78.8% (109/160)/85.2% (46/54) | 26/6 | 2 |
| Zheng et al[60], 2005 | Adult | E A C | 1d-E A M B | RUT H/UBT | 80.50% (33/41)/38.50% (15/39) | 2/1 | 2 |
| Dai et al[37], 2012 | Adult | E A C | E A F B | UBT/UBT | 78.12% (25/35)/88.57% (31/35) | 4/8 | 2 |
| Xu et al[38], 2011 | Adult | E A C | E A C B | UBT/UBT | 73.02% (46/69)/88.71% (55/67) | 7/8 | 3 |
| Liu et al[39], 2010 | Adult | R A C | R A C B | UBT/UBT | 62.90% (39/62)/88.70% (55/62) | 4/5 | 1 |
| Hu et al[13], 2012 | Adult | Lan A C | Lan A C B | RUT H UBT/UBT | 70.00% (70/100)/88.10% (89/101) | 1/2 | 3 |
| Jing et al[40], 2004 | Adult | O/R A C | O/R A C F | UBT/UBT | 85.83% (103/120)/86.7% (40/60) | 4/5 | 2 |
| Zhang et al[62], 2006 | Adult | Lan A C | Lan A C M | UBT/UBT | 69.59% (103/157)/26.78% (64/239) | 148/229 | 2 |
| Luo et al[20], 2012 | Adult | E A C | E A L | UBT RUT/UBT | 75.8% (91/120)/80.0% (96/120) | 21/19 | 2 |
| Chen et al[21], 2011 | Adult | R A C | R A D | UBT/UBT | 61.25% (49/80)/88.75% (71/80) | - | 1 |
| Xu et al[23], 2010 | Adult | E A C | E L F | H UBT RUT/UBT RUT | 75.51% (37/49)/93.87% (46/49) | 6/5 | 1 |
| Dai et al[24], 2010 | Adult | E A C | E A L | UBT RUT/UBT | 82.10% (23/28)/88.90% (26/30) | 2/2 | 2 |
| Xu et al[25], 2009 | Adult | Lan A C | Lan C M | RUT UBT H/UBT | 83.87% (26/31)/60.00% (21/35) | 4/5 | 1 |
| Wang et al[26], 2008 | Adult | E A C | A C B | RUT H/UBT | 80.0% (16/20)/85.00% (17/20) | 10/10 | 2 |
| Hu et al[27], 2008 | Adult | O A C | O A L | RUT UBT/UBT | 85.70% (36/45)/90.20% (37/45) | 2/2 | 2 |
| Su et al[28], 2005 | Child | O A C | A M B | RUT H UBT/UBT | 92.5% (74/80)/74.19% (92/124) | 16/24 | 2 |
| Mou et al[29], 2004 | Adult | O A C | O C Gm | C H RUT/RUT H UBT | 84.20% (16/19)/80.00% (20/25) | 9/12 | 1 |
| Chen et al[30], 2002 | Adult | O A C | L B F | RUT H/RUT H | 88.20% (97/110)/86.70% (92/106) | 4/0 | 1 |
| Chen et al[31], 1996 | Adult | O A C | A M B | RUT H/RUT H | 89.6% (43/48)/83.87% (78/93) | 50/59 | 2 |
| Cheng et al[32], 2010 | Adult | Lan A C | Lan A L | UBT RUT/UBT | 74.50% (111/149)/82.99% (122/148) | 5/9 | 3 |
| Zeng et al[33], 2007 | Adult | O A C | O A L | UBT/UBT | 68.30% (28/41)/86.50% (32/37) | 6/5 | 1 |
| Gao et al[34], 2005 | Adult | O A C | A C B | H/H | 83.33% (25/30)/86.67% (26/30) | 0/2 | 2 |
| Chen et al[35], 2004 | Adult | O A C | O A Am | UBT/H | 93.33% (42/45)/92.72% (51/55) | 8/3 | 2 |
| Chen et al[36], 2005 | Adult | O A C | O A Am | UBT H/UBT H | 88.50% (23/26)/86.70% (26/30) | 2/2 | 1 |
| He et al[58], 2004 | Adult | R A C | R C M | C/UBT | 85.90% (55/64)/54.70% (35/64) | - | 2 |
| Guo et al[58], 2004 | Adult | O A C | O F C/O M C /O F A | RUT S H/UBT | 84.90% (28/33)/73.74% (73/99) | 9/19 | 2 |
| Sun et al[59], 2005 | Adult | O A C | O A M | B /RUT H | 86.20% (50/58)/82.20% (37/45) | 6/5 | 2 |
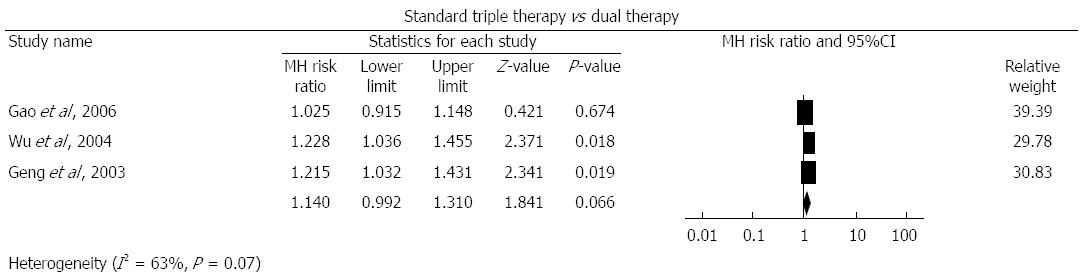
Standard triple therapy vs dual therapy: Three studies[17-19] compared standard triple therapy with dual therapy. As shown in Figure 2, the pooled RR was 1.14 (95%CI: 0.99-1.31, P = 0.066). We found evidence of heterogeneity (I2 = 63%, P = 0.07) with funnel plot asymmetry (Egger’s test coefficient 2.75 to 10.28, P = 0.03) (Table 2).
| Meta-analyses/subgroup analyses | Eradication rate with control group | RR (95%CI) | I2 |
| Standard triple therapy vs dual therapy | 78.00% | 1.140 (0.992–1.310) | 62.596% |
| 7-d subgroup | 73.08% | 1.221 (1.084 –1.374) | 0.000% |
| Adverse events | 0.651 (0.276–1.539) | 0.000% | |
| Standard triple therapy vs sequential therapy | 84.00% | 0.863 (0.824–0.904) | 37.400% |
| Adult subgroup | 82.90% | 0.899 (0.861–0.939) | 25.942% |
| Child subgroup | 87.29% | 0.779 (0.722–0.840) | 0.000% |
| 7-d subgroup | 87.52% | 0.800 (0.752–0.851) | 0.000% |
| 10-d subgroup | 80.17% | 0.849 (0.789–0.913) | 42.355% |
| 14-d subgroup | 89.72% | 0.980 (0.916–1.048) | 0.000% |
| Omeprazole subgroup | 87.37% | 0.832 (0.772–0.898) | 47.049% |
| Esomeprazole subgroup | 77.01% | 0.932 (0.871–0.998) | 40.061% |
| Pantoprazole subgroup | 87.13% | 0.846 (0.746–0.960) | 0.000% |
| Rabeprazole subgroup | 91.24% | 0.847 (0.766–0.936) | 41.871% |
| Tinidazole subgroup | 81.43% | 0.889 (0.837–0.944) | 38.688% |
| Metronidazole subgroup | 83.20% | 0.810 (0.745–0.882) | 43.630% |
| Adverse events | 1.176 (0.975–1.419) | 0.000% | |
| Standard triple therapy vs quadruple therapy | 64.90% | 1.073 (0.849–1.357) | 93.204% |
| 1-d subgroup | 28.42% | 2.367 (1.923–2.914) | 0.000% |
| 3-d subgroup | 66.67% | 1.288 (1.061–1.562) | 100.000% |
| 7-d subgroup | 86.79% | 0.790 (0.718–0.868) | 0.000% |
| 10-d subgroup | 88.04% | 0.917 (0.839–1.002) | 22.259% |
| Omeprazole subgroup | 66.67% | 1.250 (1.012–1.545) | 100.000% |
| Esomeprazole subgroup | 73.76% | 1.098 (0.699–1.725) | 88.852% |
| Lansoprazole subgroup | 45.00% | 1.391 (0.404–4.790) | 98.719% |
| Rabeprazole subgroup | 83.99% | 0.948 (0.771–1.166) | 84.733% |
| Adverse events | 0.940 (0.825–1.072) | 0.000% | |
| Standard triple therapy with other triple therapies | 79.90% | 1.010 (0.936–1.089) | 72.233% |
| Adult subgroup | 80.92% | 0.999 (0.925–1.078) | 69.085% |
| Child subgroup | 73.25% | 1.079 (0.748–1.557) | 74.810% |
| 7-d subgroup | 80.32% | 1.022 (0.949–1.100) | 60.674% |
| 10-d subgroup | 77.78% | 0.933 (0.821–1.060) | 0.000% |
| 14-d subgroup | 78.40% | 1.050 (0.712–1.549) | 93.921% |
| Omeprazole subgroup | 80.30% | 1.048 (0.976–1.125) | 49.506% |
| Esomeprazole subgroup | 84.47% | 0.911 (0.831–0.999) | 0.000% |
| Levofloxacin subgroup | 82.37% | 0.917 (0.852–0.987) | 0.000% |
| Furazolidone subgroup | 85.22% | 0.963 (0.762–1.216) | 73.898% |
| Metronidazole subgroup | 68.84% | 1.119 (0.882–1.420) | 80.863% |
| Adverse events | 1.081 (0.848–1.378) | 0.000% | |
| Eradication rate with standard triple therapy | 74.5% | ||
The pooled eradication rate of dual therapy was 78.0% based on this meta-analysis. Due to the heterogeneity, we also performed sensitivity analyses and the difference became significant when the study of Gao et al[19] was removed (RR = 1.222, 95%CI: 1.021-1.461).
Data on adverse events were available for 2 trials. The pooled RR was 0.651 (0.276-1.539), which indicated no significant difference and no evidence of heterogeneity (I2 = 0%, P = 0.699).
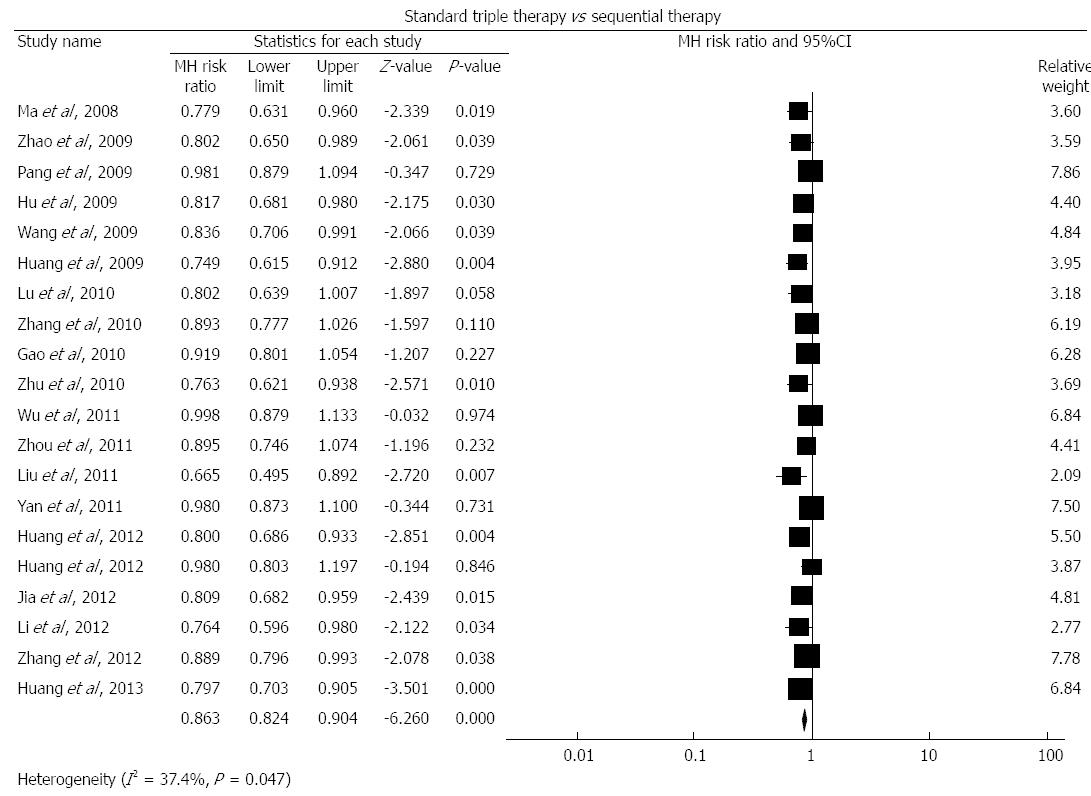
Standard triple therapy vs sequential therapy: We identified 20 studies[15,16,22,41-56,61] comparing standard triple therapy with sequential therapy. As shown in Figure 3, the pooled RR was 0.863 (95%CI: 0.824-0.904, P < 0.001). We found evidence of heterogeneity (I2 = 37.4%, P = 0.047) with funnel plot asymmetry (Egger’s test coefficient -4.86 to -1.57, P < 0.001). The pooled eradication rate of sequential therapy was 84.0% based on this meta-analysis.
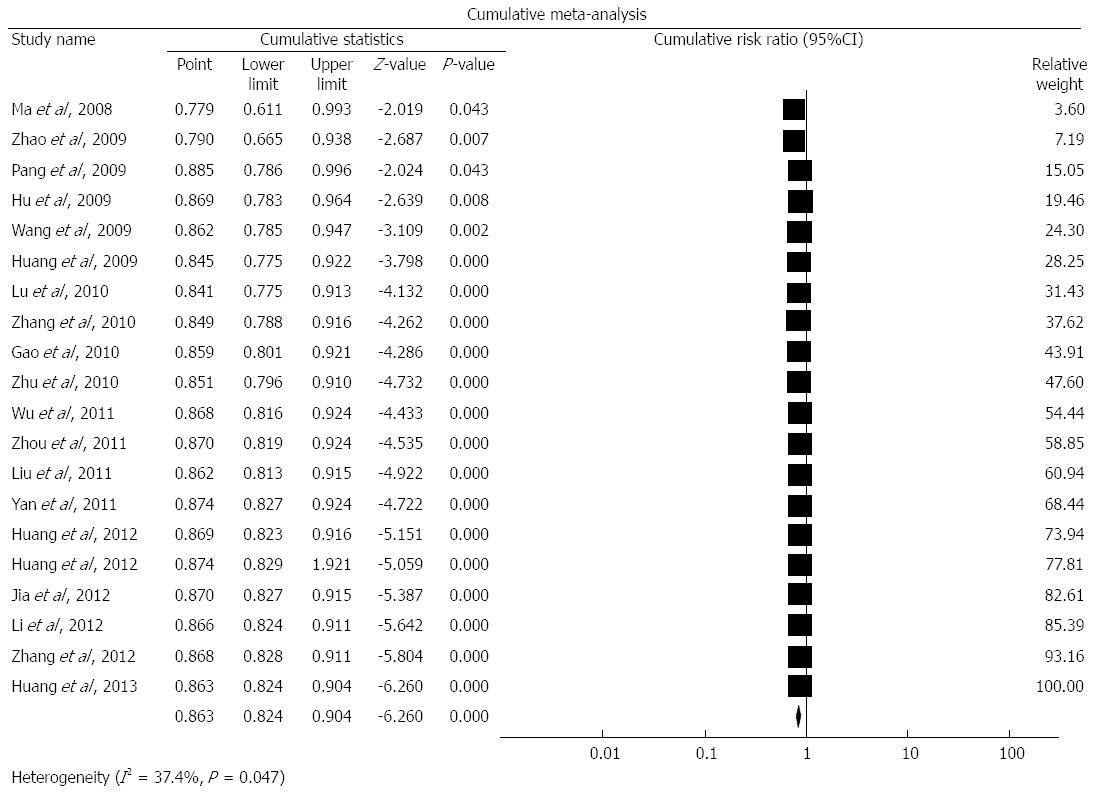
In addition, we performed a cumulative meta-analysis as shown in Figure 4. The pooled RRs for the years 2008, 2009, 2010, 2011, 2012, and 2013 were 0.779, 0.846, 0.852, 0.875, 0.876, and 0.870, respectively.
Due to the heterogeneity we also performed subgroup analyses according to age, duration of standard triple therapy (7-d, 10-d and 14-d), different PPIs in the standard triple therapy and for the different drugs used in the control group (the sequential therapy group).
In the subgroup analysis by age, the summary RRs in the adult and the child subgroups were 0.899 (95%CI: 0.861-0.939) and 0.779 (95%CI: 0.722-0.840), respectively. The pooled eradication rates of sequential treatments in the adult and the child subgroups were 82.90% and 87.29%.
For the duration of the standard triple therapy subgroup analysis, the pooled RRs in the 7-d, 10-d, and 14-d subgroups were 0.800 (95%CI: 0.752-0.851), 0.849 (95%CI: 0.789-0.913) and 0.980 (95%CI: 0.916-1.048), respectively. The pooled eradication rates of sequential treatments in the 7-d, 10-d, and 14-d subgroups were 87.52%, 80.17%, and 89.72%, respectively.
In the PPIs subanalyses, the pooled RRs in the omeprazole, esomeprazole, pantoprazole and rabeprazole subgroups were 0.832 (95%CI: 0.772-0.898), 0.932 (95%CI: 0.871-0.998), 0.846 (95%CI: 0.746-0.960), and 0.847 (95%CI: 0.766-0.936), respectively. The pooled eradication rates of sequential treatments in the omeprazole, esomeprazole, pantoprazole and rabeprazole subgroups were 87.37%, 77.01%, 87.13%, and 91.24%, respectively.
The examination of the different drugs used in the control group subanalysis showed the pooled RRs in the tinidazole and metronidazole subgroups were 0.889 (95%CI: 0.837-0.944) and 0.810 (95%CI: 0.745-0.882), respectively. The pooled eradication rates of sequential treatments in the tinidazole and metronidazole subgroups were 81.43% and 83.20%, respectively.
We also performed sensitivity analyses and found the pooled RRs were unchanged.
Data on adverse events were available for 16 trials. The pooled RR was 1.176 (95%CI: 0.975-1.419), which indicated no significant difference and no evidence of heterogeneity (I2 = 0%, P = 0.827).
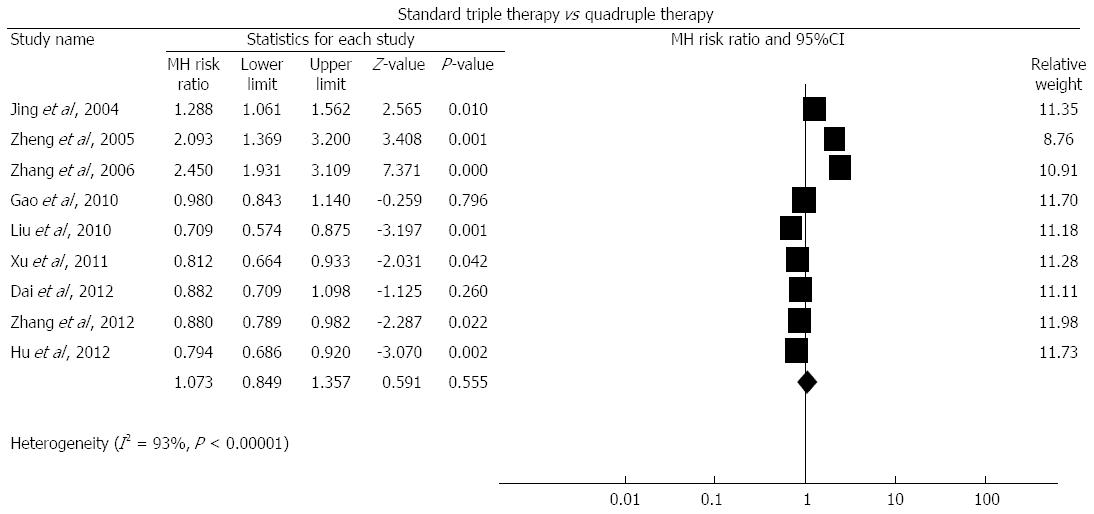
Standard triple therapy vs quadruple therapy: There were 9 studies[13,37-40,42,56,60,62] comparing standard triple therapy with quadruple therapy. As shown in Figure 5, the pooled RR was 1.073 (95%CI: 0.849-1.357), P = 0.555. We found evidence of heterogeneity (I2 = 93%, P < 0.00001) with funnel plot asymmetry (Egger’s test coefficient -2.58 to 13.51, P = 0.15). The pooled eradication rate of quadruple therapy was 64.9% based on this meta-analysis.
We performed a cumulative meta-analysis and the pooled RRs varied little with time. We also performed sensitivity analyses and found the pooled RRs were unchanged.
Due to the heterogeneity, we performed subgroup analyses according to the course of the quadruple therapy and different PPIs in the standard triple therapy.
The pooled RRs in the 1-d, 3-d, 7-d and 10-d duration of quadruple therapy subanalysis were 2.367 (95%CI: 1.923-2.914), 1.288 (95%CI: 1.061-1.562), 0.790 (95%CI: 0.718-0.868), and 0.917 (95%CI: 0.839-1.002), respectively. The pooled eradication rates of quadruple treatments in the 1-d, 3-d, 7-d and 10-d subgroups were 28.42%, 66.67%, 86.79%, and 88.04%, respectively.
For the subanalysis of PPIs, the pooled RRs in the omeprazole, esomeprazole, lansoprazole and rabeprazole subgroups were 1.250 (95%CI: 1.012-1.545), 1.098 (95%CI: 0.699-1.725), 1.391 (95%CI: 0.404-4.790), and 0.948 (95%CI: 0.771-1.166), respectively. The pooled eradication rates of quadruple treatments in the omeprazole, esomeprazole, lansoprazole and rabeprazole subgroups were 66.67%, 73.76%, 45.00%, and 83.99%, respectively.
Data on adverse events were available for 7 trials. The RR was 0.940 (95%CI: 0.825-1.072), which indicated no significant difference with no evidence of heterogeneity (I2 = 0%, P = 0.56).
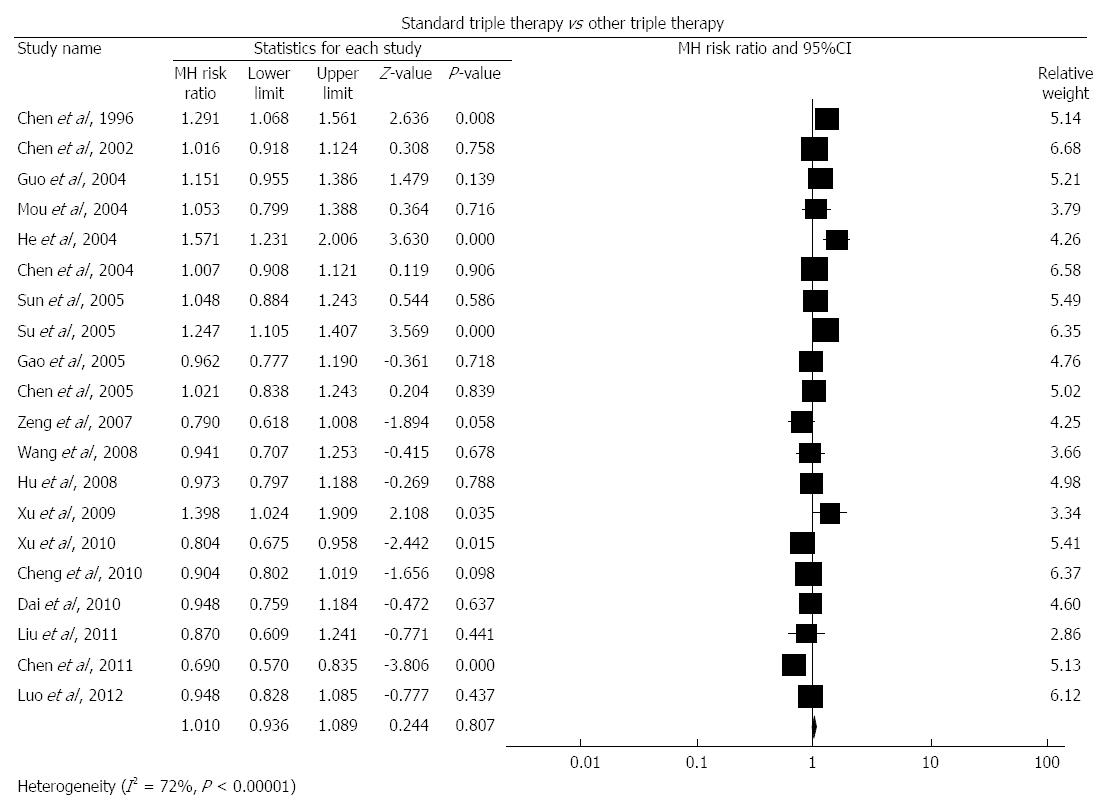
Standard triple therapy vs other triple therapies: There were 20 studies[20-36,57-59] comparing standard triple therapy with other triple therapies. As shown in Figure 6, the pooled RR was 1.01 (95%CI: 0.936-1.089, P = 0.807). We found evidence of heterogeneity (I2 = 72%, P < 0.00001) but no funnel plot asymmetry (Egger’s test coefficient -2.35 to 3.51, P = 0.68). The pooled eradication rate of other triple therapy was 79.9% based on this meta-analysis.
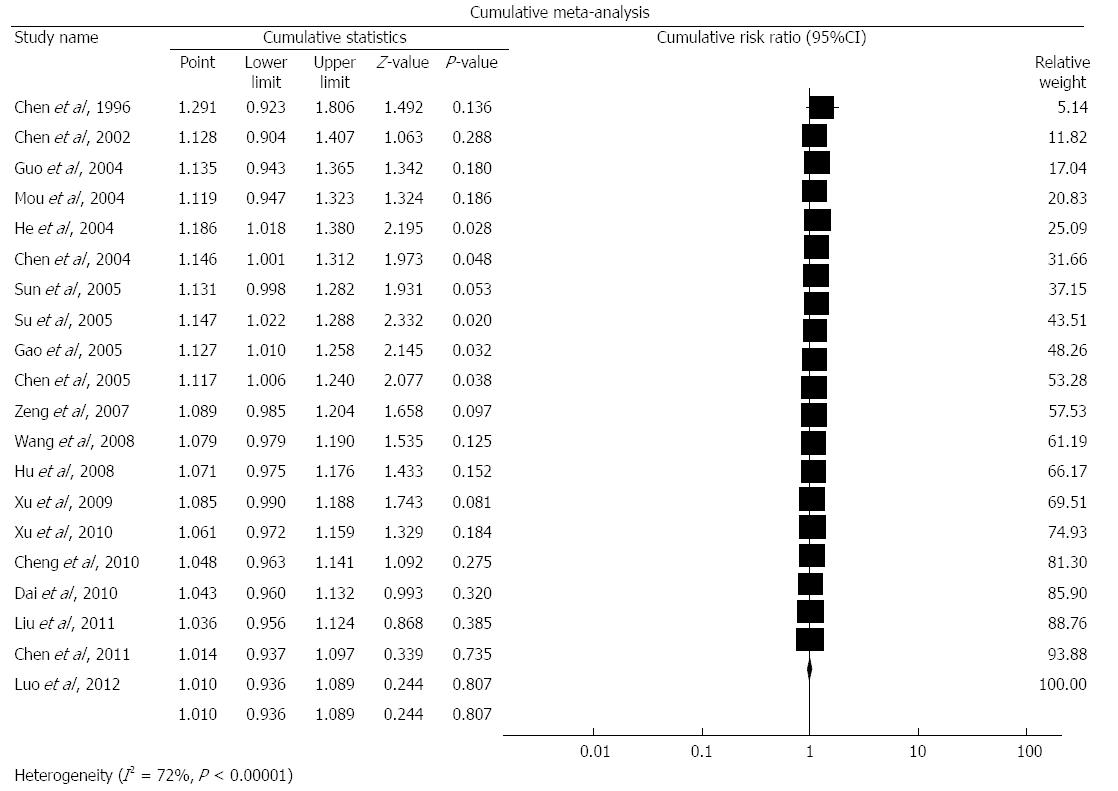
As shown in Figure 7, we performed a cumulative meta-analyses and the pooled RRs varied little with time. We also performed sensitivity analyses and found the pooled RRs were unchanged.
Due to the heterogeneity, we performed subgroup analyses according to age, duration of standard triple therapy, different PPIs in standard triple therapy, and different drugs used in the control group (other triple therapy group).
The subanalysis by age showed that the summary RRs in the adult and the child subgroups were 0.999 (95%CI: 0.925-1.078) and 1.079 (95%CI: 0.748-1.557), respectively. The pooled eradication rates of other triple therapies in the adult and the child subgroups were 80.92% and 73.25%.
For the subanalysis of duration of standard triple therapy, the pooled RRs in the 7-d, 10-d and 14-d subgroups were 1.022 (95%CI: 0.949-1.100), 0.933 (95%CI: 0.821-1.060) and 1.050 (95%CI: 0.712-1.549), respectively. The pooled eradication rates of other triple therapies in the 7-d, 10-d, and 14-d subgroups were 80.32%, 77.78%, and 78.40%, respectively.
The PPI subanalysis indicated the pooled RRs in the omeprazole subgroup and the esomeprazole subgroup were 1.048 (95%CI: 0.976-1.125) and 0.911 (95%CI: 0.831-0.999), respectively.
To examine the different drugs used in the control group subanalysis, the pooled RRs for the levofloxacin, furazolidone, and metronidazole subgroups were 0.917 (95%CI: 0.852-0.987), 0.963 (95%CI: 0.762-1.216), and 1.119 (95%CI: 0.882-1.420), respectively. The pooled eradication rates of other triple therapies in the levofloxacin, furazolidone, and metronidazole subgroups were 82.37%, 85.22%, and 68.84%, respectively.
Data on adverse events were available for 16 trials. The pooled RR was 1.081 (95%CI: 0.848-1.378), indicating no significant difference and no evidence of heterogeneity (I2 = 0%, P = 0.79).
Graham et al[63] stratified the effectiveness of the treatment regimens for H. pylori infection into the following grades based on PP analysis: excellent regimen, if the eradication rate was more than 95%; good regimen, if a 91%-95% eradication rate was achieved; borderline, if the eradication rate was 85%-89%; and unacceptable if the eradication rate was less than 85%.
The standard triple treatment includes a PPI, CLA, and AMO or metronidazole to treat H. pylori infection. This treatment has become universal since all of the consensus conferences and guidelines worldwide recommend this treatment.
The following are the primary mechanisms of standard triple therapy: (1) AMO can impede the synthesis of the cell walls of H. pylori and can increase the concentration of CLA in H. pylori infection. Thus, the combination of the two can exert synergism; and (2) PPIs that modify the pH of gastric juice can inhibit the growth of H. pylori and diminish the activity of urease. Furthermore, PPIs can improve the concentration of CLA and AMO in the stomach by raising the pH of the gastric juice.
However, this triple regimen was used worldwide and the prevalence of H. pylori resistant to CLA has increased[7,8]. Drug resistance represents the major reason for the low eradication rate of the standard triple regimen consisting of PPIs, AMO and CLA[5]. One study conducted by the H. pylori Study Group of Digestive Diseases Division of the Chinese Medical Association demonstrated that the resistance rates of H. pylori to metronidazole, CLA, and AMO were 75.6%, 27.6%, and 2.7%, respectively[64].
The Maastricht consensus report IV indicates that triple therapy with AMO and CLA is not suitable for first-line therapy when the resistance rate of H. pylori to CLA is greater than 15%-20%. However, when the resistance rate of H. pylori is lower than 15% the regimen is still recommended as the preferred first-line regimen for H. pylori infection. The standard triple therapy shows a better eradication rate in CLA-sensitive strains than in CLA-resistant strains (88% vs 18%)[65].
Our meta-analysis and systematic review showed that the standard triple therapy might not be suitable for first-line therapy in China because the pooled eradication rate is 74.5%.
According to our meta-analysis comparing the standard triple therapy with dual treatments, the eradication rate of the standard triple therapy with PPIs, AMO, and CLA was slightly higher than for dual treatments (RR = 1.14, 95%CI: 0.99-1.31). However, when the study of Gao et al[19] was removed, the difference became significant. This result may be associated with the following causes: (1) the number of studies included in this meta-analysis was insufficient. Only 3 RCTs compared the standard triple therapy with dual treatment; and (2) the quality of the studies was low. More high quality RCTs are required to determine the actual difference between the standard triple therapies and dual treatments.
In the meta-analysis comparing standard triple therapy with sequential treatments, the outcomes demonstrated that standard triple therapy was inferior to sequential treatments (RR = 0.863; 95%CI: 0.824-0.904). The subgroup analyses showed no statistical significance among those treatments. Additionally, a recent study showed sequential treatment could achieve an 89.7% eradication rate by per protocol analysis in China[15]. Thus, although the guidelines of China do not recommended sequential treatment as a first-line therapy, we suggest that it is worth further study to identify the effectiveness of sequential therapy in China. Our subgroup analyses also showed that 14-d treatments were superior to 7-d treatment. These results indicate that a longer duration might be more effective when used for H. pylori infection. The cumulative meta-analysis showed that the RRs were stable.
Our meta-analysis comparing the standard triple vs quadruple treatments showed that the eradication rate of the standard triple treatment was similar to quadruple treatments. This finding conflicts with most of the pre-existing consensus. The results of subgroup analyses showed that the 7-d quadruple treatments were superior to the standard triple treatment (RR = 0.790, 95%CI: 0.718-0.868) and that the 1-d and 3-d quadruple treatments were inferior to the standard triple treatment (RR = 2.367, 95%CI: 1.923-2.914; RR = 1.288, 95%CI: 1.061-1.562, respectively). It was interesting that the effectiveness of the 10-d standard triple treatment was equivalent to that of the 10-d quadruple treatments (RR = 0.917, 95%CI: 0.839-1.002). Although we performed subgroup analyses based on the duration, age, and PPI used in the standard triple groups, the significant heterogeneity in this meta-analysis may also affect its reliability.
Based on our meta-analysis comparing standard triple treatments with other triple treatments, the eradication rate of the standard triple treatments was similar to that of other triple treatments. This result suggested that the standard triple treatment was not inferior to other triple treatments. The eradication rates of both standard triple treatments and other triple treatments were less than 80%. We also performed subgroup analyses based on different PPIs, durations, and treatments in the control group. The results showed that the treatments containing levofloxacin were able to provide higher eradication rates than standard triple therapy and is consistent with other studies[66,67]. Interestingly, the effectiveness of the esomeprazole subgroup was inferior to the control group. There was no statistical significance in other subgroups. To determine the variations in the eradication rate of standard triple treatments compared with other triple treatments against time, we performed a cumulative meta-analysis of the chronological order of the studies’ publication dates. We found that the effectiveness of the standard triple treatment with PPI, AMO and CLA gradually reduced with time. This may be related to the increasing resistance rate of H. pylori to CLA.
To diminish bias there were 2 reviewers who performed the study selection, data extraction and the evaluation of study quality. We comprehensively analyzed the efficacy of the standard triple therapy with PPI, AMO and CLA in anti-H. pylori treatment. The subgroup analyses and sensitivity analyses made the outcomes of our meta-analyses reliable.
There were several limitations to our meta-analysis. First, most of the studies included in our meta-analysis had problems with concealing the allocation and blinding, which might have affected our results. However, we performed sensitivity analyses to determine the reliability of our results. Second, there was heterogeneity in the meta-analysis and we conducted subgroup analyses and sensitivity analyses to decrease these effects. Third, the quality of the studies included in the meta-analysis might also affect our result. Fourth, the available published languages might have exerted a bias. Thus, it is likely that our meta-analysis does not reflect all outcomes. Finally, we asked authors for unpublished data, but their lack of response may have introduced further bias.
In conclusion, the effectiveness of the standard triple therapy with PPIs, AMO, and CLA is inferior to sequential treatments and is similar to other triple treatments, but is not superior to quadruple therapy. The standard triple treatment achieves a low eradication rate for H. pylori infection and is not suitable as a first-line therapy for treatment of H. pylori infection in China.
The standard triple regimen with proton pump inhibitors (PPIs), amoxicillin (AMO) and clarithromycin (CLA) is still recommended as a first-line regimen for treatment of Helicobacter pylori infection by several groups. However, the eradication rate is decreasing and the resistance of Helicobacter pylori (H. pylori) to CLA is increasing.
In China, the efficacy of the standard triple therapy consisting of PPI, AMO and CLA, has been shown by some studies to be considerable over the past decade. However, the prevalence of antibiotic-resistant H. pylori is increasing rapidly. It is unclear if the standard triple therapy is suitable for treatment of H. pylori infection in China.
This was the first meta-analysis comparing the efficiency of standard triple therapy with other pre-existing and new therapies on the Chinese mainland. Furthermore, this meta-analysis also examined the eradication rates for H. pylori and the changes in the eradication rate of the standard triple therapy with time on the Chinese mainland.
The results indicated that standard triple therapy of PPI, AMO, and CLA might not be suitable for first-line therapy on the Chinese mainland and new agents for treatment need to be developed.
This meta-analysis study is well structured and has a great scientific merit. It provides an important contribution to H. pylori therapeutics in China.
P- Reviewer: Cavalini LT, Rotta I S- Editor: Nan J L- Editor: Cant MR E- Editor: Wang CH
| 1. | Tonkic A, Tonkic M, Lehours P, Mégraud F. Epidemiology and diagnosis of Helicobacter pylori infection. Helicobacter. 2012;17 Suppl 1:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 2. | McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 550] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 3. | Current European concepts in the management of Helicobacter pylori infection. The Maastricht Consensus Report. European Helicobacter Pylori Study Group. Gut. 1997;41:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 674] [Article Influence: 24.1] [Reference Citation Analysis (2)] |
| 4. | Malfertheiner P, Bazzoli F, Delchier JC, Celiñski K, Giguère M, Rivière M, Mégraud F. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet. 2011;377:905-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 373] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 5. | Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 736] [Article Influence: 49.1] [Reference Citation Analysis (1)] |
| 6. | Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1352] [Article Influence: 75.1] [Reference Citation Analysis (1)] |
| 7. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1589] [Article Influence: 122.2] [Reference Citation Analysis (5)] |
| 8. | Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 427] [Article Influence: 26.7] [Reference Citation Analysis (1)] |
| 9. | Megraud F, Coenen S, Versporten A, Kist M, Lopez-Brea M, Hirschl AM, Andersen LP, Goossens H, Glupczynski Y. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 632] [Article Influence: 52.7] [Reference Citation Analysis (3)] |
| 10. | O’Connor A, Molina-Infante J, Gisbert JP, O’Morain C. Treatment of Helicobacter pylori infection 2013. Helicobacter. 2013;18 Suppl 1:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Vakil N, Vaira D. Treatment for H. pylori infection: new challenges with antimicrobial resistance. J Clin Gastroenterol. 2013;47:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | De Francesco V, Giorgio F, Hassan C, Manes G, Vannella L, Panella C, Ierardi E, Zullo A. Worldwide H. pylori antibiotic resistance: a systematic review. J Gastrointestin Liver Dis. 2010;19:409-414. [PubMed] |
| 13. | Hu FL, Cheng H, Zhang XZ, An HJ, Sheng JQ, Lü NH, Xie Y, Chen ZS, Xu JM, Hu NZ. [Jinghuaweikang capsules combined with triple therapy in the treatment of Helicobacter pylori associated gastritis and duodenal ulcer and analysis of antibiotic resistance: a multicenter, randomized, controlled, clinical study]. Zhonghua Yixue Zazhi. 2012;92:679-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Zhou YQ, Xu L, Wang BF, Fan XM, Wu JY, Wang CY, Guo CY, Xu XF. Modified Sequential Therapy Regimen versus Conventional Triple Therapy for Helicobacter Pylori Eradication in Duodenal Ulcer Patients in China: A Multicenter Clinical Comparative Study. Gastroenterol Res Pract. 2012;2012:405425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Huang J, Zhou L, Geng L, Yang M, Xu XW, Ding ZL, Mao M, Wang ZL, Li ZL, Li DY. Randomised controlled trial: sequential vs. standard triple therapy for Helicobacter pylori infection in Chinese children-a multicentre, open-labelled study. Aliment Pharmacol Ther. 2013;38:1230-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Yan X, Zhou L, Song Z, Xue Y, Wang Y, Bai P, Hou X, Xu S, Chen M, Xiong L. Sequential therapy for helicobacter pylori eradication in adults compared with triple therapy in China: A multiple center, prospective, randomized, controlled trial. Helicobacter. 2011;16:77-143. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Geng HP, Zhou QD. Combined with pantoprazole, clarithromycin and amoxicillin in treatment of Helicobacter pylori infection. Zhongguo Yiyuan Yaoxue Zazhi. 2003;23:101. [DOI] [Full Text] |
| 18. | Wu TF, Huang AF. Curative effect observation of Anqi piece added on therapy in children with Helicobacter pylori infection. Shiyong Erke Linchuang Zazhi. 2004;19:782-783. [DOI] [Full Text] |
| 19. | Gao J, Li X, Wu X, Shen W, He L. Triple therapy with Rabeprazole, and Sucralfate for eradication of Helicobacter pylori in duodenal ulcer disease. Huaxi Yaoxue Zazhi. 2006;21:213-214. [DOI] [Full Text] |
| 20. | Luo LL, Zhang L, Xu JM, Hu YM, Lu CY, Wang ZH. Comparison of 7-d levofloxacin triple regimen with 10-d standard triple regimen for the first-line eradication of Helicobacter pylori. Anhui Yike Daxue Xuebao. 2012;47:845-848. [DOI] [Full Text] |
| 21. | Chen EP, Lian ZF, Chen LF. The Efficacy of combiantion of amoxicillin-clavulanate potassium and doxycycline for Helicobacter pylori infection in elderly. Zhongguo Laonianxue Zazhi. 2011;31:3049-3050. [DOI] [Full Text] |
| 22. | Liu FL, Zhen LN, Zhao Y, Xia ZW, Situ AM, Zhang SH. The efficacy of sequential therapy and standard triple therapy for eradication of Helicobacter pylori infection in children. Linchuang Erke Zazhi. 2011;29:925-928. [DOI] [Full Text] |
| 23. | Xu PR, Huang J, Zhou F. The efficacy of triple regimen with esomeiprazole levofloxacin and furazolidone for Helicobacter pylori eradication treatment. Zhonghua Yiyuan Ganranxue Zazhi. 2010;20:857-859. |
| 24. | Dai XS, Zhang XX, Chen HP, Li LP, Han SX. Clinical study of levofloxacin triple regimen for Helicobacter pylori eradication. Huaxi Yaoxue Zazhi. 2010;25:222-223. |
| 25. | Xu MH, Tang YB, Zhang GY, Chen FY, Tang LA, Leng AM, Liu JS. Random control study on eradicating of Helicobacter pylori with lansoprazole-based triple therap. Zhongguo Xiandai Yixue Zazhi. 2009;19:267-269. |
| 26. | Wang PW, Jiang HQ, Jing DD. Ranitidine bismuth citrate based triple therapy eradication of Helicobacter pylori: a randomized control study. Zhongguo Xinyao Zazhi. 2008;17:515-517. [DOI] [Full Text] |
| 27. | Hu JH, Wu JS, Chen LG. Omeprazole, levofloxacin and amoxicillin triple therapy for Helicobacter pylori infection. Shiyong Yixue Zazhi. 2008;24:1621-1623. [DOI] [Full Text] |
| 28. | Su L, Xu CD, Chen SN. Curative effects of triple therapy in children with Helicobacter pylori infection. Shiyong Erke Linchuang Zazhi. 2005;20:847-849. [DOI] [Full Text] |
| 29. | Mou FH, Wei H, Hu FL. A clinical trial of swibec-based triple regimens for Helicobacter pylori eradication. Zhongguo Xinyao Zazhi. 2004;13:1036-1039. [DOI] [Full Text] |
| 30. | Chen Y, Chen YP, Tao RF. Levofloxacin triple therapy for eradication of Helicobacter pylori infection in 106 cases. Zhongguo Xinyao Yu Linchuang Zazhi. 2002;21:437-439. [DOI] [Full Text] |
| 31. | Chen SP, Chen ZQ, Bei L, Wen SH. Omeprazole, clarithromycin and amoxicillin therapy for Helicobacter pylori infection. Zhonghua Neike Zazhi. 1996;35:8-11. |
| 32. | Cheng H, Hu FL, Zhang GX, Shi RH, Du YQ, Li ZS, Han W, Li YQ, Wu QD, Qian KD. [Levofloxacin-based triple therapy for first-line Helicobacter pylori eradication treatment: a multi-central, randomized, controlled clinical study]. Zhonghua Yixue Zazhi. 2010;90:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Zeng YM, Zhu MJ. Comparison of omeprazole, amoxicillin and clarithromycin or levofloxacin 1 week triple therapy for Helicobacter pylori eradication. Chongqing Yixue. 2007;36:1317-1318. [DOI] [Full Text] |
| 34. | Gao G, Li H, Cao J. Observation of ranitidine bismuth citrate treatment of Helicobacter pylori related peptic ulcer. Shiyong Yixue Zazhi. 2005;21:2600. [DOI] [Full Text] |
| 35. | Chen H, Li BT, Zhou HM. The efficacy of azithromycin, amoxicillin and omeprazole triple therapy for Helicobacter pylori eradication. Zhongguo Quanke Yixue. 2004;7:1858-1859. [DOI] [Full Text] |
| 36. | Chen YZ, Guo J, Chen D. Comparison of efficacy of azithromycin triple and clarithromycin triple therapy for eradication of Helicobacter pylori. Chongqing Yixue. 2005;34:1403-1404. [DOI] [Full Text] |
| 37. | Dai XS, Zhang L, Xie DL, Chen HP, Li LP, Han SX. Clinical efficacy of quadruple therapy including furazolidone on Helicobacter pylori infection. Huaxi Yaoxue Zazhi. 2012;27:595-596. |
| 38. | Xu MH, Zhang GY, Li CJ. Efficacy of bismuth-based quadruple therapy as first-line treatment for helicobacter pylori infection. Zhejiang Daxue Xuebao (Yi Xue Ban). 2011;40:327-331. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 39. | Liu BT, Liu PF, Zhao FJ. Clinical observation of compound bismuth aluminate granules combined with ray Bella, clarithromycin, amoxicillin in the treatment of Helicobacter pylori associated ulcer. Jiangsu Yiyao. 2010;36:2821-2822. |
| 40. | Jing DD, Wang P, Chen J, Zhou Y, Wang N, Wang X. Rabeprazole-based short term triple or quadruple regimens for Helicobacter pylori eradication: a randomized control study. Zhonghua Xiaohua Zazhi. 2004;24:250-251. [DOI] [Full Text] |
| 41. | Jia DQ. Efficacy and safety of eradication of Helicobacter pylori infection with levofloxacin sequential therapy of patients with duodenal ulcer. Zhongguo Neijing Zazhi. 2012;18:1304-1306. |
| 42. | Zhang J, Yang J, Wang HT, Liu YL, Duan HL. Efficacy of sequential therapy for Helicobacter pylori eradication. Tanjin Yiyao. 2012;40:79-80. [DOI] [Full Text] |
| 43. | Li L, Lin QH. Clinical Analysis of 10 Day Sequential therapy for Helicobacter pylori infection children. Xiandai Yufang Yixue. 2012;39:2440-2441. |
| 44. | Zhou W. Comparison of the efficacy of ten-day sequential therapy with conventional triple therapy for Helicobacter pylori eradication. Xiandai Yufang Yixue. 2011;38:3834-3835. |
| 45. | Wu GL, Lan Y, Zhang XJ. Sequential therapy versus standard triple therapy for Helicobacter pylori eradication. Shijie Huaren Xiaohua Zazhi. 2011;19:3100-3109. |
| 46. | Zhu LM. The efficacy and safety of amoxicillin-clavulanate potassium and rebeprazole for Helicobacter pylori infection in children. Zhongguo Shiyong Erke Zazhi. 2010;25:545-548. |
| 47. | Zhang FY, Huang XX. Sequential treatment for Helicobacter pylori eradication in peptic ulcer patients. Zhongguo Quanke Yixue. 2010;13:3813-3814. [DOI] [Full Text] |
| 48. | Lu JH, Xu MY, Shen Y, Yang WX. Comparison of the efficacy of 10 day sequential therapy and conventional triple therapy for Helicobacter pylori infection in children. Zhongguo Dangdai Erke Zazhi. 2010;12:988-990. |
| 49. | Hu SQ, Zhang M. A 10-day sequential therapy for Helicobacter pylori-infected patients: an analysis of 39 cases. Shijie Huaren Xiaohua Zazhi. 2009;17:1693-1695. [DOI] [Full Text] |
| 50. | Pang SZ, Zhao WX, Ren Y. The comparison of 10 day sequential therapy and 14 day triple therapy for eradication Helicobacter pylori. Shiyong Yixue Zazhi. 2009;25:3058-3060. [DOI] [Full Text] |
| 51. | Wang RX, Qin J. Comparison of 10-day sequential therapy with standard therapy for Helicobacter pylori. Chongqing Yixue. 2009;38:2322-2323. [DOI] [Full Text] |
| 52. | Zhao QX, Huang DY. Efficacy of tinidazole-containing sequential therapy in the eradication of Helicobacter pylori infection. Shijie Huaren Xiaohua Zazhi. 2009;17:3666-3669. [DOI] [Full Text] |
| 53. | Huang CT. The comparison of 10 day sequential therapy and standard triple therapy for eradication of Helicobacter pylori. Guangdong Yixue. 2009;30:1657. [DOI] [Full Text] |
| 54. | Ma CX, Peng GL, Zhao YK, Ma XY, Zhan LY. Treatment outcomes of functional dyspepsia patients with Helicobacter pylori infection: a comparison between sequential treatment regimen and conventional triple therapy. Dier Junyi Daxue Xuebao. 2008;29:908-911. [DOI] [Full Text] |
| 55. | Huang XX, Xiong LS, Ma S, Bai P, Dong Y, Yang X, Gao X, Liang L, Zhou L, Chen M. Efficacy of triple therapy and sequential therapy in the eradication of Helicobacter pylori in patients receiving long-term non-steroidal anti-inflammatory drugs treatment. Zhonghua Xiaohua Zazhi. 2012;32:814-817. [DOI] [Full Text] |
| 56. | Gao XZ, Qiao XL, Song WC, Wang XF, Liu F. Standard triple, bismuth pectin quadruple and sequential therapies for Helicobacter pylori eradication. World J Gastroenterol. 2010;16:4357-4362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 57. | He XX, Zhao YH, Hao YT. [Effect of CYP2C19 genetic polymorphism on treatment efficacy of Helicobacter pylori infection with rabeprazole-based triple therapy in Chinese]. Zhonghua Neike Zazhi. 2004;43:13-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 58. | Guo CY, Wu YB, Liu HL, Wu JY, Zhong MZ. Clinical evaluation of four one-week triple therapy regimens in eradicating Helicobacter pylori infection. World J Gastroenterol. 2004;10:747-749. [PubMed] |
| 59. | Sun WH, Ou XL, Cao DZ, Yu Q, Yu T, Hu JM, Zhu F, Sun YL, Fu XL, Su H. Efficacy of omeprazole and amoxicillin with either clarithromycin or metronidazole on eradication of Helicobacter pylori in Chinese peptic ulcer patients. World J Gastroenterol. 2005;11:2477-2481. [PubMed] |
| 60. | Zheng Q, Pan Y, Zhang L, Xiao SD. Comparison of the efficacy of 1-day high-dose quadruple therapy versus 7-day triple therapy for treatment of Helicobacter pylori infection. Chin J Dig Dis. 2005;6:202-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 61. | Huang J, Gong ST, Ou WJ, Pan RF, Geng LL, Huang H, He WE, Chen PY, Liu LY, Zhou LY. [A 10-day sequential therapy for eradication of Helicobacter pylori infection in children]. Zhonghua Erke Zazhi. 2012;50:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 62. | Zhang L, Shen L, Ma JL, Pan KF, Liu WD, Li J, Xiao SD, Lin SR, Classen M, You WC. Eradication of H pylori infection in a rural population: one-day quadruple therapy versus 7-day triple therapy. World J Gastroenterol. 2006;12:3915-3918. [PubMed] |
| 63. | Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014;12:177-186.e3; Discussion e12-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 64. | Chinese Helicobacter pylori Research Group, Chinese Society of Gastroenterology, Cheng H, Hu FL, Xie Y, Hu PJ, Wang JY, Lv NH, Zhang JZ, Zhang GY, Zhou Zf, Wu KL, Zhang LX, Peng XW, Dai N, Tang GD, Jiang K, Li Y, Hou XH, Bai WY, Wang MY, Wang MC, Ye HJ, Liu YL, Xu L. Prevalence of Helicobacter pylori resistance to antibiotics and its influence on the treatment outcome in China: A multicenter clinical study. Weichang Bing Xue. 2007;12:525-530. [DOI] [Full Text] |
| 65. | Mégraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53:1374-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 681] [Article Influence: 32.4] [Reference Citation Analysis (2)] |
| 66. | Gisbert JP, Pajares R, Pajares JM. Evolution of Helicobacter pylori therapy from a meta-analytical perspective. Helicobacter. 2007;12 Suppl 2:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 67. | Zhang ZF, Zhao G, Liu LN. [Effectiveness and safety of proton pump inhibitor and levofloxacin based first-line triple therapy in the eradication of Helicobacter pylori: a meta-analysis]. Zhonghua Yixue Zazhi. 2008;88:2722-2725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |