Published online Oct 28, 2014. doi: 10.3748/wjg.v20.i40.14884
Revised: March 31, 2014
Accepted: May 12, 2014
Published online: October 28, 2014
Processing time: 293 Days and 9.3 Hours
AIM: To improve the colonization rate of transplanted mesenchymal stem cells (MSCs) in the liver and effect of MSC transplantation for acute liver failure (ALF).
METHODS: MSC was modified with the chemokine CXC receptor 4 (CXCR4) gene (CXCR4-MSC) or not (Null-MSC) through lentiviral transduction. The characteristics of CXCR4-MSCs and Null-MSCs were determined by real-time quantitative polymerase chain reaction, Western blotting and flow cytometry. CXCR4-MSCs and Null-MSCs were infused intravenously 24 h after administration of CCl4 in nude mice. The distribution of the MSCs, survival rates, liver function, hepatocyte regeneration and growth factors of the recipient mice were analyzed.
RESULTS: In vitro, CXCR4-MSCs showed better migration capability toward stromal cell-derived factor-1α and a protective effect against thioacetamide in hepatocytes. In vivo imaging showed that CXCR4-MSCs migrated to the liver in larger numbers than Null-MSCs 1 and 5 d after ALF. Higher colonization led to a longer lifetime and better liver function. Either CXCR4-MSCs or Null-MSCs exhibited a paracrine effect through secreting hepatocyte growth factor and vascular endothelial growth factor. Immunohistochemical analysis of Ki-67 showed increased cell proliferation in the damaged liver of CXCR4-MSC-treated animals.
CONCLUSION: Genetically modified MSCs expressing CXCR4 showed greater colonization and conferred better functional recovery in damaged liver.
Core tip: Mesenchymal stem cell (MSC) transplantation is an effective therapy for acute liver failure (ALF), and is expected to be an alternative to liver transplantation. Many current studies have exhibited poor efficiency of MSC transplantation due to low colonization in the failing liver. We modified MSCs with the chemokine CXC receptor 4 (CXCR4) gene to promote the colonization of MSCs in failing liver depending on the stromal cell-derived factor-1α/CXCR4 axis. In vivo imaging showed that CXCR4-MSCs migrated to the liver in higher numbers than Null-MSCs 1 and 5 d after ALF. Greater colonization led to a longer lifetime and better liver function.
- Citation: Ma HC, Shi XL, Ren HZ, Yuan XW, Ding YT. Targeted migration of mesenchymal stem cells modified with CXCR4 to acute failing liver improves liver regeneration. World J Gastroenterol 2014; 20(40): 14884-14894
- URL: https://www.wjgnet.com/1007-9327/full/v20/i40/14884.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i40.14884
In recent years, mesenchymal stem cells (MSCs) have shown paracrine and immunoregulatory effects to repair damaged tissues[1,2]. A large number of studies based on stem cell transplantation has achieved impressive results and has provided new ideas for treatment of various diseases. MSC transplantation has also been used to treat a variety of end-stage liver diseases, including acute liver failure (ALF)[3-5]. However, many researchers have also found the phenomenon of poor efficacy of cell transplantation. Retrospective studies have revealed that low colonization of transplanted MSCs in the liver was the main reason restricting the efficacy of MSC transplantation[6].
A series of studies has confirmed the accelerative effect of stromal cell-derived factor (SDF)-1α in homing and survival of stem cells[7-11]. SDF-1α is a chemoattractant protein of the CXC family produced by bone marrow stromal cells. SDF-1α and its receptor, chemokine CXC receptor 4 (CXCR4), are widely expressed in a variety of cells and tissues, including immune cells, brain, heart, liver, kidney, lung and spleen[12]. A large number of studies have shown that the SDF-1α/CXCR4 axis plays an important role in stem cell homing, chemotaxis, expression of adhesion molecules, engraftment, proliferation and survival[13,14]. SDF-1α can mobilize bone marrow stem cells and promote their migration to damaged tissue by binding to CXCR4 distributed on stem cell membranes[9-11]. Zhang et al[15] have shown that the SDF-1α/CXCR4 axis can significantly improve the number of transplanted stem cells in the infarcted myocardium and cardiac function. Liu et al[16] have also found that hypoxic stimulation can improve CXCR4 expression of MSCs, which enhances functional recovery, accelerates the mitogenic response, and reduces apoptotic cell death in the kidneys. Moreover, Du et al[17] have recently shown improved homing of CXCR4-transduced MSCs to the liver post-transplantation, with improved liver function.
CXCR4 expression is endogenously regulated by tissue environmental factors such as cytokines, chemokines, stromal cells, adhesion molecules, hypoxia, tissue damage and proteolytic enzymes[18]. SDF-1α is secreted by cells within the damaged liver, and is a potent chemoattractant for cells expressing CXCR4. Overexpression of CXCR4 appears to be an effective strategy to accelerate mobilization of these cells toward the damaged area. Due to low native levels of CXCR4 expression in MSCs, they migrate sluggishly towards an SDF-1α gradient following ALF. We hypothesized that overexpression of CXCR4 in MSCs would enhance their engraftment in the damaged liver and improve liver regeneration.
This study was approved by the Institutional Animal Care and Use Committee of Nanjing University, China under the NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize suffering.
Male 7-wk-old nude mice (nu/nu) were purchased from the Laboratory Animal Center of the Affiliated Drum Tower Hospital of Nanjing University Medical School, and housed individually, placed in a ventilated cabinet under controlled air pressure and temperature conditions, and under daily cycles of alternating 12 h light/dark. They had sterile water and sterile standard pelleted rodent diet. The mice were sacrificed by cervical spine dislocation.
The human CXCR4 gene sequence was synthesized by Invitrogen (Shanghai, China) and digested by PacI/AscI. The identity of the gene was confirmed by sequencing, and was subsequently cloned into the same restriction sites on the shuttle vector pLenti6.3-hCXCR4-IRES2-EGFP, which contains enhanced green fluorescence protein (EGFP). The resulting shuttle vector was mixed with Packaging Mix (pLP1, pLP2 and pLP/VSVG; Invitrogen). The mixed vector was added into the Opti-MEM (Invitrogen) culture medium and transfected into the 293T packaging cell line. Recombinant lentivirus expressing both EGFP and CXCR4 was harvested after 2 d and viral titers were determined.
The human bone marrow MSCs, UE7T-13 cells[19,20], were used. These cells were immortalized by infection with a retrovirus carrying human telomerase reverse transcriptase (hTERT) and one of the early genes of the human papilloma virus, E7. Although hTERT has been introduced into UET7T-13 cells, it is reported that the differentiation potential of the cells is not affected[19]. MSCs were cultured overnight in six-well plates (1.0 × 105 cells/well). The lentivirus with CXCR4 gene (Lenti6.3-hCXCR4-IRES2-EGFP) or without CXCR4 gene (Lenti6.3-eGFP) were diluted by culture medium consisting of Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Grand Island, NY, United States), 2% (v/v) fetal bovine serum (Invitrogen) and 8 μg/mL polybrene (Invitrogen). MSCs were transduced using dilutions of concentrated virus equivalent to 5 × 106 infectious units (MOI = 50). MSCs transduced with Lenti6.3-eGFP served as a control. Blasticidin (Invitrogen) was added to the culture medium 48 h later. Culture medium with Blasticidin was changed every 2-3 d.
Total RNA was isolated from fresh cells using TRIzol reagent (Invitrogen). First-strand cDNA was synthesized using the Superscript II Reverse Transcriptase Kit (Invitrogen). Quantitative polymerase chain reaction (qPCR) was performed using Power SYBR Green PCR Master Mix (ABI, United States). The relative level of gene expression was normalized to an internal control (level of GAPDH) and calculated using the 2-ΔΔCT method. The sequences of primers was: human CXCR4 sense primer sequence: 5’-CCACGCCACCAACAGTCAGA-3’ and antisense primer sequence: 5’-AGGCAGGATAAGGCCAACCAT-3’; human GAPDH sense primer sequence: 5‘-GAAGGTCGGAGTCAACGGATT-3’ and antisense primer sequence: 5’-CGCTCCTGGAAGATGGTGAT-3’. Biological repeats were performed using three different samples for each genotype, and technical triplicates were carried out for each gene expression analysis. The mean relative expression of each gene between groups was used for statistical significant analysis.
Membrane proteins were obtained by membrane proteins extraction kit according to the manufacturer’s instructions (Beyotime, China). About 5 × 107 cells were centrifuged at 600 g for 5 min at 4 °C. The pellet was washed with 2 mL ice-cold PBS. The supernatant was removed without disturbing the pellet and discarded. Phenylmethylsulfonyl fluoride (protease inhibitor) was added to membrane proteins extraction reagent A 2 min before use. One milliliter of membrane proteins extraction reagent A was added to the wall of the tube and the cell pellet was mixed, resuspended and incubated for 10 min at 4 °C under gentle agitation. Cell nuclei and undisrupted cells were sedimented at 700 g and 4 °C for 10 min. The supernatant was collected without sedimentation. The cell membrane fragments were sedimented at 14000 g for 30 min at 4 °C. The supernatant was removed. Two hundred microliters of membrane proteins extraction reagent B was added to the tube and the sediment was resuspended with 5 s vortex agitation. The tube was kept on ice for 10 min. Membrane protein was extracted by centrifugation at 14000 g for 5 min at 4 °C. The supernatant (membrane fraction) was collected and stored at -20 °C until used for western blotting analysis. Membrane protein extract (20 μg) was separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The membrane was blocked with 5% milk in Tris-buffered saline solution (pH 7.6) containing 0.05% Tween-20, and incubated with primary antibodies for CXCR4 (Abcam, Cambridge, United Kingdom) overnight at 4 °C. The membrane was incubated for 1 h with horseradish-peroxidase-conjugated secondary antibody at room temperature, washed and developed with the ECL plus kit (Millipore, Billerica, MA, United States).
The rate of CXCR4 expression was determined by flow cytometry. CXCR4-MSCs were labeled by APC Mouse Anti-Human CD184 (BD Pharmingen, San Diego, CA, United States) according to manufacturer’s instructions. About 1 × 106 cells were transferred into a flow cytometry tube and centrifuged at 453 g for 5 min at 4 °C. The cells were washed with 1 mL PBS and centrifuged at 453 g for 5 min at 4 °C. The cells were resuspended in 1 mL PBS and mixed with 20 μL APC Mouse Anti-Human CD184. Incubation was carried out with the antibody at room temperature in the dark. The tube was centrifuged at 453 g for 5 min at 4 °C. The supernatant was removed and the cells were washed with 1 mL PBS and centrifuged at 453 g for 5 min at 4 °C twice. The cells were resuspended in 500 μL PBS and measured by flow cytometry.
SDF-1α was measured in liver tissue collected after injecting CCl4. For detection of SDF-1α in liver tissue, frozen tissue samples were weighed before homogenization. One hundred milligrams of tissue was minced and homogenized in 1 mL PBS with a glass homogenizer on ice. The homogenates were centrifuged at 13400 g for 5 min and the supernatants were stored at -80 °C prior to analysis. The concentration of SDF-1α was determined using ELISA kits according to the manufacturer’s instructions (R and D Systems, Minneapolis, MN, United States), and the wells were read at 450 nm on an optical plate reader. Standard curves were prepared using purified cytokine standards. Each experimental sample was run in duplicate.
Migration assays were carried out in a six-well Transwell using polycarbonate membranes with 8-μm pores (Millipore, Germany). Null-MSCs or CXCR4-MSCs at a density of 2 × 105 cells/mL in 100 μL medium (α-minimal essential medium + 0.5% fetal bovine serum) were placed in the upper chamber of the Transwell assembly. The lower chamber contained 600 μL medium with 30 ng/mL SDF-1α (Sigma, St Louis, MO, United States). After incubation at 37 °C and 5% CO2 for 10 h, the upper surface of the membrane was scraped gently to remove nonmigrating cells and washed with PBS. The membrane was fixed in 4% paraformaldehyde for 15 min and stained with 0.5% crystal violet for 10 min. The number of migrating cells was determined by counting five random fields per well under the microscope at 400 × magnification.
Hepatocytes were isolated from nude mice by a two-step collagenase perfusion procedure. The cells were seeded in the lower level of Transwell chambers at a density of 1 × 105 cells/cm2 in hepatocyte RPMI 1640 medium. On day 2, thioacetamide (TAA) (Sigma-Aldrich, Poole, Dorset, United Kingdom), a liver toxin, was added to DMEM at a final concentration of 0.2 μg/μL. At 6 h after addition of TAA, 1 × 105 Null-MSCs or CXCR4-MSCs preseeded in Transwell chambers were placed into the well containing TAA-treated hepatocytes for subsequent coculture. An empty Transwell chamber served as the control. Cell viability was determined with the WST-1 kit (Roche, Burgess Hill, West Sussex, United Kingdom) and the caspase-3 activity was determined using a Caspase-3 kit (Invitrogen, Paisley, United Kingdom).
Seventy-five nude mice were randomly divided into three groups. On day 1, 20% v/v CCl4 (8 μL/g, dissolved in olive oil) was administered intraperitoneally to establish an ALF model. On day 2, Group A (n = 25) was injected with PBS via the tail vein. Group B (n = 25) received 1 × 106 Null-MSCs and Group C (n = 25) was treated with 1 × 106 CXCR4-MSCs via the tail vein. In each group, 10 mice were picked randomly for survival analysis. The remaining mice were sacrificed on days 1, 3, 5, 7 and 14. Serum was used for biochemical analyses. The livers were dissected out and frozen in liquid nitrogen.
The MSCs were washed three times with PBS, trypsinized with 0.05% trypsin-EDTA (Gibco, United States). The MSCs were incubated with 50 μmol/L DiR buffer for 20 min at 37 °C according to the manufacturer’s protocol (Fanbo Biochemicals, Beijing, China). The DiR-labeled MSCs were centrifuged at 453 g for 5 min and the cells were resuspended in PBS. This procedure was repeated twice to ensure complete removal of any unbound dye.
Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels in mouse blood were measured with an automated biochemical analyzer. For histology, livers were fixed in 4% formaldehyde for 24 h and embedded in paraffin. Five-micrometer-thick liver sections were deparaffinized and fixed. Sections were stained with hematoxylin and eosin (Sigma-Aldrich).
The nude mice were anesthetized with chloral hydrate and placed in the In Vivo Imaging System (IVIS Spectrum, Caliper Life Sciences, Runcorn, Cheshire, United Kingdom) and images were acquired using the CCD camera at 1 and 5 d. Data analysis was performed using Living Image version 4.3.1 (Caliper Life Sciences).
Serum samples were collected from nude mice at 14 d after administration of PBS, Null-MSCs, or CXCR4-MSCs. Quantification of serum levels of hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF) was determined by ELISA as per the manufacturer’s instructions (R and D Systems).
Liver tissues were harvested at 14 d and made into paraffin sections for Ki-67 immunohistochemical staining. Anti-Ki67 antibody (Abcam) was used to detect Ki-67 protein expression in hepatocytes, and hematoxylin was used to indicate the nucleus of the hepatocytes.
All experiments were replicated a minimum of three times. Statistical analysis was performed with SPSS version 19.0, and data were expressed as means ± SD. For survival analyses, a Kaplan-Meier method was used. All other data were analyzed by the independent-sample test. P < 0.05 was considered statistically significant.
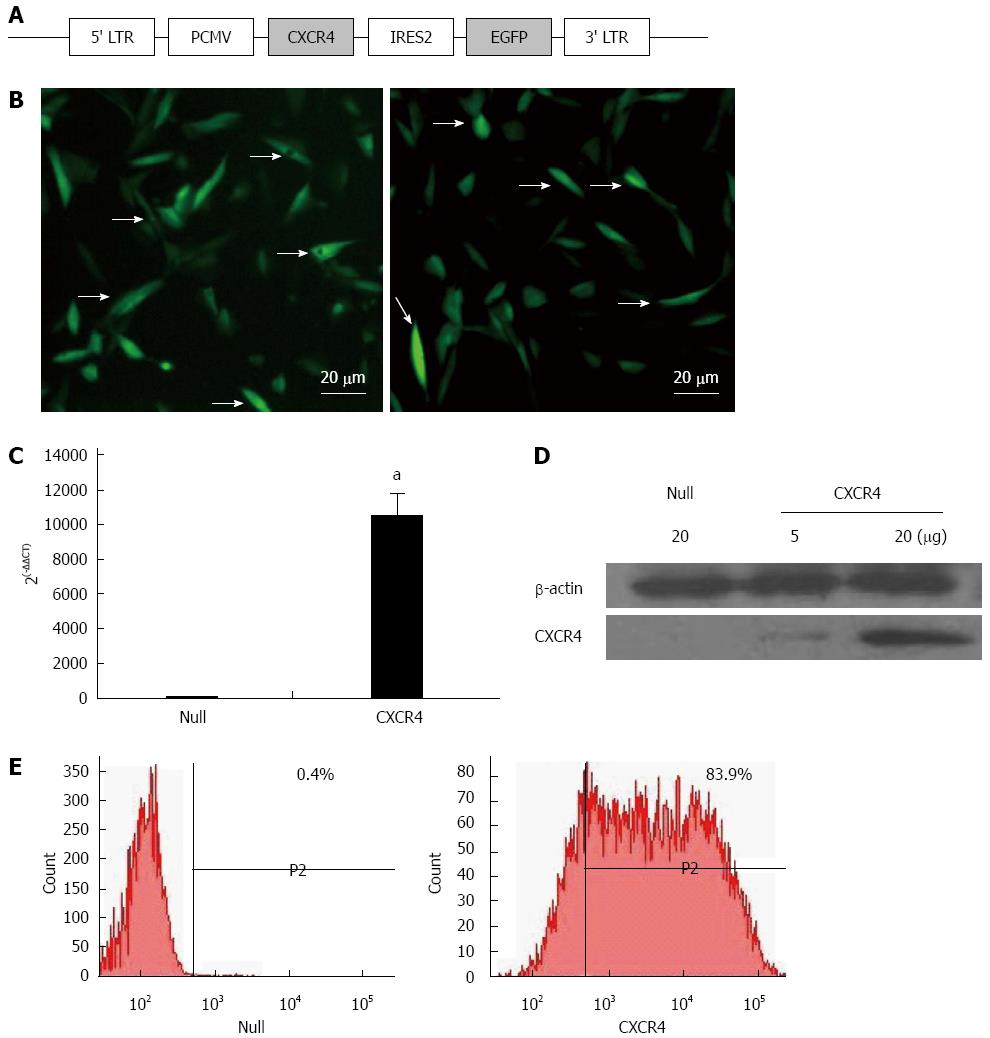
Expression of the CXCR4-EGFP fusion protein was driven by virus long terminal repeat (Figure 1A). MSCs lentivirally transduced with the Null-eGFP gene (Null-MSCs) and CXCR4-eGFP fusion gene (CXCR4-MSCs) were analyzed for expression of CXCR4 by fluorescence microscopy (Figure 1B). CXCR4 overexpression in MSCs was verified by qRT-PCR (Figure 1C). qRT-PCR showed that CXCR4 expression was > 10000-fold higher in CXCR4-MSCs compared with Null-MSCs. Overexpression of CXCR4 was confirmed by Western blotting using membrane extraction (Figure 1D). Flow cytometry showed the fraction of MSCs expressing CXCR4 and it was > 200-fold greater (83.9%) in the CXCR4 group than the Null group for CXCR4 (0.4%, Figure 1E).
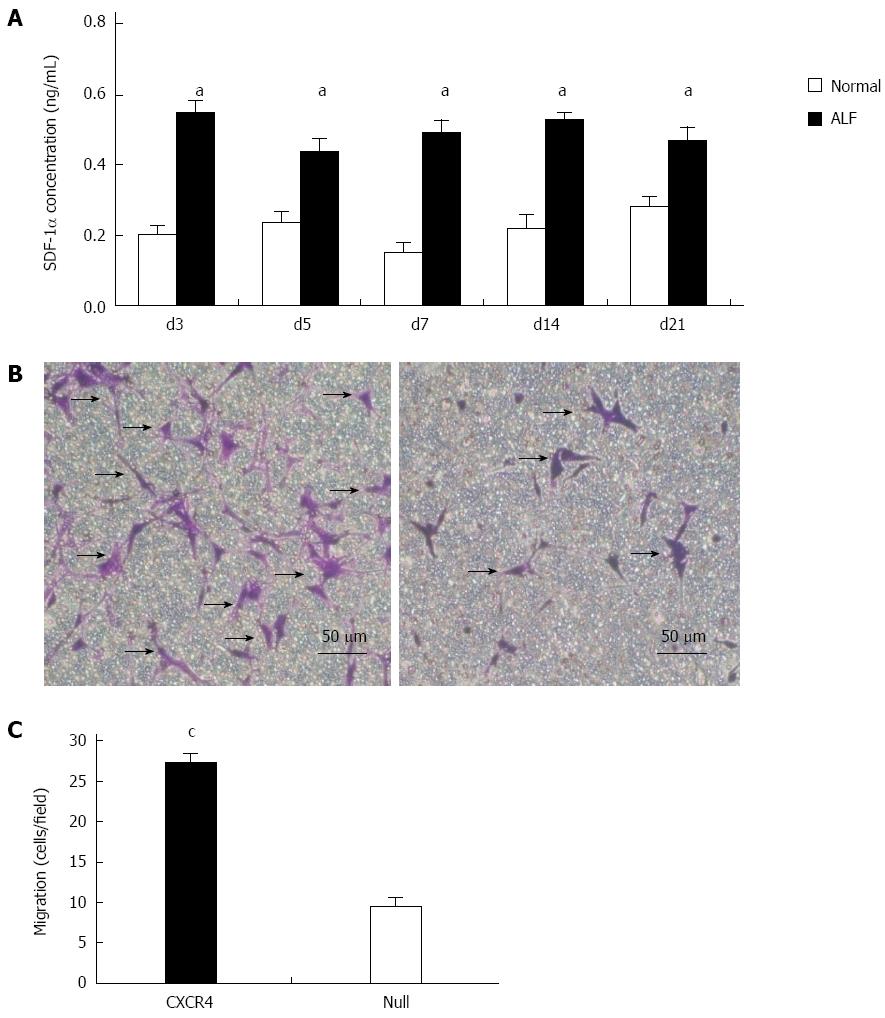
CCl4 (20% v/v, dissolved in olive oil) was administered to nude mice at a concentration of 8 μL/g. SDF-1α concentration in liver tissue homogenate was detected at 3, 5 and 7 d, and 2 and 3 wk after administration of CCl4. The results revealed that compared with the normal group, during day 3 to week 3, SDF-1α concentration increased significantly and was maintained at a relatively stable level (Figure 2A) (P < 0.05 vs normal group).
We investigated whether exogenous overexpression of CXCR4 enhanced chemotaxis of MSCs toward an SDF-1α gradient in a Transwell migration assay. CXCR4 modification of MSCs significantly increased the number of cells migrating toward SDF-1α. The number of migrating CXCR4-MSCs was more than threefold greater than the number of migrating Null-MSCs (P = 0.03) (Figure 2B and C), indicating that overexpression of CXCR4 enhanced the ability of MSCs to respond to SDF-1α-induced chemotaxis.
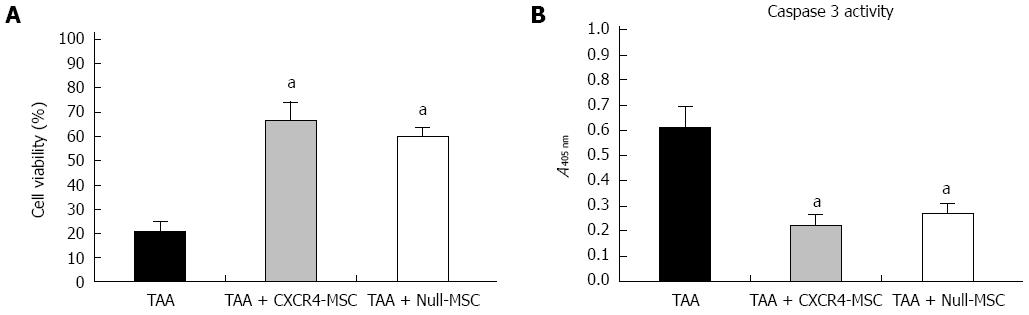
It has been reported that MSCs can secrete a broad variety of cytokines, chemokines and growth factors[21], which may prevent the adjacent cells from death and promote regeneration of injured tissue. Therefore, we tested the ability of CXCR4-MSCs and Null-MSCs to inhibit indirectly TAA-induced death of hepatocytes. We found that hepatocytes treated with TAA displayed low viability, while those administered CXCR4-MSCs or Null-MSCs showed higher viability (Figure 3A). It is known that TAA can activate the caspase-3 signaling pathway[22], and we analyzed cells for caspase-3 activity. A reduced level of caspase-3 activity in hepatocytes treated with either CXCR4-MSCs or Null-MSCs was observed (Figure 3B). These results suggested that MSCs can secrete factors that prevent cell death.
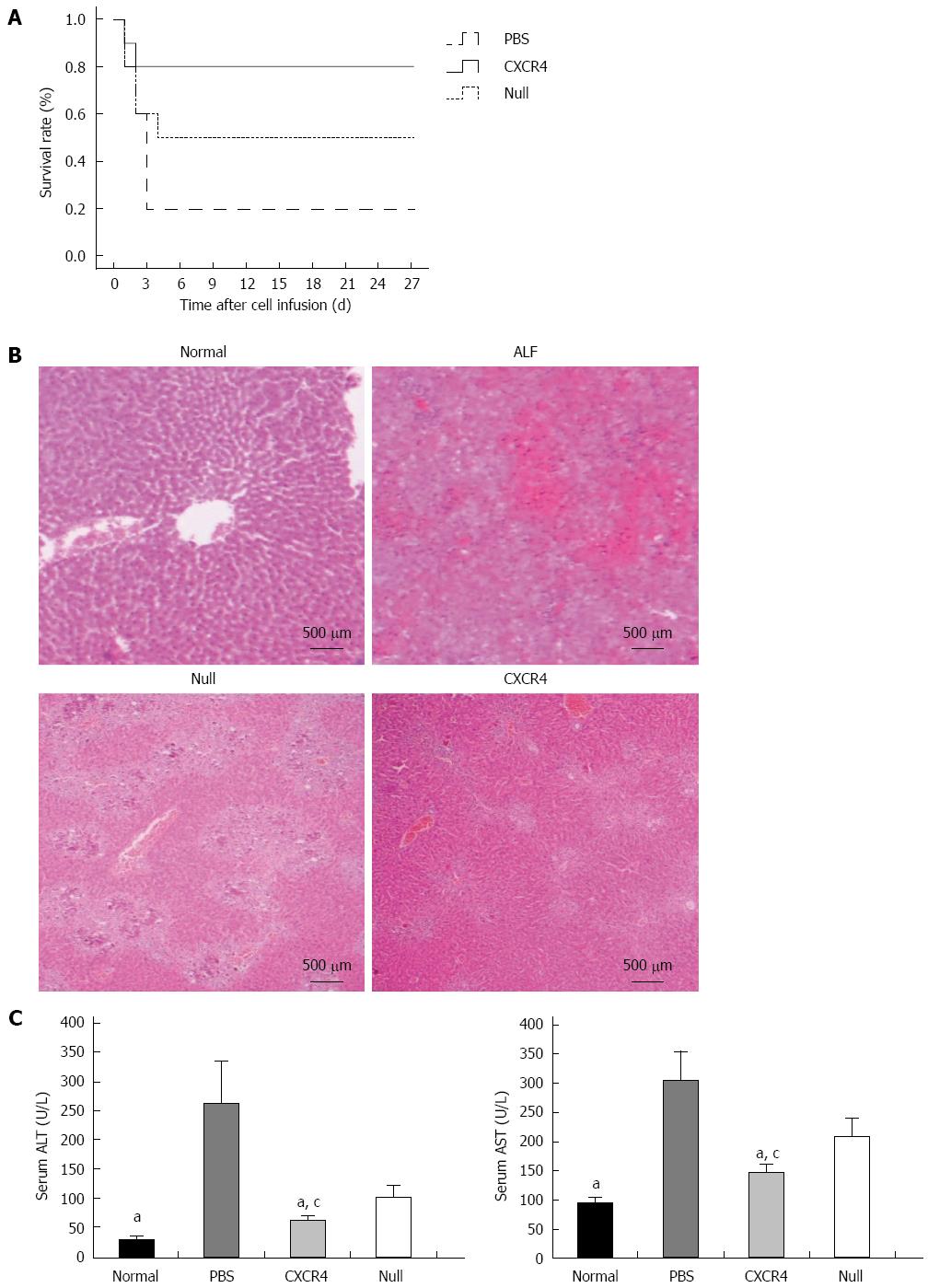
During the 4 wk after cell transplantation, most of the mice (8/10) that received only PBS died within 4 d, and 5/10 mice that received Null-MSCs survived up to 4 wk. However, 8/10 mice infused with CXCR4-MSCs survived up to 4 wk (Figure 4A). To investigate the liver histology of the mice with ALF after transplantation of MSCs, hematoxylin and eosin staining was performed. The liver histology of the normal mice showed uniform cellular morphology (Figure 4B). In contrast, failing liver showed extensive hepatocyte necrosis with hemorrhaging involving entire lobules, and the hepatocytes present had a swollen cytoplasm (Figure 4B). In the images of mice transplanted with Null-MSCs, there were many light-staining areas, indicating the presence of damaged hepatocytes (Figure 4B). However, this tendency was improved in the images of mice transplanted with CXCR4-MSCs (Figure 4B). Serum ALT and AST were detected at 7 d after cell infusion. When compared with the mice in the Null-MSCs group, the mice receiving CXCR4-MSCs showed better improvement of liver function, and liver function in both the CXCR4-MSC and Null-MSC groups was significantly better than in the PBS cohort (Figure 4C).
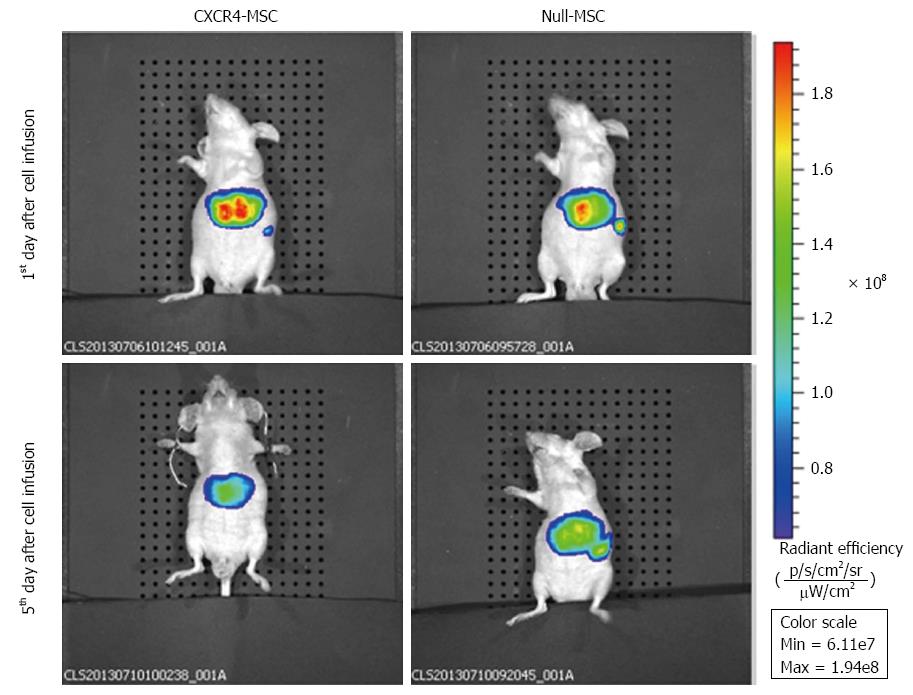
We used IVIS to identify in vivo distribution of MSCs labeled with DiR. At 1 d post-transplantation in the CXCR4 group, a strong fluorescence signal was emitted from the liver, while the fluorescence intensity was low in the spleen. In the Null group, the liver and spleen both exhibited a strong fluorescence signal and the intensity was almost the same. At 5 d following transplantation, the fluorescence signal emitted from the spleen faded in the CXCR4 group, and in the Null group, the signal intensity of the liver and spleen was also nearly the same (Figure 5). No signal could be detected in any other organs.
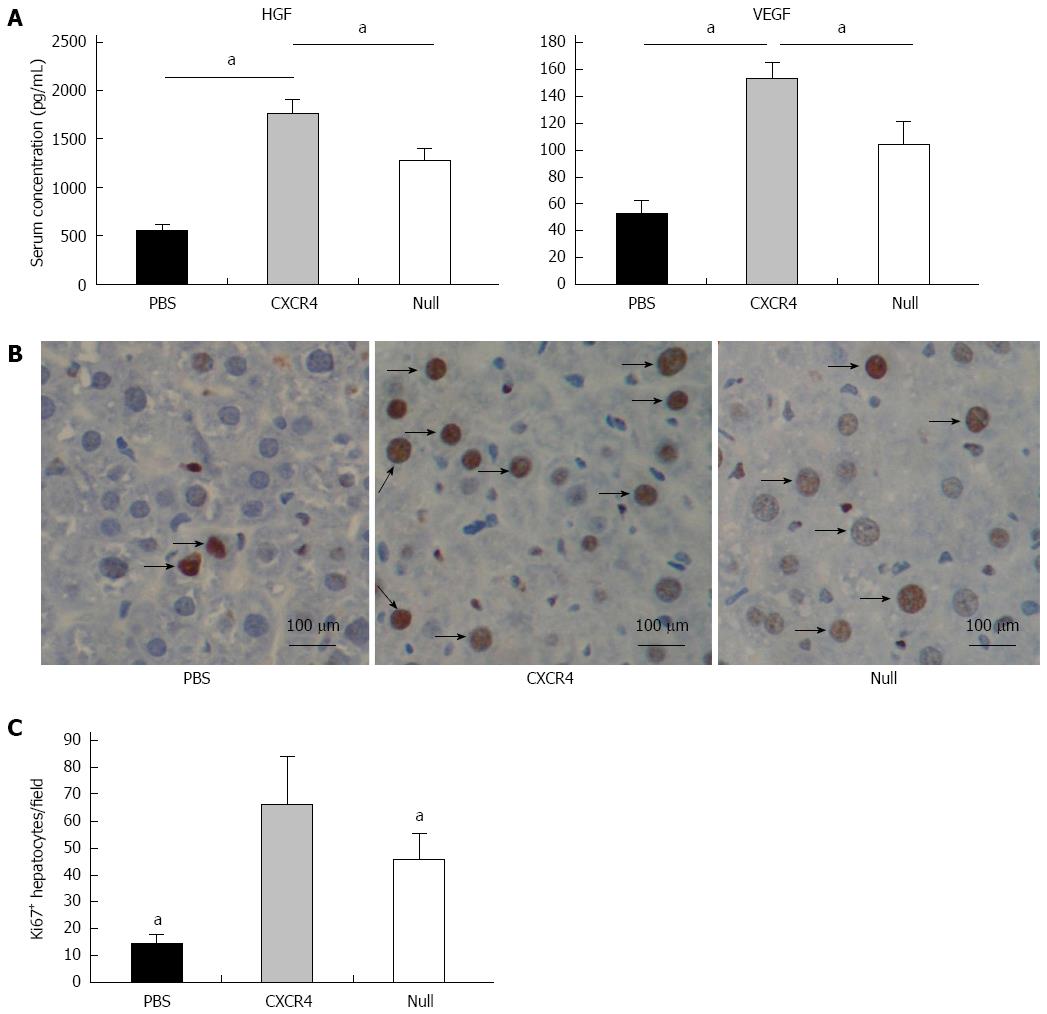
Two weeks after cell transplantation, serum showed a significant increase in HGF and VEGF in the cell transplantation group when compared with the PBS group. The level of HGF and VEGF in the CXCR4 group was approximately 1.5-fold higher than that in the Null group (Figure 6A). To evaluate whether stem cell transplantation enhanced the proliferation of hepatocytes in failing liver, the Ki67 expression level was assessed by immunohistochemistry. In the cell transplanted livers, the number of Ki67+ cells increased significantly when compared with the PBS group. More Ki67+ cells were observed in the CXCR4-MSC group than the Null-MSC group (Figure 6B and C).
Hepatocyte damage induced by liver failure stimulates the secretion of various cytokines and chemokines that are involved in mediating the process of tissue repair. SDF-1α plays an important role in the homing and recruitment of MSCs to the liver. Increased SDF-1 concentrations in the liver following induction of ALF have been reported previously[23]. In our study, we detected that the SDF-1α concentration in liver homogenate increased after liver failure and was maintained at a high level for 3 wk (Figure 2). CXCR4 is the receptor for SDF-1α and the SDF-1α/CXCR4 axis participates in the process of MSC recruitment. However, culture and proliferation of MSCs gradually downregulate the expression of CXCR4 and the cells loose their ability to migrate toward a gradient of SDF-1α concentration[24]. Thus, we used gene modification for CXCR4 overexpression to strengthen the homing of systemically delivered MSCs toward the injured liver. We used the membrane extraction kit and flow cytometry to determine the localization of CXCR4 on the cell membrane. The results showed that CXCR4-MSCs highly expressed CXCR4 compared to the unmodified MSCs (Figure 1). In vivo imaging dramatically demonstrated that ectopic distribution was negligible by use of gene modification, as opposed to unmodified MSCs (Figure 5). Therefore, it is anticipated that with genetic engineering, CXCR4-MSCs can disperse to the damaged liver and improve its function.
Stem cells have low immunogenicity, are readily cryopreserved, and show multipotent differentiation, so large numbers of trials of MSCs have been published worldwide. Many researchers have found poor efficacy of cell transplantation. An ongoing clinical trial of stem cell therapy has demonstrated that the engraftment efficiency is < 5% when transplanted into the liver via the portal vein[25]. Another study has shown that the efficiency can be improved to 20%-30%, if the stem cells are transplanted into the hepatic artery as opposed to the portal vein[26]. Even with this improvement, the majority of the cells escape to ectopic sites. To overcome this limitation, grafting strategies focus on gene modification or matrix biomaterials. We chose lentivirus to make MSCs overexpress CXCR4. In vivo imaging yielded a macro-image and showed that CXCR4-MSCs were nearly all distributed in the liver at 5 d after transplantation, whereas Null-MSCs migrated to the liver and spleen in equal proportions. This difference confirmed that our strategy enhanced the localization of cells to the liver.
To date, the mechanism of MSC-based cell therapy in liver regeneration remains controversial. Some researchers have reported that stem cells facilitate liver regeneration by transdifferentiation into primary hepatocytes[27,28]. However, this property has been challenged in subsequent reports. Some researchers have reported that only limited donor-derived cells are detected in vivo[29]. Such limited cells are not sufficient to replace the damaged hepatocytes. Therefore, there must be other mechanisms that are likely to mediate the effect of MSCs. Recently, the concept of stem cell transplantation exerting a paracrine proliferative effect on endogenous hepatocytes has gained support[21,30,31]. It has been demonstrated that MSCs can secrete a broad variety of cytokines, chemokines and growth factors, including VEGF-1, insulin-like growth factor-1, epidermal growth factor, NO, HGF, keratinocyte growth factor, angiopoietin-1, SDF-1, macrophage inflammatory protein-1a and -1b, and erythropoietin[21]. The released factors are known to be important for cell survival, proliferation and neovascularization during tissue repair. In this study, we demonstrated that the level of HGF and VEGF in mouse serum increased significantly 2 wk after cell transplantation compared with that in the PBS group. We also discovered more hepatocyte proliferation in the MSC groups relative to the PBS group. Therefore, the improvement of liver function via MSC transplantation was partially due to enhanced hepatocyte proliferation.
In summary, we showed in this study that genetic modification with CXCR4 markedly increased the homing of systemically delivered MSCs toward the failing liver, leading to reduced mortality and improved liver regeneration. We further demonstrated that MSCs exerted paracrine effects by secreting HGF and VEGF, which may have stimulated endogenous liver regeneration. The strategy of intravenous delivery of genetically modified MSCs expressing CXCR4 may be a useful and noninvasive cell therapy for ALF.
We thank all members of the laboratory for sharing reagents and advice.
Recent studies of mesenchymal stem cell (MSC)-based therapies on hepatic diseases have achieved remarkable advances. However, in vivo, their reparative capability is limited due to low colonization in the liver. To overcome this limitation, the authors genetically modified MSCs overexpressing chemokine CXC receptor 4 (CXCR4), the cognate receptor for stromal cell-derived factor (SDF)-1α, in order to maximize the capability of cell migration depending on SDF-1α.
MSCs are being considered as an optimal cell source for stem cell transplantation because of their capacity for self-renewal, immunoregulation and paracrine effects. The research hotspot is how to improve the colonization of MSCs in the target organs. SDF-1α and its receptor, CXCR4, are the most important chemokines required for homing of MSCs to injured tissues.
A large number of studies have shown that the SDF-1α/CXCR4 axis plays an important role in stem cell homing, chemotaxis, expression of adhesion molecules, engraftment, proliferation and survival. The authors constructed CXCR4-modified MSCs by lentivirus to improve the migration and homing capacity of the cells. This is believed to be the first study in which CXCR4-MSCs were used to treat acute liver failure (ALF). In order to track the distribution of MSCs in vivo, the authors labeled them with DiR, a fluorochrome with a wavelength of 700-800 nm. In vivo imaging showed that CXCR4-MSCs migrated to the damaged liver in greater numbers than Null-MSCs. The results exhibited that CXCR4-MSCs can migrate to acute failing liver and promote the recovery of liver function.
This study suggested that intravenous delivery of genetically modified MSCs expressing CXCR4 may be a useful and non-invasive cell therapy for ALF.
SDF-1α/CXCR4 axis: this axis implicates multiple signaling pathways in activating a range of cell secretion and cell adhesion molecules that take part in the homing of stem cells.
The low number of MSCs colonizing a particular diseased organ upon intravenous infusion is a concern, and a stumbling block in moving MSCs forward for clinical use. This study showed that CXCR4-modified MSCs homed better to damaged liver and improved recovery. This was a good study and the results are interesting. CXCR4-MSC transplantation could be a potential cell therapy for ALF.
P- Reviewer: Ho I S- Editor: Gou SX L- Editor: O’Neill M E- Editor: Ma S
| 1. | Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2700] [Article Influence: 168.8] [Reference Citation Analysis (0)] |
| 2. | Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1391] [Cited by in RCA: 1410] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 3. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1795] [Cited by in RCA: 1669] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 4. | Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 749] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 5. | Ng IO, Chan KL, Shek WH, Lee JM, Fong DY, Lo CM, Fan ST. High frequency of chimerism in transplanted livers. Hepatology. 2003;38:989-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 702] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 7. | Gordon MY, Levicar N, Pai M, Bachellier P, Dimarakis I, Al-Allaf F, M’Hamdi H, Thalji T, Welsh JP, Marley SB. Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells. 2006;24:1822-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, Kahn J, Spiegel A, Dar A, Samira S. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 448] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 9. | Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 540] [Cited by in RCA: 515] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 10. | Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28:299-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 458] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 11. | Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209-1216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 291] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 12. | Horuk R. Chemokines beyond inflammation. Nature. 1998;393:524-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Wojakowski W, Tendera M, Michałowska A, Majka M, Kucia M, Maślankiewicz K, Wyderka R, Ochała A, Ratajczak MZ. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213-3220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 325] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 14. | Bowie MB, McKnight KD, Kent DG, McCaffrey L, Hoodless PA, Eaves CJ. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J Clin Invest. 2006;116:2808-2816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 288] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 15. | Zhang D, Fan GC, Zhou X, Zhao T, Pasha Z, Xu M, Zhu Y, Ashraf M, Wang Y. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2008;44:281-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 225] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 16. | Liu H, Liu S, Li Y, Wang X, Xue W, Ge G, Luo X. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS One. 2012;7:e34608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 17. | Du Z, Wei C, Yan J, Han B, Zhang M, Peng C, Liu Y. Mesenchymal stem cells overexpressing C-X-C chemokine receptor type 4 improve early liver regeneration of small-for-size liver grafts. Liver Transpl. 2013;19:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Kahn J, Byk T, Jansson-Sjostrand L, Petit I, Shivtiel S, Nagler A, Hardan I, Deutsch V, Gazit Z, Gazit D. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood. 2004;103:2942-2949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 178] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 19. | Mori T, Kiyono T, Imabayashi H, Takeda Y, Tsuchiya K, Miyoshi S, Makino H, Matsumoto K, Saito H, Ogawa S. Combination of hTERT and bmi-1, E6, or E7 induces prolongation of the life span of bone marrow stromal cells from an elderly donor without affecting their neurogenic potential. Mol Cell Biol. 2005;25:5183-5195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Shimomura T, Yoshida Y, Sakabe T, Ishii K, Gonda K, Murai R, Takubo K, Tsuchiya H, Hoshikawa Y, Kurimasa A. Hepatic differentiation of human bone marrow-derived UE7T-13 cells: Effects of cytokines and CCN family gene expression. Hepatol Res. 2007;37:1068-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. [PubMed] |
| 22. | Chen LH, Hsu CY, Weng CF. Involvement of P53 and Bax/Bad triggering apoptosis in thioacetamide-induced hepatic epithelial cells. World J Gastroenterol. 2006;12:5175-5181. [PubMed] |
| 23. | Lei Y, Liu Z, Han Q, Kang W, Zhang L, Lou S. G-CSF enhanced SDF-1 gradient between bone marrow and liver associated with mobilization of peripheral blood CD34+ cells in rats with acute liver failure. Dig Dis Sci. 2010;55:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Wynn RF, Hart CA, Corradi-Perini C, O’Neill L, Evans CA, Wraith JE, Fairbairn LJ, Bellantuono I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643-2645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 579] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 25. | Puppi J, Strom SC, Hughes RD, Bansal S, Castell JV, Dagher I, Ellis EC, Nowak G, Ericzon BG, Fox IJ. Improving the techniques for human hepatocyte transplantation: report from a consensus meeting in London. Cell Transplant. 2012;21:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 26. | Khan AA, Shaik MV, Parveen N, Rajendraprasad A, Aleem MA, Habeeb MA, Srinivas G, Raj TA, Tiwari SK, Kumaresan K. Human fetal liver-derived stem cell transplantation as supportive modality in the management of end-stage decompensated liver cirrhosis. Cell Transplant. 2010;19:409-418. [PubMed] |
| 27. | Thorgeirsson SS, Grisham JW. Hematopoietic cells as hepatocyte stem cells: a critical review of the evidence. Hepatology. 2006;43:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 28. | Almeida-Porada G, Porada CD, Chamberlain J, Torabi A, Zanjani ED. Formation of human hepatocytes by human hematopoietic stem cells in sheep. Blood. 2004;104:2582-2590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005;68:1613-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 301] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 30. | Zagoura DS, Roubelakis MG, Bitsika V, Trohatou O, Pappa KI, Kapelouzou A, Antsaklis A, Anagnou NP. Therapeutic potential of a distinct population of human amniotic fluid mesenchymal stem cells and their secreted molecules in mice with acute hepatic failure. Gut. 2012;61:894-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 31. | Mintz PJ, Huang KW, Reebye V, Nteliopoulos G, Lai HS, Sætrom P, Kasahara N, Jensen S, Pai M, Gordon MY. Exploiting human CD34+ stem cell-conditioned medium for tissue repair. Mol Ther. 2014;22:149-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |