Published online Oct 28, 2014. doi: 10.3748/wjg.v20.i40.14875
Revised: June 25, 2014
Accepted: July 16, 2014
Published online: October 28, 2014
Processing time: 182 Days and 6.5 Hours
AIM: To observe the effect of Danshao Huaxian capsule (DHC) on the expression of Gremlin and bone morphogenetic protein-7 (BMP-7) in the liver of hepatic fibrosis rats.
METHODS: A total of 75 male Wistar rats were randomly divided into a normal control group (A), a CCl4-induced hepatic fibrosis model group (B), a natural recovery group (C), a low-dose DHC-treated group (D), and a high-dose DHC-treated group (E), with 15 rats in each group. Liver fibrosis was induced by subcutaneous injections of carbon tetrachloride (CCl4) and a high-lipid/low-protein diet for 8 wk, except for the rats in group A. Then, the rats in the two DHC-treated groups were administered 0.5 and 1.0 g/kg DHC by gastrogavage once per day for 8 successive weeks, respectively. By the end of the experiment, the level of transforming growth factor β1 (TGF-β1) in the liver homogenate was determined by an enzyme-linked immunosorbent assay. The mRNA and protein expression of Gremlin and BMP-7 in the liver tissue was determined by reverse-transcription polymerase chain reaction, an immunohistochemical assay, and Western blot analysis.
RESULTS: Compared with group A, the level of TGF-β1 and the mRNA and protein expression of Gremlin were significantly higher in group B (TGF-β1: 736.30 ± 24.40 μg/g vs 284.20 ± 18.32 μg/g, P < 0.01; mRNA of Gremlin: 80.40 ± 5.46 vs 49.83 ± 4.20, P < 0.01; positive protein expression rate of Gremlin: 38.46% ± 1.70% vs 3.83% ± 0.88%, P < 0.01; relative protein expression of Gremlin: 2.81 ± 0.24 vs 0.24 ± 0.06, P < 0.01), and the mRNA and protein expression of BMP-7 was significantly lower in group B (mRNA: 54.00 ± 4.34 vs 93.99 ± 7.03, P < 0.01; positive protein expression rate: 28.97% ± 3.14% vs 58.29% ± 6.02, P < 0.01; relative protein expression: 0.48 ± 0.31 vs 1.05 ± 0.12, P < 0.01). Compared with groups B and C, the degree of hepatic fibrosis was significantly improved, and the level of TGF-β1 and the mRNA and protein expression of Gremlin were significantly lowered in the two DHC-treated groups (TGF-β1: 523.14 ± 21.29 μg/g, 441.86 ± 23.18 μg/g vs 736.30 ± 24.40 μg/g, 651.13 ± 15.75 μg/g, P < 0.01; mRNA of Gremlin: 64.86 ± 2.83, 55.82 ± 5.39 vs 80.40 ± 5.46, 70.37 ± 4.01, P < 0.01; positive protein expression rate of Gremlin: 20.78% ± 1.60%, 17.43% ± 2.02% vs 38.46% ± 1.70%, 29.50% ± 2.64%, P < 0.01; relative protein expression of Gremlin: 1.95 ± 0.26, 1.65 ± 0.20 vs 2.81 ± 0.24, 2.22 ± 0.63, P < 0.01), and the mRNA and protein expression of BMP-7 was higher in the two DHC-treated groups (mRNA: 73.52 ± 4.56, 81.78 ± 5.38 vs 54.00 ± 4.34, 62.28 ± 4.51, P < 0.01; positive protein expression rate: 41.44% ± 4.77%, 47.49% ± 4.59% vs 28.97% ± 3.14%, 35.85% ± 3.50%, P < 0.01; relative protein expression: 0.71 ± 0.06, 0.81 ± 0.07 vs 0.48 ± 0.31, 0.60 ± 0.37, P < 0.01).
CONCLUSION: The therapeutic mechanism of DHC for hepatic fibrosis in rats may be associated with inhibition of the expression of Gremlin and up-regulation of the expression of BMP-7.
Core tip: Hepatic fibrosis, as a refractory disease, represents a current threat to global health. To date, no ideal drug therapies have been identified, and drugs for liver fibrosis remain in the exploratory stage of development. Previous experimental and clinical studies showed that Danshao Huaxian capsule (DHC, originally called Handan Ganle) demonstrated efficacy in treating liver fibrosis. In this study, the effect of DHC on the expression of Gremlin and bone morphogenetic protein 7 (BMP-7) in a liver fibrosis rat model was observed to explore its potential mechanism for treating liver fibrosis. We found that the therapeutic mechanism of DHC for hepatic fibrosis in rats may be associated with inhibition of the expression of Gremlin and up-regulation of the expression of BMP-7.
- Citation: Zhao XK, Cheng ML, Wu RM, Yao YM, Mu M, Zhu JJ, Zhang BF, Zhou MY. Effect of Danshao Huaxian capsule on Gremlin and bone morphogenetic protein-7 expression in hepatic fibrosis in rats. World J Gastroenterol 2014; 20(40): 14875-14883
- URL: https://www.wjgnet.com/1007-9327/full/v20/i40/14875.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i40.14875
The pathogenesis of liver fibrosis is complex. A key step in this process is the activation of hepatic stellate cells (HSCs). Many cytokines participate in the development and progression of liver fibrosis. Among them, transforming growth factor β1 (TGF-β1) is the strongest known cytokine promoter of HSCs[1-6]. Gremlin is a downstream factor of TGF-β that has been identified in recent years. After being activated by TGF-β1, the gene and protein expression of Gremlin increases and is closely associated with fibrosis[7]. Bone morphogenetic protein-7 (BMP-7) is a secreted multifunctional protein that belongs to the superfamily of TGF and can reverse the induction of fibrosis by TGF-β1[8-13]. Currently, drug therapies for liver fibrosis remain in the exploratory stage of development. Previous experimental and clinical studies showed that Danshao Huaxian capsule (DHC, originally called Handan Ganle) demonstrated efficacy in treating liver fibrosis[14-18]. In this study, the effect of DHC on the expression of Gremlin and BMP-7 in a rat model of liver fibrosis was observed to identify its potential mechanism for treating liver fibrosis.
DHC, consisting of, for example, tetrandrine, Salvia Miltiorrhiza, red peony root, Astragalus membranaceus, and ginkgo leaf, was produced by the Guiyang Pharmaceutical Factory (Batch No. 20081011) as a brown-yellow granular capsular preparation. The contents were ground into a powder before use and diluted with distilled water to the necessary concentrations.
A total of 75 male Wistar rats of clean grade, weighing 180 ± 20 g, were purchased from Experimental Animal Center, Third Military Medical University [Batch No. SCXK (Yu) 2007-0003]. Institutional and national guidelines for the care and use of animals were followed, and all experimental procedures involving animals were approved by the institutional animal ethical committee of Guiyang Medical College (Permit Number: 2008-0101). The rats were fed common forage and drank freely under natural lighting at room temperatures of 15 °C-25 °C. They were adapted to the environment 1 wk before the experiment.
Hyaluronic acid (HA), hydroxyproline (Hyp), and TGF-β1 detection kits (products of Nanjing Jiancheng Co. Ltd., Batch No. 20110228, 20110215, and 20110218, respectively); a reverse-transcription kit (product of Canada Fermentas MBI, Batch No. 00087036); an electrochemiluminescence kit (product of America Millipore Company, Batch No. 1219101); a whole protein extraction kit (product of Nanjing Keygen Biotech Co., Ltd., Batch No. KGP250); a BCA protein quantification kit (product of America Thermo Scientific Company, Batch No. 23227); and Gremlin and BMP-7 primary antibodies (product of England Abcam Company, Batch No. ad90670 and ab56023) were utilized in the study. The forward primer sequence of Gremlin was 5’-CGC CAT CCC CTC GCC TTA CAA-3’, and the reverse primer sequence of Gremlin was 5’-AAC CCT CCT CGC TCA CCG TCT-3’ (176 bp). The forward primer sequence of BMP-7 was 5’-GTA GCG CGT AGA GCC G-3’, and the reverse primer sequence of BMP-7 was 5’-CGA GTC CGT GCA TGG-3’ (345 bp). The forward primer sequence of β-actin was 5’-TCC TCC TGA GCG CAA GTA CTC T-3’, and the reverse primer sequence of β-actin was 5’-GCT CAG TAA CAG TCC GCC TAG AA-3’ (1536 bp). These primers were synthesized by Shanghai Shenggong Bioengineering Co. Ltd.
A 752-nm ultraviolet spectrophotometer (Shanghai Jinghua Sci-Tech Co. Ltd., China), nucleic acid quantification apparatus (Amersham Biosciences, United States); DA7600 nucleic acid amplification real-time fluorescence detection system (Da An Gene Co. Ltd., Sun Yat-sen University, China); Gel Doc EQ Imager (Bio-Rad, United States); and micro-image collecting system (Olympus, Japan) were utilized.
A total of 75 male Wistar rats were randomly divided into a normal control group (A), a CCl4-induced hepatic fibrosis model group (B), a natural recovery group (C), a low-dose DHC-treated group (D), and a high-dose DHC-treated group (E), with 15 rats in each group. Liver fibrosis was induced by subcutaneous injections of carbon tetrachloride (CCl4) and a high-lipid, low-protein diet for 8 wk[14], except for the rats in group A (fed a normal diet). The rats in group B were sacrificed at the end of the modeling, whereas the rats in groups A and C were fed under normal conditions. Then, the rats in the two DHC-treated groups were administered 0.5 and 1.0 g/kg DHC (the dose equivalent to 8 or 16 times that of the human adult dose used in clinical practice) by gastrogavage[14] once per day for 8 successive weeks, respectively. At the end of week 16, the rest of the rats were sacrificed after anesthesia.
Detection of alanine transaminase (ALT) and aspartate transaminase (AST) in the serum was performed with an automatic biochemical analyzer. The whole livers were removed to obtain their wet weights, and the liver index was calculated according to the liver weight (g)/the body weight (g) × 100%. A homogenate of the liver tissue was prepared. The HA, Hyp, and TGF-β1 in the liver homogenate were assessed by an enzyme-linked immunosorbent assay according to the manufacturer’s instructions.
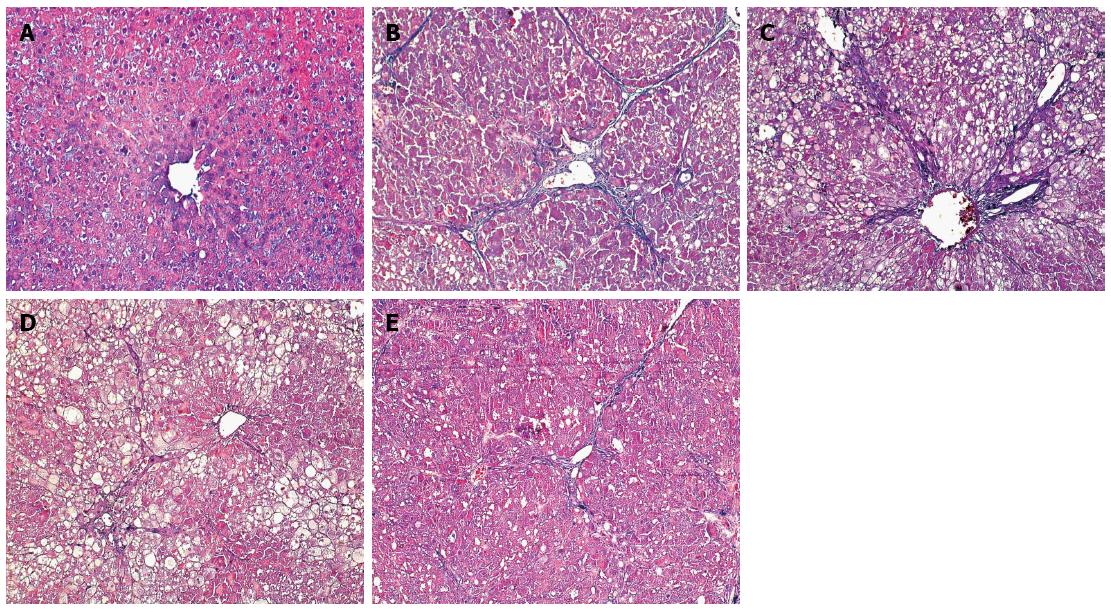
All liver tissue samples were embedded in paraffin after being fixed in 10% formalin for 24-48 h, and 4-μm sections were then obtained. We next assessed the histological changes in the liver, such as hepatocyte lesions, fibrogenesis, and structural changes, using hematoxylin and eosin (HE) and Masson fiber stains[19].
The total RNA was extracted and purified by the trizol-phenol-chloroform one-step method. The RNA concentration was determined; cDNA was synthesized after reverse transcription; and RT-PCR was performed. During the experiment, β-actin was used as an internal control to perform standardized conversions of the copy number (Ct value) of each sample. The relative differences in expression between the groups were calculated using the ∆∆Ct method.
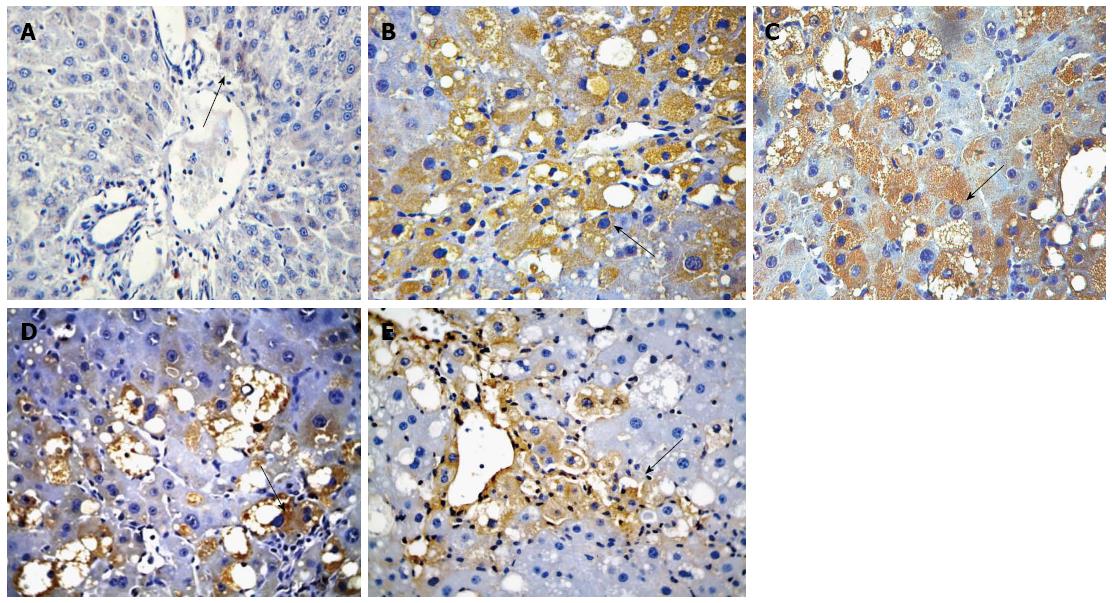
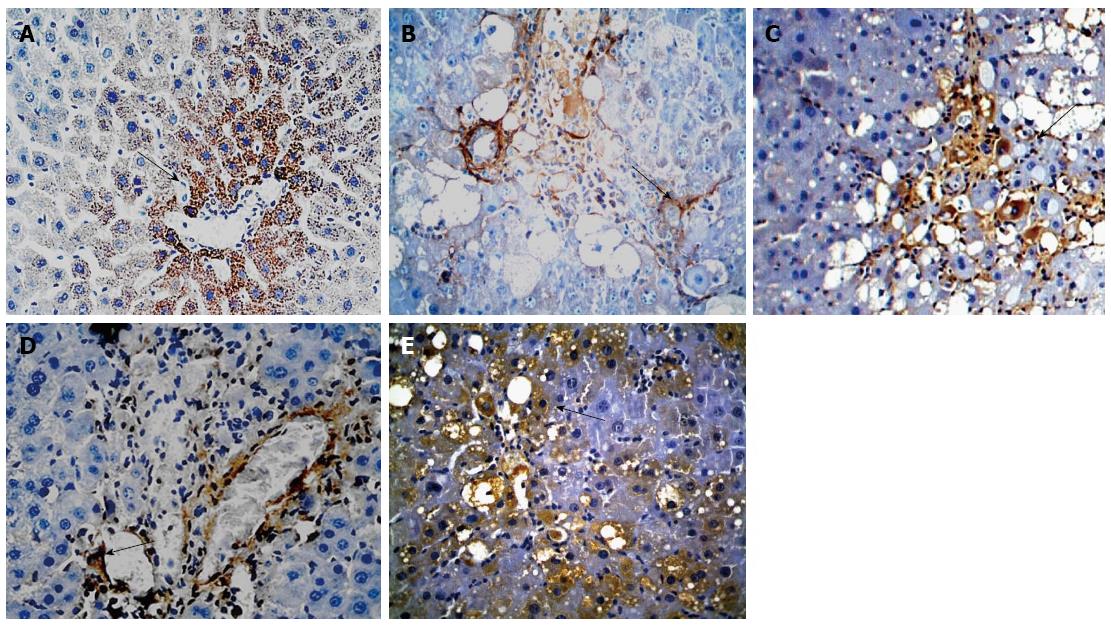
Immunohistochemistry studies were performed using the EnVision System DAKO, according to the manufacturer’s protocol. Tissue sections were deparaffinized, rehydrated, and processed for antigen retrieval using heat treatment in the presence of Tris-EDTA solution. Histological sections were then placed in 3% H2O2 for 20 min to eliminate endogenous peroxidase activity. The sections were probed with primary antibodies overnight at 4 °C, and the signal was developed with the EnVision detection kit from Gene Tech Company Limited. Phosphate-buffered saline (PBS) was used as a negative control. Yellow material in the cytoplasm was considered to indicate a positive cell. Five microscopic fields (× 400 magnification) were randomly chosen per slice, and the number of cells per field was counted[20]. The primary antibodies used in this study included anti-Gremlin (1:100) and anti-BMP-7 (1:100).
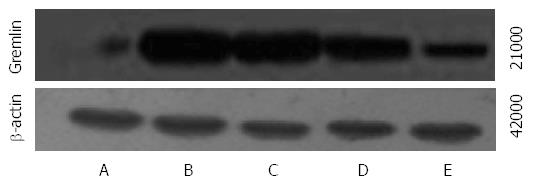
The total proteins were extracted and quantified using Bradford protein quantification kits. Protein samples of 40 μg each were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred onto polyvinylidene fluoride (PVDF) membranes and incubated with primary antibodies overnight at 4 °C. On the second day, the signal was developed with an electrochemiluminescence detection kit after incubation with the appropriate secondary antibodies. The primary antibodies used in this study included Gremlin (1:1000) and BMP-7 (1:1000). β-actin was used as the internal control.
SPSS l6.0 software was used for the statistical analyses. mean ± SD was used for the measurement data. One-factor analysis of variance (ANOVA) was used for multi-group comparisons. For inter-group comparisons, the least significant difference (LSD) method was used to assess the homogeneity of variance, whereas the Tamhane method was applied to assess the heterogeneity of variance. The rank test was used for ranked data. P < 0.05 was considered to indicate significant differences.
Two rats died in group B. In anatomical investigations, a blood clot and scattered pus pods were found in one of the rats, and a bilateral pleural effusion was seen in the other rat. In group C, one rat died when the gavage strayed into the trachea. No rats died in the other groups during the study period.
Serum levels of ALT and AST in groups B-E were greatly increased compared with those in group A but were significantly lower in groups C-E than in group B. Compared with group C, serum levels of ALT and AST in groups D and E were significantly reduced (F = 185.122, 161.858, P < 0.01 for all; Table 1).
The liver index was significantly higher in groups B and C than in group A and significantly lower in groups C-E than in group B (F = 724.560, P < 0.01 for all). There was no significant difference in the liver index between groups C, D, and E (P > 0.05 for all, Table 1).
The levels of HA, Hyp, and TGF-β1 in the liver homogenates were significantly higher in groups B-E than in group A and were significantly lower in groups C-E than in group B (P < 0.01 for all). The levels of HA, Hyp, and TGF-β1 in the liver homogenates were significantly lower in groups D and E compared with those in group C (P < 0.01 for both) and were significantly lower in group E than in group D (P < 0.01 for all, F = 5644.990, 1538.990, 1044.168; Table 2).
| Group | n | HA (ng/g) | Hyp (μg/g) | TGF-β1 (μg/g) |
| A | 15 | 1449.93 ± 121.89 | 318.20 ± 9.71 | 284.20 ± 18.32 |
| B | 13 | 8803.30 ± 200.57b | 617.77 ± 8.52b | 736.30 ± 24.40b |
| C | 15 | 7558.40 ± 161.88bd | 530.27 ± 11.88bd | 651.13 ± 15.75bd |
| D | 14 | 5249.21 ± 111.92bdf | 490.00 ± 8.13bdf | 523.14 ± 21.29bdf |
| E | 15 | 4050.73 ± 122.18bdfh | 464.07 ± 13.09bdfh | 441.86 ± 23.18bdfh |
The degree of hepatic fibrosis was significantly milder in groups C-E than in group B (P < 0.05 for all; Table 3). Masson staining showed a small amount of blue collagen fiber in the livers of the rats in group A. Obvious proliferation and widened collagen fibers were seen in the liver tissues of group B rats and were distributed throughout the hepatic lobules. The hepatic lobular structure was destroyed, with severe pseudolobule formation. Compared with group B, the expression of collagen fibers was not obviously changed in group C. Compared with group B, the degree of collagen fiber proliferation was obviously decreased in groups D and E, with some fibers extending to the hepatic lobules (Figure 1).
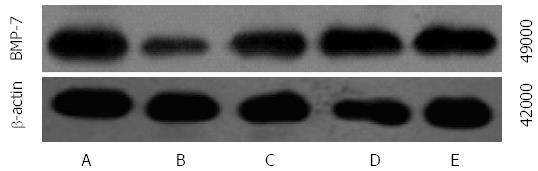
The mRNA and protein expression of Gremlin was significantly higher in groups B-E than in group A and was significantly lower in groups C-E than in group B. The mRNA and protein expression of Gremlin was significantly lower in groups D and E than in group C and was significantly lower in group E than in group D (F = 101.440, 689.269, 115.278, P < 0.01 or P < 0.05; Table 4, Figures 2 and 3).
| Group | n | Relative mRNA expression | Positive protein expression rate | Relative protein expression |
| A | 15 | 49.83 ± 4.20 | 3.83% ± 0.88% | 0.24 ± 0.06 |
| B | 13 | 80.40 ± 5.46b | 38.46% ± 1.70%b | 2.81 ± 0.24b |
| C | 15 | 70.37 ± 4.01bd | 29.50% ± 2.64%bd | 2.22 ± 0.63bd |
| D | 14 | 64.86 ± 2.83bdf | 20.78% ± 1.60%bdf | 1.95 ± 0.26bdf |
| E | 15 | 55.82 ± 5.39bdfh | 17.43% ± 2.02%bdfg | 1.65 ± 0.20bdfh |
The mRNA and protein expression of BMP-7 was significantly lower in groups B-E than in group A and was significantly higher in groups C-E than in group B. The mRNA and protein expression of BMP-7 was significantly higher in groups D and E compared with those in group C and were significantly higher in group E than in group D (F = 126.182, 86.590, 122.907, P < 0.01 for all; Table 5, Figures 4 and 5).
| Group | n | Relative mRNA expression | Positive protein expression rate | Relative protein expression |
| A | 15 | 93.99 ± 7.03 | 58.29% ± 6.02% | 1.05 ± 0.12 |
| B | 13 | 54.00 ± 4.34b | 28.97% ± 3.14%b | 0.48 ± 0.31b |
| C | 15 | 62.28 ± 4.51bd | 35.85% ± 3.50%bd | 0.60 ± 0.37bd |
| D | 14 | 73.52 ± 4.56bdf | 41.44% ± 4.77%bdf | 0.71 ± 0.06bdf |
| E | 15 | 81.78 ± 5.38bdfh | 47.49% ± 4.59%bdfh | 0.81 ± 0.07bdfh |
TGF-β1, an important cytokine in the promotion of liver fibrosis, can stimulate the activation of HSCs and increase the synthesis and secretion of the extracellular matrix through the TGF-β-Smad signal transduction pathway[21-23]. BMP-7, a secreted multifunctional protein, has extensive biological activities; participates in and regulates the proliferation, differentiation, and apoptosis of various cells; and plays important roles in the formation of tissues and organs, embryonic development, and the repair of injured tissues. Both BMP-7 and TGF-β1 belong to the superfamily of TGF-β. Their signal transduction is mutually antagonistic during the fibrotic process. BMP-7 can directly antagonize TGF-β1 signal transduction or inhibit the TGF-β1-induced epithelial-mesenchymal transition (EMT)[24-30].
Researchers[31-33] in recent years have found that Gremlin is a downstream mediator of TGF-β. Its gene was the first gene cloned from the neural crest of Xenopus laevis. The human Gremlin gene is located on chromosome 15 q13-q15, and its total mRNA length is 4175 bp. This gene encodes a highly conservative secretory protein containing 184 amino acid residues. As a natural antagonistic factor of BMP-7, Gremlin acts to promote fibrosis in the lungs, kidneys, or liver. Normally, the expression level of Gremlin is very low or virtually undetectable in the liver. Pathologically, TGF-β1 could be advantageously expressed in the microenvironment of the liver tissue, further up-regulating the expression of Gremlin. Gremlin hinders conjugation with BMP receptors by directly binding BMP-7, thereby antagonizing the biological activities of BMP-7 and promoting the incidence and development of fibrosis[7,34]. The critical potential for identifying a mutually regulating mechanism among the aforementioned three factors is obvious.
DHC, a Chinese herbal preparation, was developed according to the Chinese medical theories of blood activation, stasis removal, collateral dredging, and mass softening, and its use is supported by years of basic research and clinical studies. A previous animal study revealed that DHC could be used to treat rat hepatic fibrosis due to its up-regulation of the gene transcription and protein translation of Smad7, causing the transduction of a feedback-inhibiting fibrosis signal that promotes the activation of TGF-β/Smad signaling[35]. Based on previous studies, the observed expression of Gremlin and BMP-7 in hepatic fibrosis rats was confirmed in the present study. The effect of DHC on the expression of these proteins in the liver of rats with hepatic fibrosis was also explored.
The present results indicated that the mRNA and protein expression of Gremlin in the liver tissue, as well as the level of TGF-β1 in the liver homogenate of the hepatic fibrosis rats in group B, was significantly higher than that in group A, whereas the mRNA and protein expression of BMP-7 in the liver tissue of group B rats was significantly lower than that of group A rats. These results are similar to the results found in the literature[36], indicating that, during the progression of rat liver fibrosis, the up-regulation of TGF-β1 expression can further activate the downstream signaling molecule Gremlin. Then, the heightened TGF-β1 expression can inhibit the expression of BMP-7 and antagonize BMP-7-mediated signal transduction, potentially representing a pathogenic mechanism for CCl4-induced hepatic fibrosis in rats.
After the 8-wk DHC treatment, serum levels of ALT and AST, the liver indexes, and the HA and Hyp levels in liver homogenates were significantly lower than those in group B rats. Histopathological examination showed a significantly attenuated level of liver fibrosis in groups D and E, indicating that DHC improved liver function, lessened the degree of liver fibrosis, and demonstrated efficacy in treating CCl4-induced liver fibrosis. The detection of TGF-β1 in the rat liver homogenate showed that the level of TGF-β1 was significantly lower in groups D and E than in groups B and C. The assessment of Gremlin and BMP-7 in the rat liver tissue showed that the mRNA and protein expression of Gremlin was significantly lower in groups D and E than in groups B and C, whereas the mRNA and protein expression of BMP-7 was significantly higher in groups D and E than in groups B and C. Meanwhile, there was a significant difference in the aforementioned two indices between groups D and E, indicating the existence of a dose-effect relationship. We inferred that the therapeutic mechanism of DHC for hepatic fibrosis in rats may be associated with inhibition of the expression of TGF-β1 and Gremlin and up-regulation of the expression of BMP-7.
Hepatic fibrosis, a refractory disease, represents a serious threat to human health. Currently, all potential optimal drug therapies remain in the exploratory stage of development. Current research suggests that traditional Chinese medicine could play a role in treating hepatic fibrosis, but further study is necessary to support this possibility.
Previous experimental and clinical studies showed that Danshao Huaxian capsule (DHC) demonstrated efficacy in treating hepatic fibrosis. Within this specific field of research, the authors focused this study on determining the effect of DHC on the expression of Gremlin and bone morphogenetic protein-7 (BMP-7) in a rat model of hepatic fibrosis.
This study showed that DHC has a therapeutic effect on carbon tetrachloride (CCl4)-induced hepatic fibrosis in rats. The effect of DHC is likely achieved through inhibition of transforming growth factor β1 (TGF-β1) and Gremlin expression, as well as upregulation of BMP-7.
The results of this study indicate that DHC is a potential therapeutic drug that could be used to treat hepatic fibrosis.
Hepatic fibrosis is a progressive condition with serious clinical complications arising from the abnormal proliferation and amassing of tough fibrous scar tissue. The participation of various cell types, interlinked cellular events, and large number of mediator molecules make the fibrotic process very complex and dynamic. DHC is a traditional Chinese medicine consisting of tetrandrine, Salvia Miltiorrhiza, red peony root, Astragalus membranaceus, and ginkgo leaf that acts as a potential therapeutic drug to treat hepatic fibrosis.
This is a good descriptive study in which the authors analyze the protective effect of DHC on liver fibrosis induced by CCl4 in rats. The results are interesting and have possible significant clinical utility, indicating that DHC is a potential therapeutic drug for hepatic fibrosis.
P- Reviewer: De Ponti F, Xu J S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76-99. [PubMed] |
| 2. | Si XH, Yang LJ. Extraction and purification of TGFbeta and its effect on the induction of apoptosis of hepatocytes. World J Gastroenterol. 2001;7:527-531. [PubMed] |
| 3. | Shen M, Chen K, Lu J, Cheng P, Xu L, Dai W, Wang F, He L, Zhang Y, Chengfen W. Protective effect of astaxanthin on liver fibrosis through modulation of TGF-β1 expression and autophagy. Mediators Inflamm. 2014;2014:954502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Yang JW, Hien TT, Lim SC, Jun DW, Choi HS, Yoon JH, Cho IJ, Kang KW. Pin1 induction in the fibrotic liver and its roles in TGF-β1 expression and Smad2/3 phosphorylation. J Hepatol. 2014;60:1235-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Fan X, Zhang Q, Li S, Lv Y, Su H, Jiang H, Hao Z. Attenuation of CCl4-induced hepatic fibrosis in mice by vaccinating against TGF-β1. PLoS One. 2013;8:e82190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Klironomos S, Notas G, Sfakianaki O, Kiagiadaki F, Xidakis C, Kouroumalis E. Octreotide modulates the effects on fibrosis of TNF-α, TGF-β and PDGF in activated rat hepatic stellate cells. Regul Pept. 2014;188:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Sethi A, Jain A, Zode GS, Wordinger RJ, Clark AF. Role of TGFbeta/Smad signaling in gremlin induction of human trabecular meshwork extracellular matrix proteins. Invest Ophthalmol Vis Sci. 2011;52:5251-5259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Chen BL, Peng J, Li QF, Yang M, Wang Y, Chen W. Exogenous bone morphogenetic protein-7 reduces hepatic fibrosis in Schistosoma japonicum-infected mice via transforming growth factor-β/Smad signaling. World J Gastroenterol. 2013;19:1405-1415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Bi WR, Xu GT, Lv LX, Yang CQ. The ratio of transforming growth factor-β1/bone morphogenetic protein-7 in the progression of the epithelial-mesenchymal transition contributes to rat liver fibrosis. Genet Mol Res. 2014;13:1005-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 53] [Reference Citation Analysis (0)] |
| 10. | Aktug Demir N, Kolgelier S, Inkaya AC, Sumer S, Demir LS, Pehlivan FS, Arslan M, Arpaci A. Are bone morphogenetic protein-7 (BMP-7) serum levels correlated with development of hepatic fibrosis? J Infect Dev Ctries. 2014;8:605-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Zhong L, Wang X, Wang S, Yang L, Gao H, Yang C. The anti-fibrotic effect of bone morphogenic protein-7(BMP-7) on liver fibrosis. Int J Med Sci. 2013;10:441-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Bi WR, Jin CX, Xu GT, Yang CQ. Bone morphogenetic protein-7 regulates Snail signaling in carbon tetrachloride-induced fibrosis in the rat liver. Exp Ther Med. 2012;4:1022-1026. [PubMed] |
| 13. | Scherner O, Meurer SK, Tihaa L, Gressner AM, Weiskirchen R. Endoglin differentially modulates antagonistic transforming growth factor-beta1 and BMP-7 signaling. J Biol Chem. 2007;282:13934-13943. [PubMed] |
| 14. | Geng XX, Yang Q, Xie RJ, Luo XH, Li CX, Cheng ML. Effects of Dan-Shao-Hua-Xian on the expression of collagen type I and III in rats with hepatic fibrosis. Hepatobiliary Pancreat Dis Int. 2004;3:558-563. [PubMed] |
| 15. | Geng XX, Yang Q, Xie RJ, Luo XH, Han B, Ma L, Li CX, Cheng ML. In vivo effects of Chinese herbal recipe, Danshaohuaxian, on apoptosis and proliferation of hepatic stellate cells in hepatic fibrotic rats. World J Gastroenterol. 2005;11:561-566. [PubMed] |
| 16. | Yang Q, Xie RJ, Geng XX, Luo XH, Han B, Cheng ML. Effect of Danshao Huaxian capsule on expression of matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 in fibrotic liver of rats. World J Gastroenterol. 2005;11:4953-4956. [PubMed] |
| 17. | Wang HY, Zhou XQ, Cheng ML. Expression of PPAR-gamma in rat liver fibrosis and the effect of Dan-shao-hua-xian capsule on its expression pattern. Zhonghua Ganzangbing Zazhi. 2007;15:859-860. [PubMed] |
| 18. | Wu J, Lu T, Cheng ML. Therapeutic effects of the Chinese medicine Han-Dan-Gan-Le, on arsenic-induced liver injury in Guizhou, China. Zhongguo Difangbingxue Zazhi. 2006;25:86-89. |
| 19. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [PubMed] |
| 20. | Scharl A, Vierbuchen M, Conradt B, Moll W, Würz H, Bolte A. Immunohistochemical detection of progesterone receptor in formalin-fixed and paraffin-embedded breast cancer tissue using a monoclonal antibody. Arch Gynecol Obstet. 1990;247:63-71. [PubMed] |
| 21. | Ikushima H, Miyazono K. Biology of transforming growth factor-β signaling. Curr Pharm Biotechnol. 2011;12:2099-2107. [PubMed] |
| 22. | Gressner OA, Lahme B, Siluschek M, Rehbein K, Weiskirchen R, Gressner AM. Connective tissue growth factor is a Smad2 regulated amplifier of transforming growth factor beta actions in hepatocytes--but without modulating bone morphogenetic protein 7 signaling. Hepatology. 2009;49:2021-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Inagaki Y, Okazaki I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007;56:284-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 403] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 24. | Weiskirchen R, Meurer SK. Bone morphogenetic protein-7 in focus: a member of the transforming growth factor-beta superfamily is implicated in the maintenance of liver health. Hepatology. 2007;45:1324-1325. [PubMed] |
| 25. | Khan I, Agarwal P, Thangjam GS, Radhesh R, Rao SG, Kondaiah P. Role of TGF-β and BMP7 in the pathogenesis of oral submucous fibrosis. Growth Factors. 2011;29:119-127. [PubMed] |
| 26. | Xu Y, Wan J, Jiang D, Wu X. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition in human renal proximal tubular epithelial cells. J Nephrol. 2009;22:403-410. [PubMed] |
| 27. | Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, Kalluri R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282:23337-23347. [PubMed] |
| 28. | Yang G, Zhu Z, Wang Y, Gao A, Niu P, Tian L. Bone morphogenetic protein-7 inhibits silica-induced pulmonary fibrosis in rats. Toxicol Lett. 2013;220:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Pegorier S, Campbell GA, Kay AB, Lloyd CM. Bone morphogenetic protein (BMP)-4 and BMP-7 regulate differentially transforming growth factor (TGF)-beta1 in normal human lung fibroblasts (NHLF). Respir Res. 2010;11:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Yang T, Chen SL, Lu XJ, Shen CY, Liu Y, Chen YP. Bone morphogenetic protein 7 suppresses the progression of hepatic fibrosis and regulates the expression of gremlin and transforming growth factor β1. Mol Med Rep. 2012;6:246-252. [PubMed] |
| 31. | Rodrigues-Diez R, Lavoz C, Carvajal G, Rayego-Mateos S, Rodrigues Diez RR, Ortiz A, Egido J, Mezzano S, Ruiz-Ortega M. Gremlin is a downstream profibrotic mediator of transforming growth factor-beta in cultured renal cells. Nephron Exp Nephrol. 2012;122:62-74. [PubMed] |
| 32. | Costello CM, Cahill E, Martin F, Gaine S, McLoughlin P. Role of gremlin in the lung: development and disease. Am J Respir Cell Mol Biol. 2010;42:517-523. [PubMed] |
| 33. | Michos O, Panman L, Vintersten K, Beier K, Zeller R, Zuniga A. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development. 2004;131:3401-3410. [PubMed] |
| 34. | Boers W, Aarrass S, Linthorst C, Pinzani M, Elferink RO, Bosma P. Transcriptional profiling reveals novel markers of liver fibrogenesis: gremlin and insulin-like growth factor-binding proteins. J Biol Chem. 2006;281:16289-16295. [PubMed] |
| 35. | Yang T, Xie RJ, Luo XH, Yang Q. Effects of traditional Chinese medicine prescription Dan-shao-hua-xian Capsule on the expression of Smads in rat liver of hepatic fibrosis. Zhongguo Bingli Shengli Zazhi. 2010;26:1807-1812. |
| 36. | Myllärniemi M, Lindholm P, Ryynänen MJ, Kliment CR, Salmenkivi K, Keski-Oja J, Kinnula VL, Oury TD, Koli K. Gremlin-mediated decrease in bone morphogenetic protein signaling promotes pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:321-329. [PubMed] |