Published online Oct 28, 2014. doi: 10.3748/wjg.v20.i40.14855
Revised: May 5, 2014
Accepted: June 21, 2014
Published online: October 28, 2014
Processing time: 223 Days and 4.9 Hours
AIM: To investigate the role of acyl-CoA synthetase 5 (ACSL5) activity in Wnt signaling in intestinal surface epithelia.
METHODS: Several cell lines were used to investigate the ACSL5-dependent expression and synthesis of Wnt2B, a mitochondrially expressed protein of the Wnt signaling family. Wnt activity was functionally assessed with a luciferase reporter assay. ACSL5-related biochemical Wnt2B modifications were investigated with a modified acyl-exchange assay. The findings from the cell culture models were verified using an Apcmin/+ mouse model as well as normal and neoplastic diseased human intestinal tissues.
RESULTS: In the presence of ACSL5, Wnt2B was unable to translocate into the nucleus and was enriched in mitochondria, which was paralleled by a significant decrease in Wnt activity. ACSL5-dependent S-palmitoylation of Wnt2B was identified as a molecular reason for mitochondrial Wnt2B accumulation. In cell culture systems, a strong relation of ACSL5 expression, Wnt2B palmitoylation, and degree of malignancy were found. Using normal mucosa, the association of ACSL5 and Wnt2B was seen, but in intestinal neoplasias the mechanism was only rudimentarily observed.
CONCLUSION: ACSL5 mediates antiproliferative activities via Wnt2B palmitoylation with diminished Wnt activity. The molecular pathway is probably relevant for intestinal homeostasis, overwhelmed by other pathways in carcinogenesis.
Core tip: Acyl-CoA synthetase 5 (ACSL5) plays a key role in fatty acid metabolism. Besides its proapoptotic effects along the crypt-villus-axis ACSL5 also functions as an antiproliferative modifier of Wnt signaling activity. In the presence of ACSL5, Wnt2B was S-palmitoylated and thereby enriched in mitochondria, which was paralleled by a significant decrease in Wnt activity. The molecular pathway is probably of relevance for the homeostasis of the intestinal barrier.
- Citation: Klaus C, Schneider U, Hedberg C, Schütz AK, Bernhagen J, Waldmann H, Gassler N, Kaemmerer E. Modulating effects of acyl-CoA synthetase 5-derived mitochondrial Wnt2B palmitoylation on intestinal Wnt activity. World J Gastroenterol 2014; 20(40): 14855-14864
- URL: https://www.wjgnet.com/1007-9327/full/v20/i40/14855.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i40.14855
The intestinal barrier consists of several highly specialized cellular types, which differentiate along the crypt-villus axis (CVA) or crypt-plateau axis (CPA) assisted by lymphatic tissue[1]. One hallmark of the intestinal surface epithelium is a continuous renewal within a few days from transit-amplifying cells and 4-6 undifferentiated epithelial stem cells located at the crypt basis[2]. Cells migrate from the crypt to the villus tip. At the transition between crypt and villus, they differentiate into enterocytes, enteroendocrine cells and goblet cells[3,4]. At the villus tip the cells undergo apoptosis and are scaled off in the lumen. Within the united cell structure, various signaling cascades are responsible for the transfer of information[5]. Disruption of these signal transductions is associated with cell degeneration and is of relevance for tumor formation. Because of this sensitive balance, proliferating signaling cascades and destruction events are interacting and regulate each other.
In the modifier concept of intestinal homeostasis, long-chain fatty acid metabolism, mediated by mitochondrial acyl-CoA synthetase 5 (ACSL5) is suggested to play an important role. ACSL5 belongs to the acyl-CoA synthetase family catalyzing activation of long-chain fatty acids by thioester formation with coenzyme A. ACSL5 is found in an ascending gradient along the crypt-villus axis and modifies apoptosis susceptibility of enterocytes towards TRAIL-derived apoptosis. ACSL5-derived long chain acyl-CoAs are assumed to be responsible for proapoptotic activities in enterocytes at the villus tip[6]. ACSL5 locates on chromosome 10q25.1-q25.2[7]. The functional protein is found in mitochondrial membranes[8,9].
Canonical Wnt signaling is of central relevance for the physiology of crypt-villus axis (CVA) and 19 Wnt molecules have been identified so far[10-12]. Wnt activation starts with a ligand-receptor linking to the cysteine-rich domain of transmembrane receptors from the Frizzled (Fzd) family and co-receptors from the low-density lipoprotein receptor-related protein (LRP) family. Following Wnt activation, β-catenin accumulates in the cytoplasm, translocates into the nucleus, and then activates transcription factors of the T-cell factor/lymphoid enhancing factor (Tcf/Lef) family by displacing Groucho proteins and recruiting co-activating proteins like BCL9/PYG and CBP[13,14]. As a result, gene expression of c-MYC, c-JUN, Cyclin D1 and others, involved in growth, differentiation, cell cycle progression, migration, and cell survival is induced[15]. Aberrations in Wnt signaling are frequently associated with colorectal carcinogenesis[16,17]. Various factors are able to modify Wnt-activity, like the lipid metabolism[18].
Wnt2B (also: Wnt13) is a positive regulator of the Wnt-β-catenin-Tcf-pathway[19-21]. The gene is located on human chromosome 1p13 and homologous to the proto-oncogene Wnt2 on chromosome 7q31[22]. Varying mRNA isoforms are generated by alternative splicing, differing in N-terminus, protein processing and subcellular localization. Katoh et al[23] identified in 2001 two splice variants, differing N-terminally, Wnt2B1 and Wnt2B2. They showed an increased level of Wnt2B2 in gastrointestinal tumors and an activating role in β-catenin/Tcf signaling cascade. Poulain et al[24] describe the interplay between Wnt2 and its isoform Wnt2bb during liver development. Struewing et al[25] identified three different isoforms, Wnt13A, Wnt13B and Wnt13C. While Wnt13A is glycolyzed and secreted as a typical Wnt protein, Wnt13B and Wnt13C are found intracellularly with a mitochondrial and nuclear localization. Wnt13B exists in two forms, L-Wnt13B with an N-terminal mitochondrial target sequence and mitochondrially localized, and S-Wnt13B nuclear[25-27].
Palmitoylation increases protein hydrophobicity and membrane associations as well as protein/protein and protein/lipid interactions that are essential for efficient signal transduction[28]. Even proteins involved in canonical Wnt signaling are described as fatty-acid-modified which influences their secretion and activity[29,30].
The working hypothesis of the present study was that modification of Wnt activity by a molecular interaction between mitochondrial ACSL5 enzyme activity and mitochondrial localized Wnt2B could exist.
Cloning of full-length human ACSL5 cDNA (GeneBank accession Nos. AB033899, AB033920) was performed as described previously[6]. Briefly, RNA was isolated from human intestinal mucosa, reverse transcribed, PCR-based amplified, and cloned into the pENTRY vector of the GATEWAY system (Invitrogen, Darmstadt, Germany). CMV-controlled expression constructs were generated by recombination into the pcDNA_DEST40 vector. Full-length sequencing was performed to control cDNA correctness. The human intestinal epithelial cell line CaCo2 was stable transfected with either ACSL5 expression constructs (clone 3/25) or the empty vector pcDNA_DEST40 (clone P14; control) using lipofectamine (Invitrogen) followed by subcloning. Transfection was controlled by PCR, Western blot, and immunostainings.
For cell culture experiments, established cell lines were used. CaCo2 cells (CaCo2; ATCC: HTB-37), stable clone P14 (CaCo2VEC+; transfection control), and stable clone 3/25 (CaCo2ACSL5+; ACSL5 transfectant) were cultured as previously described[6]. HEK293 (ATCC: CRL-3022), HT29 (ATCC: HTB-38), SW480 (ATCC: CCL-228), and HCT116 (ATCC: CCL-247) were cultured as recommended.
Palmostatin B is a substance with a molecular weight of 376 kDa. Cells and tissue were incubated with 30 μmol/L/50 μmol/L palmostatin B for 30 min. For the activation of Wnt signaling, cells were treated with 200 ng/mL recombinant Wnt3A (R&D systems 5036-WN, Minneapolis, United States) and incubated for 1 h.
Mitochondria were isolated and purified as described previously[6,31]. Briefly, cells were removed by trypsination, tissue was cut into small pieces and homogenized, washed with PBS at 4 °C, and suspended in 250 mmol/L sucrose, 10 mmol/L HEPES, 1 mmol/L EDTA pH = 7.4 followed by nitrogen cavitation (30 bar, 15 min). The cell or tissue lysates were cleared by an initial centrifugation step followed by pellet subfractionation (31000 ×g for 5 min) in 30 mL of a self-generating gradient of 30% percoll solution (250 mol/L sucrose, 5 mmol/L HEPES, 0.2 mmol/L EDTA). Fractions of 2 mL each were collected from the bottom of the gradient. Accumulation of mitochondria was controlled with measurement of succinate dehydrogenase enzyme activity and immunolabeling of cytochrome c oxidase IV subunit (CoxIV) in Western blotting. Fractions enriched with mitochondria were processed for protein preparation and analysis.
Protein G Sepharose beads (GE Healthcare 17-0618-01, Buckinghamshire, England) were washed in lyse buffer twice. The pellet from mitochondria isolation was resuspended with 500 μL lyses buffer and protease inhibitor, incubated at room temperature for 5 min and centrifuged 20 min at 300 g. The supernatant was transferred to sepharose beads, 1 μg anti-Wnt2B (Abcam 50575, Cambridge, England) was added. The samples were rotated for 2 h at 4 °C, then washed in lyse buffer 3 times and centrifuged. The pellet was directly used for acyl-biotin exchange.
For the acyl-biotin exchange, the pellet of isolated and immunoprecipitated mitochondria was resolved in 250 μL 1 mol/L hydroxylamine(hydrochlorid) and incubated for 1 h at 900 rpm. The resulting pellet after centrifugation for 3000 g for 2 min was treated with 10 mL 320 μmol/L Biotin-BMCC and rotated for 2 h at 4 °C. After washing with PBS the pellet was charged with sample buffer for Western blot.
Total RNA isolated with the Chomczynski procedure[32] was used for cDNA synthesis with the Reverse Transcription System Kit according to manufacturer’s protocol (Promega). The LightCycler (Roche) and the LightCycler Faststart DNA Master Plus SYBR Green I kit (Roche) were used to perform qRT-PCR measurements. Routine PCR runs consisted of 40 cycles with [95 °C/15 s; 63 °C/15 s; 72 °C/20 s]. Primer sequences: ACSL5: 5-TTT TTG TAC ACG GGG AGA GC-3; 5-ACA GGC TGT CAA TTT GGG TC-3; Wnt2B: 5-GTG TCC TGG CTG GTT CCT TA-3; 5-AGC TGG TGC AAA GGA AAG AA-3; β-catenin: 5-GCC GGC TAT TGT AGA AGC TG-3; 5-TGA TGT CTT CCC TGT CAC CA-3. The comparative cycle threshold method with cyclophilin or GAPDH as the house keeping gene was used to calculate relative mRNA levels.
Following the Chomczynski procedure[32], proteins were extracted, separated with SDS-PAGE, and electro transferred to a PVDF Immobilon-P membrane (Millipore, Bedford, MA). For immunodetection, the following primary antibodies were used as recommended by the manufacturers: anti-ACSL5 (Abnova 51703, Taipei, Taiwan, 1:500) anti-Wnt2B (Abcam 50575, Cambridge, England, 1:500), anti-Cox IV (Cell Signalling Technology, 4850, Frankfurt, Germany, 1:500), and anti-β-actin (Sigma, A5441, Taufkirchen, Germany, 1:1000). HRP-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, Heidelberg, Germany) (1:10000) and Pierce (High Sensitivity Streptavidin HRP Conjugate, Pierce, Rockford, United States) and visualized with enhanced chemiluminescence (Pierce, Rockford, IL). For molecular weight estimation, the peqGold prestained Protein Marker V (Peqlab, Erlangen, Germany) was used.
For immunohistochemistry, sections of paraffin-embedded tissues were processed following routine procedures. Briefly, tissue sections were dewaxed, incubated in blocking solution followed by the first antibody or isotype-matched immunoglobulin. The primary antibodies included anti-ACSL5 (Abnova 51703; dilution 1:50), anti-Wnt2B (Abcam 50575; dilution 1:500), anti-Ki67 (Medac RM-9106-R7; dilution 1:3), and anti-β-catenin (BD Transduction 610153; dilution 1:25). The antigens were uncovered by pre-treatment with Citrate buffer pH 6.0. Slides were scanned with Nano Zoomer 2.0 HT (Hamamatsu, Japan) and analyzed with NDP.view (Hamamatsu, Japan).
RNA interference on ACSL5 transcripts was performed as described previously[33]. Briefly, 75 nmol/L double-stranded small interfering RNAs Hs_ACSL5_6 HP and Hs_ACSL5_7 HP (Qiagen) were used for transfection experiments as described by the manufacturer. In control experiments, non-silencing siRNAs were used. Knockdown efficiency was evaluated by fluorescence microscopy, qRT-PCR, and anti-ACSL5 immunoblotting.
Cells were transiently transfected with pGL4[luc2CP/TCF-LEF RE/Hygro] (Firefly luciferase vector, Promega, Mannheim, Germany) and pGL4.70[hRluc] (Renilla luciferase vector, Promega, Mannheim, Germany). Wnt signaling was activated by the addition of 200 ng/mL recombinant Wnt3A (R&D Systems, Wiesbaden, Germany) and incubated for 1 h at 37 °C. The Dual luciferase Assay (Promega, Mannheim, Germany) was performed as recommended by manufacturer.
Apcmin/+ mice, originating from the Jackson Laboratory[34], were maintained in temperature-controlled rooms with a 12 h light/dark cycle in the animal facility of the University Hospital of Aachen. All studies were approved by the authority for environment conservation and consumer protection of the state North Rhine-Westphalia (Germany). Mice were sacrificed at the indicated time points, tissues were sampled for histological and RNA analysis. For each investigated time-point and condition at least 5 mice were investigated.
The use of human tissues was approved by the local ethics committee at the University Hospital RWTH Aachen (EK 179/12). A total of 12 patients were included in this study. Neither neo-adjuvant chemotherapy nor radiation was applied prior to surgery for sporadic human colorectal adenocarcinomas (all graded G2 or G3). Tissue specimens were fixed in formalin and embedded in paraffin. For immunostainings against ACSL5, Wnt2B, Ki67, or β-catenin, tissues were sectioned and dewaxed. In each slide, the staining intensity of normal mucosa and adenocarcinoma was evaluated. The staining intensity of a staining control was used as standard.
Data demonstrate findings of experiments reproduced at least three times. Excel-based algorithms and procedures were used in data processing. Standard deviations from the means are indicated by error bars. The t-test was applied to calculate differences between groups. P-values of less than 0.05 were considered to be significant. Immunoblots were calculated with densitometry and data processing with the ImageJ Quant 5.1 software (National Institutes of Health, United States; http://rsb.info.nih.gov/ij).
To prove the possibility of molecular interaction between ACSL5 and Wnt signaling, CaCo2 cells were used. CaCo2 is an established cell line derived from human colon adenocarcinoma with mutated APC and disrupted Wnt signaling[35]. Besides wild-type cells we used a stable transfected ACSL5 clone (clone 3/25) and a vector control (clone P14).
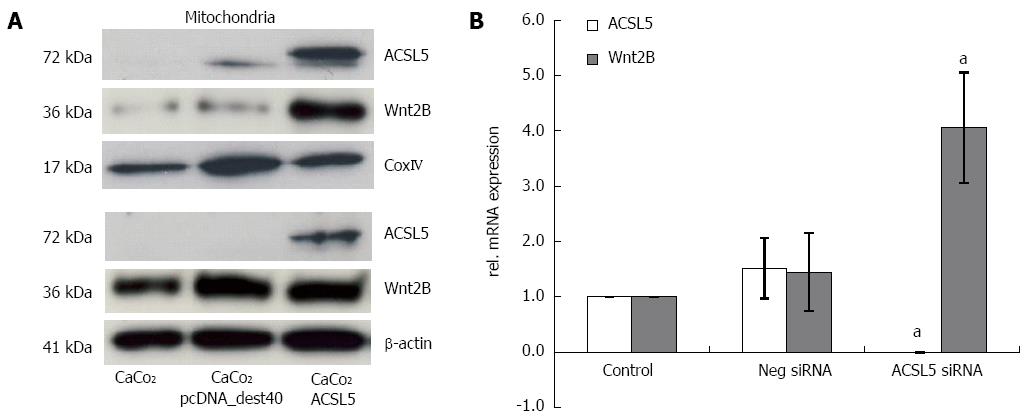
To characterize the cellular compartment of ACSL5 and Wnt2B, CaCo2 cells were fractionated into mitochondria and cytoplasm, and protein expression was measured by Western blotting. As reference proteins, β-actin (cytoplasm) and CoxIV (mitochondria) were used. An overexpression of ACSL5 caused significantly increased Wnt2B levels in the mitochondrial fraction, while cytoplasmatic levels remained unchanged (Figure 1A). To evaluate changes in Wnt2B mRNA levels in dependence on ACSL5, realtime PCR was performed. Samples were CaCo2 cells in which ACSL5 was knocked down by siRNA. In the absence of ACSL5, Wnt2B showed significantly increased mRNA expression (Figure 1B).
In contrast to CaCo2, where the functional analysis of Wnt activity is hampered due to mutated APC[35], HEK293 cells are preferentially used for studies on Wnt, because the cascade activity is intact[36]. These cells derived from a human embryonic kidney cell line were transformed by DNA of human adenovirus 5[37]. ACSL5 is physiologically not detectable in these cells. Thus, conditions were ideal to characterize the putative influence of ACSL5 on Wnt signal transduction in HEK293 cells by on-/off system (with transient ACSL5 expression). Both cell lines, CaCo2 and HEK293, were furthermore transiently transfected with Wnt2B and/or luciferase Tcf reporter pTOPflash and control construct pFOPflash. Wnt signaling was induced by adding recombinant Wnt3A and detected luminometry.
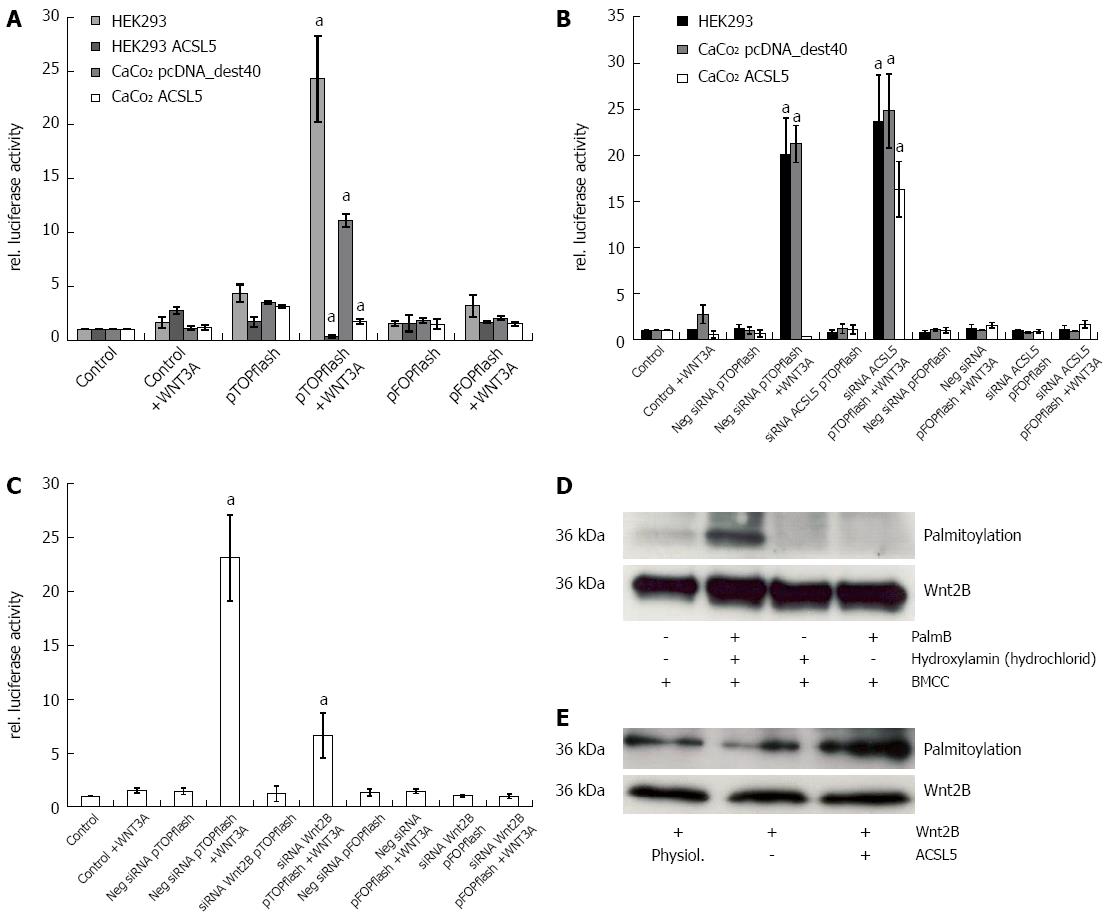
Cells with intact Wnt signaling (HEK293) showed distinct reporter gene expression after activation with Wnt3A. CaCo2 control transfectants (clone P14) reproduced this observation in half the expression intensity of HEK293. In the presence of increased ACSL5 expression, no statistically significant Wnt activation was detectable; reporter gene expression was significantly decreased (Figure 2A). To verify these results, ACSL5 was knocked down by siRNA in HEK293 and CaCo2, then pTOPflash and pFOPflash were transiently transfected and cells activated with Wnt3A. The luminometric detection showed a re-activation of Wnt signaling in ACSL5 transfected CaCo2 cells (Figure 2B). To characterize the role of Wnt2B in canonical Wnt signaling (wild type), the experiment was modified by reducing Wnt2B expression. A Wnt2B knockdown showed a decreased reporter gene expression in HEK293 cells (Figure 2C). This observation indicates a decreased activation of the canonical Wnt pathway in dependence on Wnt2B expression. In summarize, Wnt2B was identified as an activator of canonical Wnt signaling regulated by mitochondrial ACSL5.
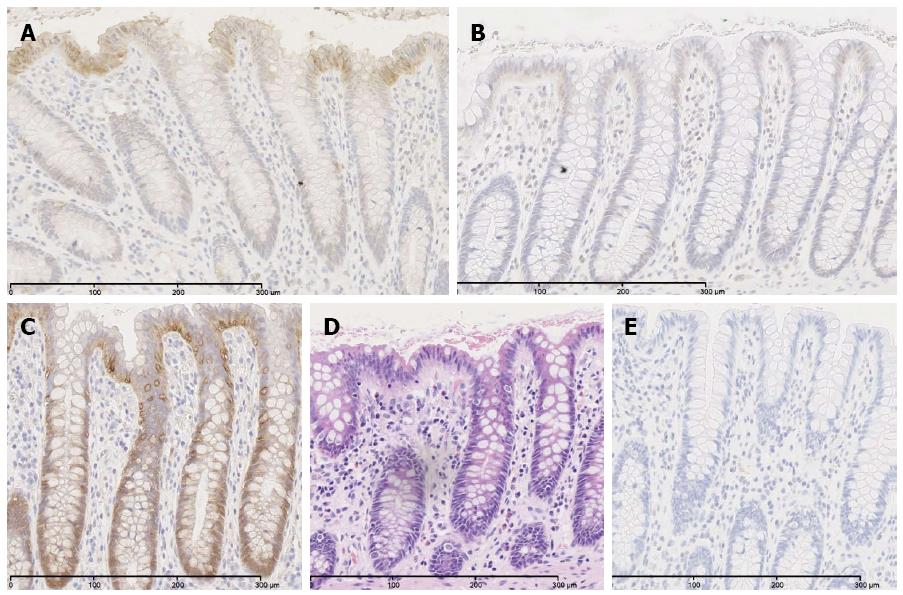
In order to prove palmitoylation as molecular mechanism of ACSL5-induced arrest of Wnt2B in mitochondria, an acyl-biotin-exchange assay was used[38]. To conserve palmitoylation of Wnt2B for the subsequent measure, cells were treated with palmostatin B. Afterwards mitochondria were isolated. Wnt2B was then immunoprecipitated in the mitochondrial fraction by sepharose beads by specific antibodies. The resulting samples were treated by acyl-biotin exchange, SDS-PAGE and analyzed by Western blotting. Figure 2D shows the established palmitoylation assay, figure 2E palmitoylation in relation to Wnt2B protein expression, depending on ACSL5. Increased ACSL5 expression resulted in significantly increased palmitoylation of Wnt2B in relation to Wnt2B protein expression, knocking down ACSL5 resulted in decreased palmitoylation of Wnt2B. The functional data are in line with the descriptive findings in normal human intestinal mucosa. Both ACSL5 and Wnt2B were expressed in an increasing gradient along the CVA, whereas Wnt activity, shown by nuclear β-catenin expression, was inverse (Figure 3).
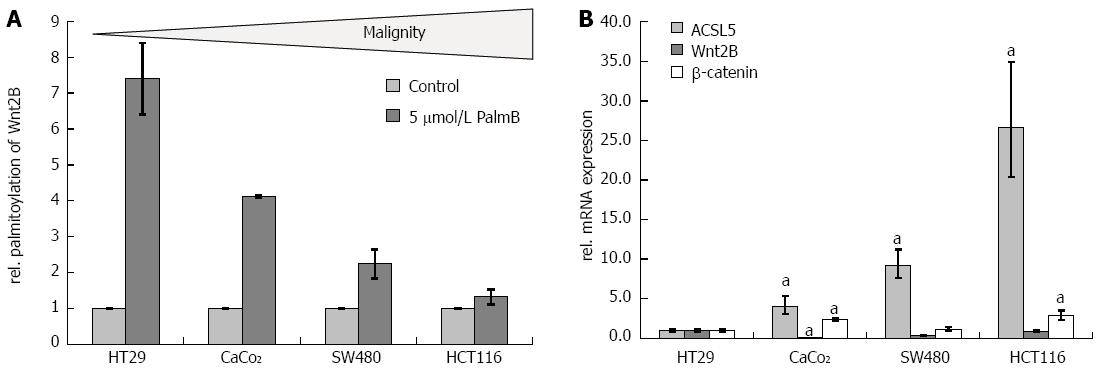
The palmitoylation status of Wnt2B, the main criterion of ACSL5-Wnt interaction, was tested in several human colon carcinoma cell lines of different malignancy statuses including HT29, CaCo2, SW480, and HCT116. Using the origin and power of cellular differentiation, the cell lines were characterized as low (HT29, CaCo2), intermediate (SW480), or high (HCT116) grade malignity[39]. In the palmitoylation exchange assay, cells with increasing malignity reveal significantly decreased palmitoylation of Wnt2B in mitochondria (Figure 4A). According to the in vivo situation, the analysis of ACSL5 showed that decreased palmitoylation correlates not immediate with a decreased ACSL5 expression. Expression studies in total cell lysates showed increased mRNA expression of ACSL5 with only marginally varying Wnt2B and β-catenin expression (Figure 4B).
In a next step we aimed to characterize the molecular association of ACSL5 and Wnt2B in intestinal neoplasias, i.e., adenomas and adenocarcinomas. In adenomas of the Apcmin/+ mouse model at 21 wk of age, translocation of β-catenin from cytoplasm into the nucleus was found (Figure 5B). Adenomatous or rather tumorous areas with decreased ACSL5 expression showed a tendency towards decreased expression of Wnt2B compared to surrounding normal tissue. These results suggest decoupling of ACSL5-Wnt interaction in adenomas.
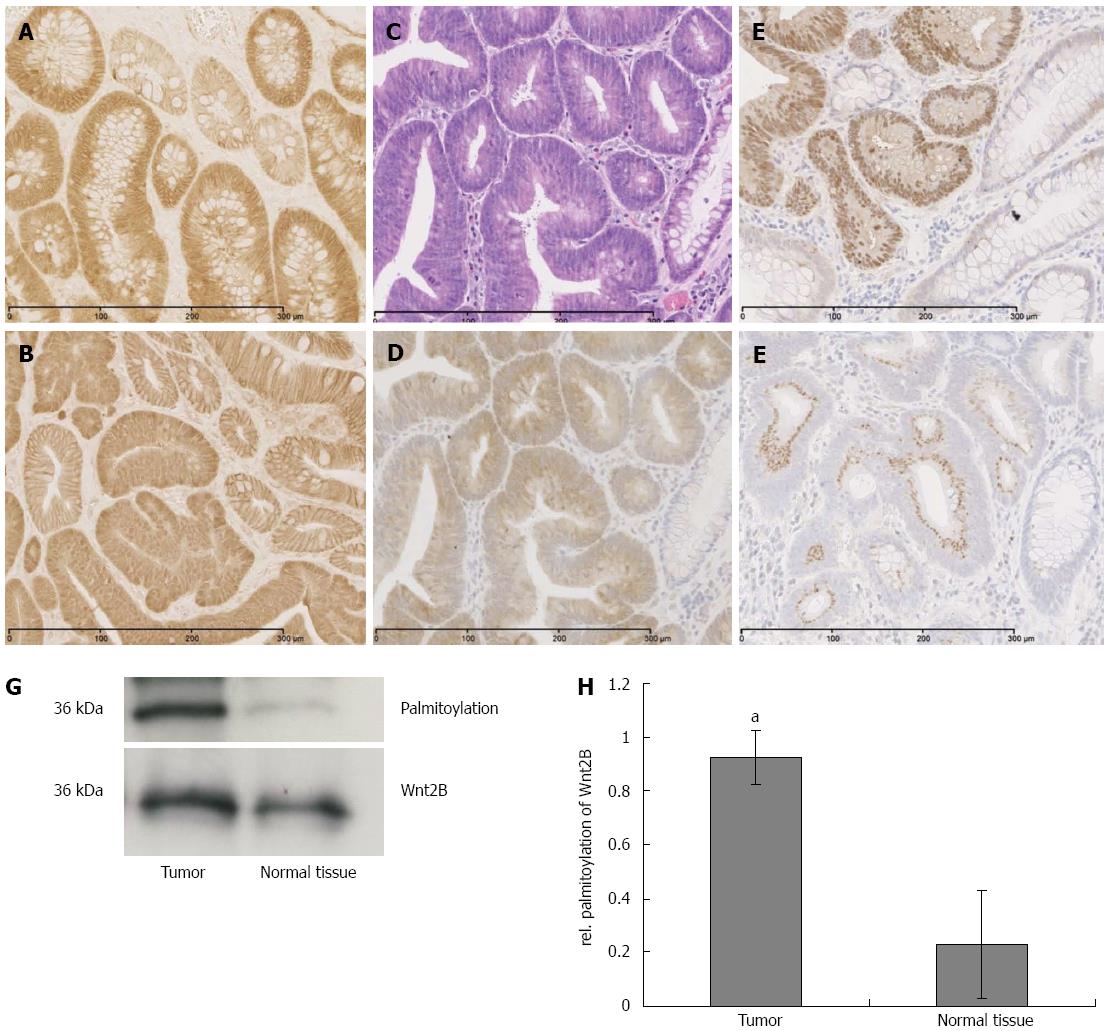
In human sporadic adenocarcinoma of the colon, a significant upregulation of ACSL5 and Wnt2B in carcinoma was shown (Figure 5C-F). Wnt2B was detectable both nuclearly and cytoplasmatically. In parallel an activation of Wnt signaling was found, shown by the translocation of β-catenin into the nucleus (Figure 5E). In the tumors, palmitoylation of Wnt2B was increased (Figure 5G and H) reflecting the in vitro findings.
Homeostasis of intestinal barrier is amongst other things maintained by regulatory signaling pathways like Wnt, which are essential for physiologic cell reagibility and influence the throughput of epithelial cells. Thus these signaling cascades are of central relevance for navigating enterocytes towards proliferation, differentiation, and apoptosis.
By its distinct and preferential mitochondrial localization on the inner and outer mitochondrial membrane, ACSL5 has a special ranking among acyl-CoA synthetases. It is of important relevance in mitochondrial fatty-acid metabolism and energy homeostasis of cells by the channeling of acyl-coA and promotion of ß-oxidation[8,40]. In addition, ACSL5 was identified as a functional regulator in the cellular homeostasis of apoptosis, survival, and differentiation. To analyze a putative link between ACSL5 and Wnt signaling, a luciferase reporter assay to detect Wnt activity by Tcf reporter[41] was established in two cell culture models. In HEK293 cells with intact Wnt signaling, ACSL5 was not detectable. In CaCo2 cells, characterized by a mutation in the APC, ACSL5 was strongly expressed. By means of both cell culture models it was coherently shown that ACSL5 expression has an inhibitory effect on Wnt activation. The findings with CaCo2 indicate that the ACSL5-Wnt interaction could be downstream of APC.
Lipid modifications of proteins are of extreme physiological and pathophysiological relevance. A functionally important variance of lipid modification is protein palmitoylation, biochemically an O- or S-derived binding of palmitate. Palmitoylation of a protein alters its function and is an important regulator in various signaling pathways[38]. Inaccurate palmitoylation is involved in the pathogenesis of several diseases including cancer[42]. It is known that various molecules of canonical Wnt signaling are modified by palmitoylation, possibly influencing their activity and localization[29]. To date, ACSL5 as promoter of Wnt palmitoylation has not been not characterized. However it has been shown that ACSL5-derived lipid modification of proteins regulating intestinal cell properties functions by palmitate addition as well[43]. This characteristic is presumably important in different signaling cascades and receptor structures[44]. Both in cell culture models and in murine and human tissue it was shown that overexpressed or downregulated ACSL5 alters expression and synthesis of Wnt2B in mitochondria and cytoplasm. This observation argues for an ACSL5-derived change in localization of Wnt2B. At high expression of mitochondrial ACSL5, Wnt2B is also increased in the mitochondrion; after ACSL5 knockout, Wnt2B expression is shifted into the cytoplasm. As an underlying mechanism palmitoylation was identified here, because the palmitoylation pattern of Wnt2B changed with varying ACSL5. This observation suggests an ACSL5-derived localization change of Wnt2B by palmitoylation, indirectly influencing activation of Wnt signaling.
Regarding a potential uncoupling of ACSL5-Wnt interaction in intestinal carcinogenesis, the relevance of this newly identified pathway was analyzed in different intestinal tumor cell lines dependent on their potential malignity. HT29 is the lowest-maligned cell line, increasing via CaCo2 and SW480 to HCT116 with the highest malignity grade[39]. With potentially increasing malignity, palmitoylation of Wnt2B in mitochondria was downregulated, with increasing ACSL5 mRNA expression and constant mRNA expression of Wnt2B and β-catenin as indicators for Wnt activation in whole lysates. Colon carcinoma cell lines of different origin and malignity status showed a broad range in Wnt2B palmitoylation. It is assumed from these data that our observed mechanism differs in its expressivity, maybe caused by other Wnt-regulatory factors or locoregional reasons. Maybe the localization of Wnt2B shifts towards the cytoplasm with increasing malignity and ACSL5 is no longer able to palmitoylate and arrest Wnt2B to mitochondrion. Other studies also describe a loss of palmitoylation in molecules with antineoplastic properties[45].
Following the cell-culture-based experiments, murine and human intestinal normal or diseased tissues were investigated. Both molecules, ACSL5 and Wnt2B, were downregulated in highly proliferating tissue and Wnt2B palmitoylation was decreased. In a direct comparison to tumor-surrounding normal tissues, the carcinoma showed significantly increased expression of ACSL5 and Wnt2B. Wnt2B was found cytoplasmatically and nuclearly. Mitochondria isolated from carcinoma showed significant increased palmitoylation of Wnt2B. Because of the mitochondrial localization of both target molecules, the observed expression changes, and in particular the mitochondrial palmitoylation of Wnt2B, ACSL5 seems to play a central role. Other ACSL isoforms, in particular ACSL1b and -6, were described in correlation with palmitoylation[43]: palmitoylation of G-Alpha S is essential for its function. It was inhibited by blocking of the ACSL activity by Triacsin C. As a result, the G-Alpha S signaling pathway in oocytes was inhibited and resulted in a mis-maturation in the human reproduction cycle.
Our data reveal that in addition to ACSL5 as a modifier of apoptosis, the enzyme is able to regulate Wnt signaling/activity. Its functionality is mediated by palmitoylation and results in Wnt signaling inhibition, which could antagonize early carcinogenesis. Based on the manifold cross-linking of different pathways particularly in intestinal epithelia, a key role of ACSL5 and other ACSL isoforms in other proliferation- and differentiation-associated pathways or apoptosis could be possible. The ACSL5 influence seems to be circumvented by other mechanisms with increasing malignity and disproportionate Wnt activation. The palmitoylation pattern changes independently and seems to underlie other regulatory mechanisms as only ACSL5-based expression. Electrostatic interactions could play an important role and lead to an association of Wnt2B and β-catenin instead of mitochondria and ACSL5.
In conclusion, experimental evidence exists for an inhibitory ACSL5 activity on Wnt signaling via palmitoylation of Wnt2B with mitochondrial Wnt2B arrest. The molecular pathway could be of relevance in carcinogenesis.
The intestinal surface epithelium is characterized by a continuous cellular renewal along the crypt-villus axis (CVA) or crypt-plateau axis. Cells migrate from the crypt to the villus tip and differentiate by this time. At the villus tip the cells undergo apoptosis and are scaled off in the lumen. Various signaling cascades build a sensitive balance and regulate each other. Mitochondrial acyl-CoA synthetase 5 (ACSL5) is expressed in an ascending gradient along the CVA and promotes enterocytic apoptosis at the villus tip. Canonical Wnt signaling is a proliferating signaling pathway. An interaction of ACSL5 with members of Wnt signaling pathway could be of central relevance for intestinal homeostasis.
ACSL5 is involved in fatty acid metabolism, its main function is the activation of long-chain fatty acids. In the area of prevention of intestinal diseases and tumor formation, the research hotspot is how ACSL5 modulates canonical Wnt signaling and regulates proliferation along the CVA.
Earlier studies showed the ascending expression of ACSL5 along the crypt-villus axis and a functional influence on the apoptosis susceptibility of enterocytes. The modifying properties on canonical Wnt signaling were so far unknown. Palmitoylation was identified as the fundamental mechanism for the molecular interaction between mitochondrial ACSL5 enzyme activity and mitochondrial localized Wnt2B.
The study results suggest that in addition to ACSL5 as a modifier of apoptosis, the enzyme is able to regulate Wnt signaling/activity. Its functionality is mediated by palmitoylation and results in Wnt signaling inhibition, which could antagonize early carcinogenesis.
Palmitoylation: palmitoylation is a reversible posttranslational protein-modification, consisting of a covalent thioester-bond between palmitoyl-CoA and cysteine. Cellular effects of palmitoylation are increased hydrophobicity, transformational change, interaction with membranes, or integration in membranes.
This is a very interesting paper aimed to characterize molecular mechanisms of antiproliferative ACSL5 activity, assumed to be a modifier of enterocytic maturation, on Wnt signaling.
P- Reviewer: de Magistris L, Serafino A, Zou C S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Gordon JI, Hooper LV, McNevin MS, Wong M, Bry L. Epithelial cell growth and differentiation. III. Promoting diversity in the intestine: conversations between the microflora, epithelium, and diffuse GALT. Am J Physiol. 1997;273:G565-G570. [PubMed] |
| 2. | Potten CS. Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos Trans R Soc Lond B Biol Sci. 1998;353:821-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 381] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 3. | Heath JP. Epithelial cell migration in the intestine. Cell Biol Int. 1996;20:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 149] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux JF, Pignodel C. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. 2011;192:767-780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 318] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 5. | Solanas G, Cortina C, Sevillano M, Batlle E. Cleavage of E-cadherin by ADAM10 mediates epithelial cell sorting downstream of EphB signalling. Nat Cell Biol. 2011;13:1100-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Gassler N, Roth W, Funke B, Schneider A, Herzog F, Tischendorf JJ, Grund K, Penzel R, Bravo IG, Mariadason J. Regulation of enterocyte apoptosis by acyl-CoA synthetase 5 splicing. Gastroenterology. 2007;133:587-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Mashima T, Sato S, Sugimoto Y, Tsuruo T, Seimiya H. Promotion of glioma cell survival by acyl-CoA synthetase 5 under extracellular acidosis conditions. Oncogene. 2009;28:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Lewin TM, Kim JH, Granger DA, Vance JE, Coleman RA. Acyl-CoA synthetase isoforms 1, 4, and 5 are present in different subcellular membranes in rat liver and can be inhibited independently. J Biol Chem. 2001;276:24674-24679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 227] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Coleman RA, Lewin TM, Van Horn CG, Gonzalez-Baró MR. Do long-chain acyl-CoA synthetases regulate fatty acid entry into synthetic versus degradative pathways? J Nutr. 2002;132:2123-2126. [PubMed] |
| 10. | Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1536] [Cited by in RCA: 1659] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 11. | Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4088] [Cited by in RCA: 4449] [Article Influence: 234.2] [Reference Citation Analysis (0)] |
| 12. | Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1521] [Cited by in RCA: 1530] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 13. | Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429-22433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 969] [Cited by in RCA: 1030] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 14. | Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 1215] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 15. | Harris TJ, Peifer M. Decisions, decisions: beta-catenin chooses between adhesion and transcription. Trends Cell Biol. 2005;15:234-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1377] [Cited by in RCA: 1448] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 17. | Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2659] [Cited by in RCA: 2766] [Article Influence: 138.3] [Reference Citation Analysis (0)] |
| 18. | Kuniyasu H. The Roles of Dietary PPARgamma Ligands for Metastasis in Colorectal Cancer. PPAR Res. 2008;2008:529720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Blasband A, Schryver B, Papkoff J. The biochemical properties and transforming potential of human Wnt-2 are similar to Wnt-1. Oncogene. 1992;7:153-161. [PubMed] |
| 20. | Shimizu H, Julius MA, Giarré M, Zheng Z, Brown AM, Kitajewski J. Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ. 1997;8:1349-1358. [PubMed] |
| 21. | Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, Yamaguchi TP, Morrisey EE. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17:290-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 334] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 22. | Katoh M. Frequent up-regulation of WNT2 in primary gastric cancer and colorectal cancer. Int J Oncol. 2001;19:1003-1007. [PubMed] |
| 23. | Katoh M, Kirikoshi H, Terasaki H, Shiokawa K. WNT2B2 mRNA, up-regulated in primary gastric cancer, is a positive regulator of the WNT- beta-catenin-TCF signaling pathway. Biochem Biophys Res Commun. 2001;289:1093-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Poulain M, Ober EA. Interplay between Wnt2 and Wnt2bb controls multiple steps of early foregut-derived organ development. Development. 2011;138:3557-3568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Struewing IT, Toborek A, Mao CD. Mitochondrial and nuclear forms of Wnt13 are generated via alternative promoters, alternative RNA splicing, and alternative translation start sites. J Biol Chem. 2006;281:7282-7293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Bunaciu RP, Tang T, Mao CD. Differential expression of Wnt13 isoforms during leukemic cell differentiation. Oncol Rep. 2008;20:195-201. [PubMed] |
| 27. | Tang T, Rector K, Barnett CD, Mao CD. Upstream open reading frames regulate the expression of the nuclear Wnt13 isoforms. Biochem Biophys Res Commun. 2008;366:1081-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4693] [Cited by in RCA: 4782] [Article Influence: 191.3] [Reference Citation Analysis (0)] |
| 29. | Doubravska L, Krausova M, Gradl D, Vojtechova M, Tumova L, Lukas J, Valenta T, Pospichalova V, Fafilek B, Plachy J. Fatty acid modification of Wnt1 and Wnt3a at serine is prerequisite for lipidation at cysteine and is essential for Wnt signalling. Cell Signal. 2011;23:837-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Gao X, Arenas-Ramirez N, Scales SJ, Hannoush RN. Membrane targeting of palmitoylated Wnt and Hedgehog revealed by chemical probes. FEBS Lett. 2011;585:2501-2506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Schotte P, Van Criekinge W, Van de Craen M, Van Loo G, Desmedt M, Grooten J, Cornelissen M, De Ridder L, Vandekerckhove J, Fiers W. Cathepsin B-mediated activation of the proinflammatory caspase-11. Biochem Biophys Res Commun. 1998;251:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15:532-54, 532-54. [PubMed] |
| 33. | Reinartz A, Ehling J, Leue A, Liedtke C, Schneider U, Kopitz J, Weiss T, Hellerbrand C, Weiskirchen R, Knüchel R. Lipid-induced up-regulation of human acyl-CoA synthetase 5 promotes hepatocellular apoptosis. Biochim Biophys Acta. 2010;1801:1025-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1131] [Cited by in RCA: 1162] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 35. | Sääf AM, Halbleib JM, Chen X, Yuen ST, Leung SY, Nelson WJ, Brown PO. Parallels between global transcriptional programs of polarizing Caco-2 intestinal epithelial cells in vitro and gene expression programs in normal colon and colon cancer. Mol Biol Cell. 2007;18:4245-4260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Gujral TS, MacBeath G. A system-wide investigation of the dynamics of Wnt signaling reveals novel phases of transcriptional regulation. PLoS One. 2010;5:e10024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Shein HM, Enders JF. Transformation induced by simian virus 40 in human renal cell cultures. I. Morphology and growth characteristics. Proc Natl Acad Sci USA. 1962;48:1164-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 146] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8:74-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 906] [Cited by in RCA: 891] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 39. | Flatmark K, Maelandsmo GM, Martinsen M, Rasmussen H, Fodstad Ø. Twelve colorectal cancer cell lines exhibit highly variable growth and metastatic capacities in an orthotopic model in nude mice. Eur J Cancer. 2004;40:1593-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Mashek DG, Li LO, Coleman RA. Rat long-chain acyl-CoA synthetase mRNA, protein, and activity vary in tissue distribution and in response to diet. J Lipid Res. 2006;47:2004-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 41. | Takemaru KI, Moon RT. The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. J Cell Biol. 2000;149:249-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 377] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 42. | Draper JM, Smith CD. Palmitoyl acyltransferase assays and inhibitors (Review). Mol Membr Biol. 2009;26:5-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Wang HW, Fang JS, Kuang X, Miao LY, Wang C, Xia GL, King ML, Zhang J. Activity of long-chain acyl-CoA synthetase is required for maintaining meiotic arrest in Xenopus laevis. Biol Reprod. 2012;87:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Kaemmerer E, Peuscher A, Reinartz A, Liedtke C, Weiskirchen R, Kopitz J, Gassler N. Human intestinal acyl-CoA synthetase 5 is sensitive to the inhibitor triacsin C. World J Gastroenterol. 2011;17:4883-4889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Galluzzo P, Caiazza F, Moreno S, Marino M. Role of ERbeta palmitoylation in the inhibition of human colon cancer cell proliferation. Endocr Relat Cancer. 2007;14:153-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |