Published online Oct 28, 2014. doi: 10.3748/wjg.v20.i40.14598
Revised: December 24, 2013
Accepted: April 27, 2014
Published online: October 28, 2014
Processing time: 349 Days and 7 Hours
Hepatitis B virus (HBV) infection is a leading cause of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma worldwide. Due to the shared modes of transmission, coinfection with HBV and human immunodeficiency virus (HIV) is not uncommon. It is estimated that 10% of HIV-infected patients worldwide are coinfected with HBV. In areas where an HBV vaccination program is implemented, the HBV seroprevalence has declined significantly. In HIV/HBV-coinfected patients, HBV coinfection accelerates immunologic and clinical progression of HIV infection and increases the risk of hepatotoxicity when combination antiretroviral therapy (cART) is initiated, while HIV infection increases the risk of hepatitis events, cirrhosis, and end-stage liver disease related to chronic HBV infection. With the advances in antiviral therapy, concurrent, successful long-term suppression of HIV and HBV replication can be achieved in the cART era. To reduce the disease burden of HBV infection among HIV-infected patients, adoption of safe sex practices, avoidance of sharing needles and diluent, HBV vaccination and use of cART containing tenofovir disoproxil fumarate plus emtricitabine or lamivudine are the most effective approaches. However, due to HIV-related immunosuppression, using increased doses of HBV vaccine and novel approaches to HBV vaccination are needed to improve the immunogenicity of HBV vaccine among HIV-infected patients.
Core tip: We provide an updated review of hepatitis B virus (HBV) coinfection among human immunodeficiency virus (HIV)-infected patients, focusing on the epidemiology, management and prevention of HBV infection. The mutually detrimental interactions between HBV and HIV are discussed. Three updated treatment guidelines for the management of patients with HIV/HBV coinfection are summarized. We also review the published data on the effectiveness or efficacy of HBV vaccination studies, with emphasis on the different approaches to improvement of the serologic responses to conventional HBV vaccine among HIV-infected patients.
- Citation: Sun HY, Sheng WH, Tsai MS, Lee KY, Chang SY, Hung CC. Hepatitis B virus coinfection in human immunodeficiency virus-infected patients: A review. World J Gastroenterol 2014; 20(40): 14598-14614
- URL: https://www.wjgnet.com/1007-9327/full/v20/i40/14598.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i40.14598
Hepatitis B virus (HBV) infection is a leading cause of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (HCC) worldwide[1]. Due to the shared modes of transmission, coinfection with HBV and human immunodeficiency virus (HIV) is not uncommon. It is estimated by the Joint United Nations Program on HIV/AIDS that 10% of 33 million HIV-infected patients has concurrent chronic HBV infection[2]. The prevalence or incidence of HBV infection among HIV-infected patients may vary widely with risks for HIV and HBV transmission, implementation of HBV vaccination programs, and the geographic regions with different levels of endemicity of HBV infection in the general population[2]. HBV and HIV have a mutually detrimental impact in that HIV infection accelerates HBV-related liver damage, leading to earlier cirrhosis and end-stage liver disease[3,4], and the presence of HBV infection complicates the management of HIV infection, impairs CD4 recovery, accelerates immunologic progression, and increases the morbidity and mortality of HIV-infected patients[4-8]. In this article we review the epidemiology, interactions between HIV and HBV, and management and prevention of HBV infection in HIV-infected patients in the era of combination antiretroviral therapy (cART) that often contains 1 or 2 nucleos(t)ide reverse-transcriptase inhibitors (NRTIs) that are active against HBV as well as HIV.
According to the World Health Organization[9], the world can be divided into 3 areas based on the levels of endemicity of HBV infection that are defined by the prevalence of chronic HBV infection: low endemicity, < 2%; intermediate endemicity, 2%-8%; and high endemicity, > 8%. In areas of high endemicity of chronic HBV infection, the transmission of HBV mainly occurs through perinatal transmission (predominantly in East and Southeast Asia) or in young children through close household contact or through medical or traditional scarification procedures (predominantly in Africa)[2,9]. Given the shared transmission routes of HIV and HBV, coinfection with HBV and HIV is common. Approximately 10% of the HIV-infected population in Asia and Africa has concurrent chronic HBV infection with coinfection more common in areas of high prevalence for both viruses[2,10]. The rate can be as high as 25% in countries where the viruses are highly endemic[10]. In areas where HBV is less endemic (North America, Europe, and Australia), HBV and HIV are most often acquired during adolescence or adulthood through sexual transmission or injection drug use[10]. In Western Europe and the United States, the overall prevalence of chronic HBV infection among HIV-infected persons is estimated to be 6%-14%[3,4,11-13], including 4%-6% of HIV-infected heterosexuals[3,12], 9%-17% of HIV-infected men who have sex with men (MSM)[3,11,12], and 7%-10% of injecting drug users[3,4,11,12]. Previous studies have shown that seropositivity for syphilis and HIV infection, the number of lifetime sexual partners, and receptive anal intercourse are associated with increased risk of HBV infection in MSM[14-16].
In a recent review of global epidemiology of HBV infection[17], the prevalence of HBV infection has been shown to be decreasing, particularly evident in central sub-Saharan Africa, tropical and central Latin America, southeast Asia and central Europe. Expanded programs of immunization against HBV have been proposed to significantly contribute to such an observation[17]. In areas that implemented universal neonatal HBV vaccination program such as Taiwan and Alaska, the incidence of acute HBV infection[18,19], prevalence of chronic HBV infection[19,20], and incidence of HCC in children have significantly declined[19,21], so has mortality due to chronic liver disease as well as HCC in persons aged 5-29 years[22].
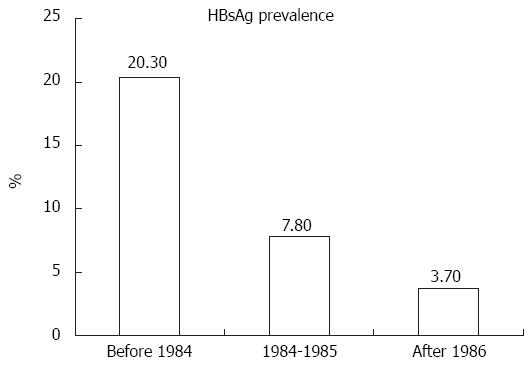
Recent studies that evaluated the long-term impact of universal neonatal HBV vaccination on HBV seroprevalence among HIV-infected populations and persons at high risk for HIV transmission in Taiwan also demonstrated decreasing trends in chronic HBV infection in those persons born after implementation of neonatal HBV vaccination and catch-up vaccination programs (Figure 1)[23,24]. The prevalence of hepatitis B surface antigen (HBsAg) positivity in HIV-infected patients born after July 1984, when the nationwide HBV vaccination program in Taiwan was initially implemented to vaccinate newborns of HBsAg-positive mothers, has declined to 3.3% vs 20.3% in those born before July 1984 (P < 0.05)[23]. Furthermore, the prevalence of HBsAg positivity was similar between HIV-infected MSM and HIV-uninfected MSM (3.7% vs 2.4%) who were born in the era of universal HBV vaccination (in or after 1986), despite the fact that HIV-infected MSM were more likely to have syphilis (21.2% vs 2.8%) and had a higher prevalence of HBV core antibody (anti-HBc) positivity (26.3% vs 19.6%), while HIV-infected MSM born in 1984-1985 had a significantly higher prevalence of HBsAg positivity than HIV-infected MSM born in or after 1986 (7.8% vs 3.7%)[24]. Additionally, syphilis and positive anti-HCV were significantly associated with HBsAg positivity in HIV-infected patients born in the era of universal HBV vaccination.
Based on the extent of genetic diversity in HBV sequences, HBV can be divided into 10 genotypes (A to J) and several subtypes[25,26]. Genotypes A to D are more prevalent, with genotype A in sub-Saharan Africa, Northern Europe, and West Africa; genotypes B and C in Asia; and genotype D in Africa, eastern Europe, the Mediterranean region, and India. Other HBV genotypes are less prevalent with genotype E in West Africa; genotype F in Central and South America; genotype G in France, Germany, and the United States; and genotype H in Central America. Unlike other genotypes, the 2 newly identified genotypes, I and J, have yet to establish characteristic geographic and ethnic distribution. Genotype I is known as a recombinant of genotypes A, C, and G, and was found in Vietnam and Laos[27]. Genotype J was first identified in Ryukyu Island, Japan[28].
Many retrospective and prospective studies have been conducted to determine the impact of HBV genotypes on disease outcomes among the general population. Although some controversial results were observed likely due to the transmission route and age when HBV infection occurs, which are closely correlated with seroprevalence of HBV in the geographic areas studied, several studies suggested that patients infected with genotypes C and D had lower rates of seroconversion than patients infected with genotypes A and B, which is likely correlated with the relatively delayed onset of spontaneous HBV envelope antigen (HBeAg) seroconversion and HBsAg seroclearance[29-31]; infection with genotype C was associated with an increased risk of HCC than with genotype B in retrospective, prospective, and case-control studies[32-36]; and patients infected with genotype C tended to have higher HBV viral load and higher frequency of basal core promoter A1762T/G1764A mutation than those with genotype B[34,35,37]. In addition, although HBV genotyping before antiviral therapy is not recommended by current guidelines[38], the impact of HBV genotypes on clinical responses to interferon (IFN) have been described. First, in HBeAg-positive patients receiving standard IFN-α, the sustained virologic response rate was higher in patients infected with genotypes A and B than those with genotypes C and D[39-41]. Even among HBeAg-negative patients treated with IFN-α, HBsAg clearance was significantly higher in patients with genotype A (20%) than those with genotypes B (6%), C (9%), and D (6%)[42]. While Chien et al[43] first reported that the sustained response rate to lamivudine (LAM) was much higher in patients with genotype B than those with genotype C, subsequent studies demonstrated similar therapeutic responses or risk of emergence of LAM resistance among patients infected with different HBV genotypes[44-46]. No statistically significant difference was observed in response to adefovir dipivoxil (ADV)[47] and telbivudine (LdT)[48], either.
In HIV/HBV-coinfected patients, HBV genotype A is the most prevalent in Western countries[49], although the distribution of HBV genotypes might also vary according to the risk factors, geographic origin, and coinfection with other hepatitis viruses[50,51]. The impact of HBV genotypes on the course of HBV infection observed included more advanced fibrosis in patients infected with non-A genotypes[49], especially with genotype G[52], although a recent study with more than 5 years of follow-up demonstrated that infection with HBV genotype G was not significantly associated with severe liver disease and had no impact on fibrosis progression[53]. In another study in HIV/HBV-coinfected patients receiving long-term LAM-containing ART by Sheng et al[54], patients coinfected with genotype B were more likely to experience acute exacerbations of hepatitis, HBeAg seroconversion, LAM resistance, and liver disease-related death than those coinfected with genotype C.
It is suggested that a persistent state of immune activation in patients with chronic HBV infection could up-regulate HIV replication[55], and an in vitro study showed that HBV X protein could induce ongoing HIV replication and long-term repeated transcription of HIV by synergizing with kappa B-like enhancer and T-cell activation signals[56,57]. Early prospective cohort studies of HIV/HBV-coinfected patients revealed a 3.6 to 6.8-fold relative risk of progression to AIDS compared to those without coinfection[58,59]. However, other reports failed to confirm these results[3,60]. To minimize the influence of duration of HIV infection, a prospective observational cohort of adult patients with primary HIV infection (seroconversion window ≤ 6 mo)[7] has shown that HBV coinfection (adjusted hazards ratio, 3.46; 95%CI: 1.16-10.32) was an independent predictor of immunologic progression that was defined as the occurrence of a CD4 cell count < 350 cells/μL 3 mo or more after diagnosis of primary HIV infection[7]. Chun et al[6] examined the interactions of HBV and HIV using the composite endpoint of AIDS-defining illnesses and death among HIV-infected individuals who had a seroconversion window of ≤ 3 years in a large cohort, which revealed that the hazards ratio for an AIDS or death event was almost double (adjusted hazards ratio, 1.80; 95%CI: 1.20-2.69) for those with HBV coinfection. The adverse impact of HBV on HIV was also recently demonstrated by the Swiss HIV Cohort Study[8], in which patients who tested positive for HBsAg had significantly impaired CD4 recovery during the first 3 years of cART despite similar virologic effectiveness of antiretroviral therapy compared to patients without HBV infection [504 cells/μL (95%CI: 496-511) vs 449 cells/μL (95%CI: 428-469)].
Compared to HIV-uninfected subjects, patients with HIV infection have a higher risk of chronicity after acute HBV infection[61]. A higher proportion of chronic HBs antigenemia has been found in HIV-infected patients because HIV destroys CD4 cells which compromises host immunity against HBV[62]. A previous study on pregnant women with chronic HBV infection in Zambia showed that those with HIV coinfection had a 3-fold higher HBeAg-positive rate than HIV-uninfected pregnant women (25% vs 8.5%, P < 0.05)[63]. Another similar study showed that HBV DNA was detected in 26.7% of pregnant women with HIV/HBV coinfection vs 9.4% of those with HBV infection alone (P = 0.06)[64]. Clinical observational studies have demonstrated that HIV/HBV-coinfected patients may have faster progression of hepatic fibrosis and a higher risk of cirrhosis, end-stage liver disease, and HCC than HBV-monoinfected patients[4,65]. Similarly, compared with HIV-monoinfected patients, those with HIV/HBV coinfection, especially HBV genotype B, had a higher risk of acute hepatitis, hepatic decompensation, and liver-related mortality[4,54,66]. Superinfection or coinfection with hepatitis D virus may further exacerbate the complications in patients with HIV/HBV coinfection, which has recently been observed to increase in incidence in an area which was used to be hyperendemic for HBV infection in the general population[67,68].
The goal of antiviral therapy for HBV is to suppress HBV DNA replication, reduce necroinflammatory activity, and prevent progression to cirrhosis and HCC. At present, seven therapeutic agents, including IFN, pegylated-interferon (peg-IFN), LAM, emtricitabine (FTC), ADV, entecavir (ETV), LdT and tenofovir disoproxil fumarate (TDF) are approved by the US Food and Drug Administration (FDA) for the treatment of chronic HBV infection[69]. The characteristics of anti-HBV therapeutic agents are shown in Table 1[70-81]. LAM, FTC and TDF have both anti-HBV and anti-HIV activities. According to current treatment guidelines for HIV-infected adult patients, when patients meet the criteria to start cART, 2 agents active against HBV should be included and the most commonly chosen agent is TDF in combination with either FTC or LAM[69,82]. If TDF is not available or not well tolerated, either ADV or ETV in combination with either FTC or LAM are recommended (Table 2)[82-84]. CART regimens containing LAM as the only agent with anti-HBV activity should be avoided due to the high risk of emergence of HBV with LAM resistance during therapy[85-90].
| Characteristics | Interferon alfa-2b1 | Pegylated interferon alfa-2a1 | Lamivudine | Emtricitabine | Adefovir | Entecavir | Telbivudine | Tenofovir disoproxil fumarate |
| Antiviral effect | Immune modulation | Immune modulation | Interference of HBV DNA synthesis | Interference of HBV DNA synthesis | Interference of HBV DNA synthesis | Interference of HBV DNA synthesis | Interference of HBV DNA synthesis | Interference of HBV DNA synthesis |
| HIV-1activity | No | No | Yes | Yes | No, at low dose2 | Yes | No7 | Yes |
| Dosage and administration | 10 million IU SC or IM 3 times a week | 180 mg SC once a week | 300 mg/d oral | 200 mg/d oral | 10 mg/d oral | 0.5 mg/d oral5 | 600 mg/d oral | 300 mg/d oral |
| Defined treatment duration | 48 wk | 48 wk | Indefinite | Indefinite | Indefinite | Indefinite | Indefinite | Indefinite |
| Undetectable HBV DNA | - | - | 40%-84% at 1 yr | 53% at 2 yr | 8.6% and 5.7% at 36 wk 36 and 48, respectively8 | 38% by the end of study (mean follow-up, 74 wk)9 | - | Up to 91% at 5 yr |
| HBeAg seroconversion | 0%-20% | 0%-20% | 22%-35% at 1 yr | 14% at 48 wk | 9% at 144 wk | - | - | 50% of TDF use; 57% of TDF plus FTC use at 5 yr |
| Tolerability | Poor | Poor | Excellent | Excellent | Good | Excellent | Good | Good |
| Major adverse events | Leukopenia, depression | Leukopenia, depression | - | - | Nephrotoxicity (3%) | - | - | Nephrotoxicity (1%-3%) |
| Viral resistance barrier | No | No | Low (50% at 2 yr and 90% at 4 yr) | Intermediate (18% at 2 yr) | High | High | - | High |
| HBV resistancemutations | No | No | M204I/VL180MA181T/VV173L | M204I/VL180MA181T/V | N236TA181T/V | M204I/V4L180MT184I/A/G/LS202I/GI169T | M204IA181T/V | A194T3A181T/VN236T |
| Cross-resistance to LAM | No | No | - | Yes | No | No | Yes | No |
| Interaction with other antiretrovirals | No | No | No | No | No | No | Zidovudine; stavudine6 | Didanosine; atazanavir6 |
| Agents for HBV treatment only | Agents for HBV/HIV treatment | Timing of cART initiation | Comments | |
| DHHS 2013[82] | ADV, ETV, or LdT | TDF can safely be used: TDF/LAM-containing cART or TDF/FTC-containing cART TDF cannot safely be used: ETV plus LAM-cART, or ETV plus LAM-cART | HBV: NA HIV: prioritized; regardless of CD4 count | CART may attenuate liver disease progression by preserving or restoring immune function and reducing HIV-related immune activation and inflammation |
| EACS 2013[83] | ADV and LdT | LAM-naïve cART including TDF/LAM or FTC LAM-experienced Add or substitute 1 NRTI with TDF as part of cART HBV treatment indicated Early ART including TDF/FTC or LAM PEF-IFN (if genotype A, high ALT, low HBV DNA) | HBV: HBV DNA > 2000 IU/mL; significant liver fibrosis (F2-F4) even when HBV-DNA is below 2000 IU/mL and liver enzymes are not elevated HIV: CD4 < 500 cells/μL or symptomatic HIV, cirrhosis, or HBV treatment indicated | The optimal treatment duration for nucleos(t)ide analogues with anti-HBV activity has not yet been determined and experts recommend life-long therapy if anti-HBV nucleos(t)ides are given as part of ART In persons with HBV genotype A, high ALT and low HBV DNA, Peg-IFN might be used for a total treatment period of 48 wk. The addition of an NRTI-based anti-HBV regimen has not been proved to increase Peg-IFN efficacy In persons with liver cirrhosis, stopping effective anti-HBV treatment is not recommended in order to avoid liver decompensation due to flares of liver enzymes Anti-HBV therapy may be stopped cautiously in HBeAg-positive persons who have achieved HBe seroconversion for at least 6 mo or after confirmed HBs seroconversion in those who are HBeAg-positive The addition of ETV to TDF in persons with low persistent HB -replication has not been statistically proved to be efficient and should therefore be avoided Caution is warranted when switching from a TDF-based regimen to drugs with a lower genetic barrier, e.g., FTC or LAM, in particular in LAM-pretreated cirrhotic persons as viral breakthrough due to archived YMDD mutations is likely to occur |
| BHIVA 2013[84] | NA | CD4 count > 500 cells/μL: TDF/FTC-cART Unwilling or unable to receive TDF/FTC: ADV or 48 wk of PEG-IFN plus cART CD4 count < 500 cells/μL: Wild-type HBV: TDF/FTC-cART or TDF/LAM-cART LAM/FTC-resistant HBV or HIV: TDF as the sole anti-HBV active agent TDF is contraindicated: ETV plus cART | HBV: HBV DNA > 2000 IU/mL; more than minimal fibrosis on liver biopsy (Metavir > F2 or Ishak > S2) or indicative of > F2 by TE (FibroScan > 9.0 kPa) regardless of HBV DNA HIV: CD4 < 500 cells/μL or patients requiring HBV therapy | At least 2 baseline HBV DNA measurements 3 to 6 mo apart to guide initiation of therapy 6-mo HBV DNA measurements for routine monitoring of therapy An ALT level below the upper limit of normal (30 IU/L for men; 19 IU/L for women) should not be used to exclude fibrosis or as a reason to defer HBV therapy |
Peg-IFN is superior to conventional IFN in the treatment of chronic HBV infection because of its long-acting characteristics and weekly administration[91]. The response rate of HBeAg seroconversion and suppression of HBV replication to peg-IFN with or without LAM among HBV-monoinfected patients ranges from 24% to 32%[91,92], compared with 0% to 20% among HBV/HIV-coinfected patients[71]. Nevertheless, IFN should be avoided in patients with low CD4 counts due to significant lymphocytopenia related to IFN[93]. As IFN has potential anti-HIV effects[94] without resulting in emergence of IFN-resistant HIV[93], IFN can be used in those who may need anti-HBV therapy but not anti-HIV therapy (e.g., CD4 count ≥ 500 cells/μL). However, IFN is contraindicated in patients with decompensated liver disease because of concerns about hepatic failure and deaths during IFN treatment[95].
LAM has activity against both HIV and HBV at the daily dose of 300 mg and 100 mg, respectively. This agent is well tolerated with few adverse effects[96]. The rates of HBeAg seroconversion and HBV viral suppression (HBV DNA < 400 copies/mL) among HBV/HIV-coinfected patients receiving LAM 300 mg daily for 1 year ranged from 22% to 35% and 40% to 84%, respectively[85-88]. However, the genetic barrier to LAM resistance is low and LAM resistance rates may be as high as 50% after 2 years and 90% after 4 years of LAM therapy in HIV/HBV-coinfected patients[85-90].
FTC is a cytosine analogue that is structurally similar to LAM, and the daily dose for both HBV and HIV is 200 mg. The resistance profile and efficacy of FTC against HIV and HBV are also similar to LAM[97,98]. After 2 years of treatment with FTC, 53% of HBV-monoinfected patients had undetectable serum HBV DNA (< 4700 copies/mL), 33% seroconverted to anti-HBe, and 85% had normal alanine aminotransferase (ALT) levels. The rate of mutations in the YMDD motif was 18% at 2 years of FTC treatment[99]. In a small cohort of 16 HBV/HIV-coinfected patients treated with TDF and FTC for 48 wk, 94% patients had undetectable serum HBV DNA and 14% of them seroconverted to anti-HBe[100].
LAM may promote the selection of resistant mutations in the HBV DNA polymerase gene at the YMDD motif, rtM204V/I, which predisposes to FTC and LdT cross-resistance[101]. Furthermore, the common LAM-resistant mutations, rtL180M and rtM204V, are 2 of the 3 major mutations required for the development of ETV resistance. In addition, the rtA181T/V mutation confers cross-resistance to ADV[102]. Therefore, the combination of LAM with other anti-HBV agents without cross-resistance as part of antiretroviral therapy is the most effective approach to prevent against emergence of LAM-resistant HBV strains among HIV/HBV-coinfected patients.
ADV had sustained antiviral activity against LAM-resistant HBV strains in 29 HIV/HBV-coinfected patients throughout 144 wk of treatment, with 25% achieving undetectable HBV DNA and 9% HBeAg seroconversion[103]. In a prospective randomized controlled study of 52 HBV/HIV-coinfected patients, the anti-HBV activity of ADV was comparable to TDF (average change in serum HBV DNA from baseline to week 48, -4.44 log copies/mL for TDF and -3.21log10 copies/mL for ADV)[78]. The incidence of HBV resistant to ADV is less frequent than that to LAM[104,105]. Mutations at rtN236T and rtA181V, which confer resistance to ADV, occurred in 29% of the patients receiving 5 years of ADV treatment[105]. These mutations are potentially cross-resistant to TDF, and rtA181V is partially cross-resistant to LAM[102]. The rate of ADV resistance is markedly reduced when ADV is added to LAM rather than used as sequential monotherapy in patients with LAM-resistant HBV infection[106,107]. In addition, no genotypic resistance of HIV to ADV was found after 3 years of therapy[103].
TDF is a potent agent and effective against LAM-resistant HBV[108] and ADV-resistant HBV[109]. In a study that included 110 HIV/HBV-coinfected patients with 57% being HBeAg-positive at baseline, TDF-containing cART led to high rates of HBeAg seroconversion after 5 years of treatment: 21% in the LAM group, 50% in the TDF group and in 57% in the TDF plus FTC group[110]. During a median observation period of 83 mo, 91% achieved suppression of HBV replication[110]. In a meta-analysis of available data from 23 studies that included 550 HBV/HIV-coinfected patients treated with TDF[111], the overall proportion achieving suppression of HBV replication was 57.4%, 79.0% and 85.6% at 1, 2 and 3 years, respectively, and prior or concomitant 3TC or FTC did not impact the virologic response of HBV infection to TDF; furthermore, virologic rebound on TDF treatment was rare. Those findings of dual anti-HBV and anti-HIV activity and a high genetic barrier to resistance have made TDF an attractive option for the treatment of both viruses in patients with HIV/HBV coinfection. However, TDF may cause renal impairment (1%-3%), which includes Fanconi’s syndrome, tubular dysfunction, increases in serum creatinine, and, in rare cases, acute renal failure. Therefore, regular monitoring of renal function in patients receiving TDF-containing regimens is advised[112].
ETV is a guanosine analogue that is highly active against wild-type HBV at a daily dose of 0.5 mg and LAM-resistant HBV at 1 mg. It has been demonstrated that ETV reduced HBV DNA by 4.20log10 copies/mL in HIV/HBV-coinfected patients with HBV resistant to LAM at 48 wk of therapy[113]. ETV has been found to be associated with a 1-log10 reduction of plasma HIV RNA load and mutation in HIV polymerase (rtM184V) that confers resistance to both LAM and FTC[114]. ETV resistance is the result of 3 major mutations, rtL180M, rtM204V and either rtT184G/S, rtS202I or rtM250V. The first 2 mutations also confer resistance to LAM[115]. Therefore, ETV is not recommended as monotherapy in HIV/HBV-coinfected patients.
Data on the antiviral effect of LdT against HBV in HIV/HBV-coinfected patients are sparse. In HBV-monoinfected patients, LdT decreased HBV DNA levels by 6.45 log10 copies/mL in HBeAg-positive and by 5.23 log10 copies/mL in HBeAg-negative patients[116,117]. LdT has greater anti-HBV efficacy than LAM or ADV, and selects for resistance mutations at an intermediate rate. Resistant mutations were selected in 11% of HBeAg-negative and 25% of HBeAg-positive patients after 2 years of treatment with LdT[116,117]. In an in vitro and human study, LdT was not shown to exert antiviral activity against HIV-1[77], while a transient reduction in HIV-1 RNA between 2 and 3 log10 copies/mL after 24 wk of telbivudine therapy was seen in 2 of 3 patients without showing genotypic resistance mutations to antiretrovirals[81].
Serum HBV DNA level is a marker of viral replication and efficacy of antiviral treatment in individuals with chronic HBV infection. Maintaining suppression of HBV replication using anti-HBV therapy may reduce the progression of liver fibrosis, reverse advanced fibrosis, reduce the development of cirrhosis, and prevent hepatic decompensation and HCC in patients with advanced fibrosis or cirrhosis. In a prospective cohort study of 3653 HBsAg-positive participants (aged 30-65 years) in Taiwan[118], the incidence of HCC increased with increasing serum HBV DNA levels at study entry in a dose-response relationship, from 108 per 100000 person-years for patients with an HBV DNA level of < 300 copies/mL to 1152 per 100000 person-years for those with an HBV DNA level of 1 million copies/mL or greater; the corresponding cumulative incidence rates of HCC were 1.3% and 14.9%, respectively. A high serum HBV DNA level (≥ 10000 copies/mL) is a significant risk predictor of HCC independent of HBeAg, serum alanine aminotransferase level, and cirrhosis of the liver[118].
In a systemic review of 21 studies conducted among 3881 anti-HBV NRTI-treated (for at least 24 mo or more) and 534 untreated patients, HCC developed less frequently in anti-HBV NRTI-treated patients (2.8% vs 6.4%, P = 0.003) during a 46 mo (range, 32-108 mo) observation period[119]; furthermore, HCC developed significantly less frequently in patients remaining in virologic remission than in those with virologic breakthrough or no response (2.3% vs 7.5%, P < 0.001)[119]. In a recent report conducted in an HIV-uninfected population, long-term ETV treatment reduced the incidence of HCC in HBV-infected patients and the treatment effect was greater in patients at higher risk of HCC[120]. These findings provide supportive evidence to the well-known association between the biologic gradients of HBV DNA levels and risk of HCC[118].
If LAM-resistant HBV is present, LAM can be continued for the management of HIV as LAM-resistant HIV has reduced viral fitness in vitro and slower progression in vivo. TDF, ADV and ETV are active against LAM-resistant HBV[78,103,108,109,113,121]. A previous study comparing the efficacy of TDF and LAM combination therapy vs TDF after LAM failure for the treatment of HBV in HIV/HBV-coinfected patients revealed no statistically significant difference in terms of HBeAg loss or HBV suppression[122]. ETV is less preferred because LAM resistance predisposes to ETV resistance[115]. However, a small cohort of 13 patients with positive HBeAg and detectable HBV DNA who had received > 6 mo of TDF/FTC therapy, add-on ETV to TDF/FTC-experienced patients achieved undetectable HBV DNA load in 4 (30%) and normal ALT levels in 8 (62%)[79].
During anti-HBV treatment, monitoring of liver functions (alanine aminotransferase, aspartate aminotransferase, and total bilirubin) is advised every 3 to 6 mo and serum HBV DNA levels every 6 to 12 mo[83]. The presence of detectable serum HBV DNA with the use of sensitive assays after 24 wk of therapy suggests a suboptimal response or treatment failure, and add-on therapy with agents without cross-resistance should be considered at this stage[102].
The advances in therapy for HIV infection have prolonged the life expectancy of HIV-infected patients receiving cART[123], which has led to a greater need for treating HBV-related chronic complications. The 2 major adverse outcomes in patients with chronic HBV infection are cirrhosis and HCC, both of which can lead to liver-related death[124]. A low CD4 cell count in HIV/HBV-coinfected patients has been associated with increased risk of cirrhosis and HCC[10,125,126]. Overall, less treatable cases and lower survival rates have been described in HIV-infected patients following the diagnosis of HCC[4]. New treatment strategies are available for advanced HCC, but data are limited for HIV/HBV-coinfected patients. Case reports suggest some benefit from sorafenib treatment in HIV/HBV-infected patients with newly diagnosed HCC[127-130]. For most patients with end-stage liver disease, orthotopic liver transplantation remains the only therapeutic option. Accumulated experience in North America and Europe indicated that the patient and graft survival rates in selected HIV-infected recipients of liver transplants were almost similar to those of HIV-uninfected recipients[131,132]. Therefore, HIV infection by itself is not a contraindication to liver transplantation. Together with screening of patients at risk and an early diagnosis, aggressive treatment of HCC, including treatment of relapses and maintenance of HIV and HBV suppression, are the best management strategies for HCC in people living with HIV. All patients should receive anti-HBV NRTIs, and hepatitis B immune globulin indefinitely post-transplantation with a decrease in dose frequency after 12 mo[131]. It is recommended that patients with liver disease should start referral and workup for liver transplantation if they become symptomatic with liver disease[133], which includes the development of hepatic encephalopathy, ascites, variceal bleeding, or liver dysfunction with albumin < 3 g/dL and prolongation of prothrombin time by > 5 s[133].
Although the modes of transmission of HBV are the same as those for HIV, HBV is transmitted more efficiently than HIV[134,135]. Other than adoption of safe sex practices and avoidance of sharing needles and diluent, HBV vaccination remains the most effective approach to prevent against HBV infection and its chronic consequences. According to the HIV treatment guidelines by the US Department of Health and Human Services[82], pre-vaccination screening should include HBsAg, anti-HBsAg antibody (anti-HBs), and anti-HBc. Serological markers may be time-dependent variables in HIV-infected patients, which are associated with host immunity and viral activities; and, therefore, periodic measurements are recommended[5]. The presence of anti-HBs at levels of > 10 international units/L (IU/L) is consistent with seroprotection and at levels of > 100 IU/L is associated with long-term protection[136,137]. Anti-HBs antibody titers decrease over time and can fall below protective concentrations.
HBV vaccine series should be administered on the standard schedule (3 × 20-μg doses, administered intramuscularly at 0, 1, and 6 mo) if HBsAg, anti-HBs antibody, and anti-HBc antibody are all negative. Approximately 90% to 95% of healthy adults have protective anti-HBs titers after standard doses of HBV vaccines[138,139]. However, only 17.5% to 71% of HIV-infected patients could retain protective anti-HBs[137,139-148] (Table 3). In HIV-infected patients, variable immune responses to HBV vaccine have been shown to be associated with dysfunction of CD4 T cells, specific B-cell defects, and hyper-immune activation status and genes within the human leukocyte antigen complex[149-151].
| Ref. | Year | Study design | n | Dose (μg) | Schedules/ administration | Age, median, yr | CART | HIV-1 VL, RNA copies/mL < 10000 | CD4, median, cells/μL | Response rate | Predictors |
| Standard-dose vaccination | |||||||||||
| Rey et al[141] | 2000 | Prospective | 20 | 3 × 20 | 0, 1, 2 mo, IM | 30.5 | 85% | NA | 470 | 55% | CD4 > 500 cells/μL |
| Tedaldi et al[143] | 2004 | Retrospective, cross-sectional | 198 | 52.5% ≥ 3 × 20 | NA | 41 | 70.7% | > 75% | 406 | 37.2% | Higher CD4; HIV-1 VL < level of detection |
| Overton et al[142] | 2005 | Retrospective | 194 | 3 × 10 (97%-99%) | 0, 1 to 3, 6-9 mo, IM | 34.1 | 82.0% | 38.1% (< 400) | 290 | 17.5% | HIV-1 VL < level of detection |
| Ungulkraiwit et al[148] | 2007 | Prospective | 65 | 3 × 20 | 0, 1, 6 mo, IM | 39 | 88% | > 75% | 345 | 46% | Higher CD4; young age |
| Paitoonpong et al[137] | 2008 | Prospective | 28 | 3 × 20 | 0, 1, 6 mo, IM | 35 | 100% | 100% (< 50) | 324 | 71.4% | Higher CD4; use of efavirenz |
| Kim et al[144] | 2008 | Retrospective | 97 | 3 × 20 | 0, 1, 6 mo, IM | 39 | 31% | 24% (< 400) | 325 | 44% | Nadir CD4 > 200 cells/μL; young age ( < 40 yr); HIV-1 VL < level of detection |
| Irungu et al[139] | 2013 | Prospective | 293 | 3 × 20 | 0, 1 to 3, 6 mo, IM | 31 | HIV-1 uninfected | 85.7% | CD4 > 500 cells/μL; female | ||
| 310 | 0% | 65.7% | 557 | 64.2% | |||||||
| Alternative strategies | |||||||||||
| Fonseca et al[140] | 2005 | RCT | 94 | 3 × 20 | 0, 1, 6 mo, IM | 37 | 85.1% | 80.9% | ≥ 350, 59.6% | 34% | CD4 > 350 cells/μL; HIV-1 VL < 10000 copies/mL |
| 98 | 3 × 40 | 87.8% | 75.5% | ≥ 350, 57.1% | 47% (P = 0.07) | ||||||
| Cornejo-Juárez et al[147] | 2006 | RCT | 39 | 3 × 10 | 0, 1, 6 mo, IM | 35.6 | 56.4% | ≤ 20000, 56.6% | ≥ 200, 48.8% | 61.5% | CD4 ≥ 200 cells/μL |
| 40 | 3 × 40 | 34.1 | 72.5% | ≤ 20000, 55.6% | ≥ 350, 47.5% | 60% (P = 0.89) | |||||
| Potsch et al[145] | 2010 | Prospective | 47 | 3 × 40 | 0, 1, 2, 6 mo, IM | 36 | 79% | < 80, 70% | 402 | 89% | HIV-1 VL < 80 copies/mL |
| Launay et al[146] | 2011 | RCT | 145 | 3 × 20 | 0, 1, 6 mo, IM | 43 | 86% | < 50, 79% | 516 | 65% (95%CI: 56-72) | Young age; four-dose |
| 148 | 4 × 40 | 0, 1, 2, 6 mo, IM | 42 | 80% | < 50, 77% | 509 | 82% (95%CI: 77-88) | ||||
| 144 | 4 × 4 | 0, 1, 2, 6 mo, ID | 43 | 86% | < 50, 78% | 482 | 77% (95%CI: 56-72) | ||||
In HIV-infected patients, those with CD4 cell counts ≥ 350 cells/μL had a higher seroconversion rate (anti-HBs ≥ 10 IU/L) than those with CD4 cell counts < 350 cells/μL (39.3% vs 26.3%)[140]. Failure of anti-HBs seroconversion and lower anti-HBs titers after HBV vaccination in HIV-infected patients have been shown to be associated with detectable plasma HIV RNA, lower CD4 cell counts[142,147,152,153], age, HCV coinfection, occult HBV infection, alcohol abuse, and the general health status of the host[144,148,154,155]. A favorable response to cART may improve serological response[137,156] (Table 3).
Based on these data, early vaccination is recommended in HIV-infected patients before CD4 cell counts decline. These also strengthen the arguments for universal HBV vaccination of individuals at risk for HIV infection before they become HIV-infected and their immunosuppression worsens. Post-vaccination testing is recommended 1 to 2 mo after administration of the final dose of the primary vaccine series to determine the response to the vaccine. The height of the antibody titers is associated with the durability of effective antibody[136].
To improve the response rate and long-term persistence of antibodies, numerous studies have tried to use a variety of strategies such as increased doses, intradermal vaccination, and co-administration of immunomodulators. A fundamental strategy is to ensure that patients have optimal adherence to the vaccination schedule. A study conducted in a clinic specializing in the care of HIV-infected adults revealed that 7.5% had evidence or documentation of prior HBV vaccination at screening, and only 49% of those eligible for vaccination completed the standard vaccination schedule[157]. Other studies have also reported completion rates ranging from 29% to 62%[12,158,159].
For patients undergoing hemodialysis and for adults with general immune suppression, higher vaccine doses given on a standard schedule (3 × 40-μg doses administered intramuscularly at 0, 1, 6 mo) are recommended[160,161]. However, appropriate vaccine dosage has not been well defined in HIV-infected patients. In 1 double-blinded, randomized, controlled trial in 210 HIV-infected adults, 94 in the standard-dose group (3 × 20-μg doses at 0, 1, 6 mo) and 98 in the double-dose group (3 × 40-μg doses at 0, 1, 6 mo) completed the study[140]. There was no overall benefit in the double-dose group (seroconversion rate 47% vs 34%, P = 0.07), but a statistically significant higher seroconversion rate was found in patients with CD4 cell counts ≥ 350 cells/μL and receiving double doses (64.3% vs 39.3%, P = 0.008). The double-dose strategy also improved seroconversion compared with standard-dose strategy in patients with an HIV viral load < 10000 copies/mL (58.3% vs 37.3%, P = 0.01). In a small double-blinded, randomized controlled trial comparing a 40-μg dose to a 10-μg dose in 3 administrations[147], the increased dose of HBV vaccine did not increase the response rate in HIV-infected subjects (60.0% vs 61.5%, P = 0.89). Stratified by CD4 cell count or viral load, CD4 cell count ≥ 200 cells/μL was the only significant factor associated with the response rate and no difference was observed between the 2 different vaccine doses.
For travelers or subjects exposed to HBV, an accelerated vaccination schedule of 3 doses at 0, 1, and 2 mo, followed by a booster at 12 mo, can be given to achieve rapid protection[162,163]. A randomized study was designed to evaluate the protective efficacy of an accelerated vaccination schedule (n = 407; 3 × 10-μg doses administered intramuscularly at 0, 1 and 3 wk) in comparison to a standard schedule (n = 434; 3 × 10-μg doses at 0, 4 and 24 wk) in HIV-infected individuals[164]. The study showed that compliance to the accelerated schedule was better than that to the standard schedule (91.8% vs 82.7%), but the overall response rate was higher in the standard schedule arm [50% vs 38.7%; difference, 11.3% (95%CI: 4.3-18.3)]. Noninferiority was demonstrated only in patients with CD4 cell counts > 500 cells/μL.
Potsch and colleagues reported a higher response rate (89%) using a modified HBV vaccination schedule that administered 4 × 40-μg doses intramuscularly at 0, 4, 8 and 24 wk, with 79% achieving antibody titers above 100 IU/L[145]. A subsequent study confirmed these results with response rates of 83% and 91% following vaccination with 3 and 4 double doses, respectively[165].
An alternative vaccine delivery method, the intradermal route, driven by the fact that the dermis and epidermis of human skin are rich in antigen-presenting cells, could permit vaccine dose sparing, as 20% of the antigen dose has elicited good vaccine responses. It has shown improved immunogenicity in patients with chronic kidney disease[166]. However, there are significant operational challenges, such as reformulation, changing from a single- to a multiple-dose presentation, development of intradermal delivery devices and training health workers. An open-label, multicenter, 1:1:1 parallel-group, randomized trial compared the standard HBV vaccination schedule (3 × 20-μg doses administered intramuscularly at 0, 4, and 24 wk; n = 145), 4 double doses (4 × 40-μg doses administered intramuscularly at 0, 4, 8 and 24 wk; n = 148), and 4 intradermal low-doses (4 × 4-μg doses administered intradermally at 0, 4, 8 and 24 wk; n = 144) in HIV-infected adults with CD4 cell counts ≥ 200 cells/μL[146]. At week 28, both the 4 intramuscular double-dose group (82%) and the 4 intradermal low-dose group (77%) showed statistically significant higher response rates than the standard regimen. The four-dose schedule allowed for the possibility of overcoming age, a negative predictor for response in the standard schedule. However, data on long-term persistence of immunity are yet to be seen, and patients with CD4 cell counts of < 200 cells/μL were not evaluated.
HBV vaccination appeared to be safe in HIV-infected patients compared with HIV-uninfected persons and has no effect on HIV viral load, progression to AIDS or depletion of CD4 cell counts[140,141,146,147]. In the study by Launay et al[146], 1 serious hepatic cytolysis event possibly related to the vaccine was reported in the 4 intramuscular double-dose group. A higher incidence of injection site adverse events was reported in the 4 intramuscular double-dose group compared with the standard group, but these adverse events were generally mild.
The use of newer adjuvants may also augment hepatitis B vaccine efficacy. Standard hepatitis B vaccines contain aluminum adjuvants. Two new adjuvants in addition to a commercial HBV vaccine have been evaluated in randomized trials in HIV-infected patients[167-170]. The granulocyte macrophage colony-stimulating factor (GM-CSF), a cytokine produced primarily by activated T and B lymphocytes that increases neutrophil count, improves APC function, and is involved in the development and perpetuation of cellular immune responses, has been studied as an adjuvant in HIV-infected individuals[167-169]. GM-CSF is safe with expected side effects in HIV-infected subjects when administered as an adjuvant. While 1 study showed promise for the role of adjuvant to augment immune response[169], no additive benefits were noted in the 2 other trials[167,168]. CPG 7909, is an oligodeoxynucleotide containing immunostimulatory CpG motifs, which activates human B and plasmacytoid dendritic cells via Toll-like receptor 9. A randomized, double-blind controlled trial was conducted in HIV-infected adults on effective antiretroviral therapy who underwent HBV vaccination (3 × 40-μg administered intramuscularly at 0, 1, and 2 mo) with/without 1 mg CPG 7909[170]. The study showed that significantly more CPG 7909 recipients than control subjects maintained seroprotective titers for up to 60 mo in vaccine-naive participants and in those who had previously experienced vaccine failure[170]. While more studies are warranted to determine optimal vaccination strategies in patients with advanced immunosuppression, the vaccination series should be initiated at first visit regardless of CD4 cell count.
Some health-care practitioners may weigh the risk of vaccination delay and the likelihood of HBV infection in patients when making decisions to postpone vaccination until cART is started and virologic suppression is achieved to improve serologic response to vaccination. A cohort study in Japan examined the prophylactic effect against HBV in HIV-infected patients who had not received HBV vaccination and were negative for HBsAg, anti-HBs, and anti-HBc at baseline[171]. The incidence rate of HBV infection was lower during LAM- or TDF-containing cART (0.669 incident infections in 100 person-years of follow-up) than during no antiretroviral therapy (6.726 incident infections in 100 person-years) and other antiretroviral therapy (5.263 incident infections in 100 person-years) (P < 0.001). A similar trend was also noted in Taiwan[172].
In this review, we have found in the published data that the prevalence or incidence of HBV infection among HIV-infected patients is likely to decrease in areas where HBV vaccination programs are implemented and the coverage of cART containing TDF plus LAM or FTC is high. The challenges in the prevention of HBV transmission are to ensure that HIV-monoinfected patients have optimal adherence to protected sex and an HBV vaccination schedule, and to identify novel approaches or novel adjuvants to improve vaccination effectiveness. While the experience with management of HBV/HIV-coinfected patients using cART containing TDF plus LAM or FTC is accumulating in clinical practice, early diagnosis of HIV infection and initiation of cART to achieve durable suppression of both HIV and HBV replication in those with coinfection are warranted to ensure long-term success in the prevention of HBV-related chronic complications. With the progress made in liver transplantation over the past decades, early referral for workup for liver transplantation is advised when HIV/HBV-coinfected patients become symptomatic with liver disease.
P- Reviewer: Farzin R, Manesis EK, Mudawi HMY, Said ZNA, Yang YF S- Editor: Gou SX L- Editor: Webster JR E- Editor: Zhang DN
| 1. | Hoffmann CJ, Thio CL. Clinical implications of HIV and hepatitis B co-infection in Asia and Africa. Lancet Infect Dis. 2007;7:402-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 227] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 2. | Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection--a global challenge. N Engl J Med. 2012;366:1749-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 3. | Konopnicki D, Mocroft A, de Wit S, Antunes F, Ledergerber B, Katlama C, Zilmer K, Vella S, Kirk O, Lundgren JD. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS. 2005;19:593-601. [PubMed] |
| 4. | Thio CL, Seaberg EC, Skolasky R, Phair J, Visscher B, Muñoz A, Thomas DL. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet. 2002;360:1921-1926. [PubMed] |
| 5. | Sheng WH, Kao JH, Chen PJ, Huang LM, Chang SY, Sun HY, Hung CC, Chen MY, Chang SC. Evolution of hepatitis B serological markers in HIV-infected patients receiving highly active antiretroviral therapy. Clin Infect Dis. 2007;45:1221-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Chun HM, Roediger MP, Hullsiek KH, Thio CL, Agan BK, Bradley WP, Peel SA, Jagodzinski LL, Weintrob AC, Ganesan A. Hepatitis B virus coinfection negatively impacts HIV outcomes in HIV seroconverters. J Infect Dis. 2012;205:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Tsai MS, Chang SY, Lo YC, Yang CJ, Sun HY, Liu WC, Wu PY, Hung CC. Hepatitis B virus (HBV) coinfection accelerates immunologic progression in patients with primary HIV infection in an area of hyperendemicity for HBV infection. J Infect Dis. 2013;208:1184-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Wandeler G, Gsponer T, Bihl F, Bernasconi E, Cavassini M, Kovari H, Schmid P, Battegay M, Calmy A, Egger M. Hepatitis B virus infection is associated with impaired immunological recovery during antiretroviral therapy in the Swiss HIV cohort study. J Infect Dis. 2013;208:1454-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | World Health Organization. Hepatitis B. Available from: http//www.who.int/mediacentre/factsheets/fs204/en/. |
| 10. | Thio CL. Hepatitis B and human immunodeficiency virus coinfection. Hepatology. 2009;49:S138-S145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 11. | Denis F, Adjide CC, Rogez S, Delpeyroux C, Rogez JP, Weinbreck P. [Seroprevalence of HBV, HCV and HDV hepatitis markers in 500 patients infected with the human immunodeficiency virus]. Pathol Biol (Paris). 1997;45:701-708. [PubMed] |
| 12. | Kellerman SE, Hanson DL, McNaghten AD, Fleming PL. Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in human immunodeficiency virus-infected subjects. J Infect Dis. 2003;188:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 193] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Roca B, Suarez I, Gonzalez J, Garrido M, de la Fuente B, Teira R, Geijo P, Cosin J, Perez-Cortes S, Galindo MJ. Hepatitis C virus and human immunodeficiency virus coinfection in Spain. J Infect. 2003;47:117-124. [PubMed] |
| 14. | Rosenblum L, Darrow W, Witte J, Cohen J, French J, Gill PS, Potterat J, Sikes K, Reich R, Hadler S. Sexual practices in the transmission of hepatitis B virus and prevalence of hepatitis delta virus infection in female prostitutes in the United States. JAMA. 1992;267:2477-2481. [PubMed] |
| 15. | Osmond DH, Charlebois E, Sheppard HW, Page K, Winkelstein W, Moss AR, Reingold A. Comparison of risk factors for hepatitis C and hepatitis B virus infection in homosexual men. J Infect Dis. 1993;167:66-71. [PubMed] |
| 16. | Piot P, Goilav C, Kegels E. Hepatitis B: transmission by sexual contact and needle sharing. Vaccine. 1990;8 Suppl:S37-40; discussion S41-3. [PubMed] |
| 17. | Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1217] [Cited by in RCA: 1328] [Article Influence: 102.2] [Reference Citation Analysis (0)] |
| 18. | Su WJ, Liu CC, Liu DP, Chen SF, Huang JJ, Chan TC, Chang MH. Effect of age on the incidence of acute hepatitis B after 25 years of a universal newborn hepatitis B immunization program in Taiwan. J Infect Dis. 2012;205:757-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | McMahon BJ, Bulkow LR, Singleton RJ, Williams J, Snowball M, Homan C, Parkinson AJ. Elimination of hepatocellular carcinoma and acute hepatitis B in children 25 years after a hepatitis B newborn and catch-up immunization program. Hepatology. 2011;54:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 20. | Chen SM, Kung CM, Yang WJ, Wang HL. Efficacy of the nationwide hepatitis B infant vaccination program in Taiwan. J Clin Virol. 2011;52:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1328] [Cited by in RCA: 1196] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 22. | Chiang CJ, Yang YW, You SL, Lai MS, Chen CJ. Thirty-year outcomes of the national hepatitis B immunization program in Taiwan. JAMA. 2013;310:974-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 23. | Sun HY, Ko WC, Tsai JJ, Lee HC, Liu CE, Wong WW, Su SC, Ho MW, Cheng SH, Yang CH. Seroprevalence of chronic hepatitis B virus infection among taiwanese human immunodeficiency virus type 1-positive persons in the era of nationwide hepatitis B vaccination. Am J Gastroenterol. 2009;104:877-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Sun HY, Cheng CY, Lee NY, Yang CJ, Liang SH, Tsai MS, Ko WC, Liu WC, Wu PY, Wu CH. Seroprevalence of hepatitis B virus among adults at high risk for HIV transmission two decades after implementation of nationwide hepatitis B virus vaccination program in Taiwan. PLoS One. 2014;9:e90194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | McMahon BJ. The influence of hepatitis B virus genotype and subgenotype on the natural history of chronic hepatitis B. Hepatol Int. 2009;3:334-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 26. | Kurbanov F, Tanaka Y, Mizokami M. Geographical and genetic diversity of the human hepatitis B virus. Hepatol Res. 2010;40:14-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 27. | Olinger CM, Jutavijittum P, Hübschen JM, Yousukh A, Samountry B, Thammavong T, Toriyama K, Muller CP. Possible new hepatitis B virus genotype, southeast Asia. Emerg Infect Dis. 2008;14:1777-1780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 28. | Tatematsu K, Tanaka Y, Kurbanov F, Sugauchi F, Mano S, Maeshiro T, Nakayoshi T, Wakuta M, Miyakawa Y, Mizokami M. A genetic variant of hepatitis B virus divergent from known human and ape genotypes isolated from a Japanese patient and provisionally assigned to new genotype J. J Virol. 2009;83:10538-10547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 336] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 29. | Kao JH, Chen PJ, Lai MY, Chen DS. Genotypes and clinical phenotypes of hepatitis B virus in patients with chronic hepatitis B virus infection. J Clin Microbiol. 2002;40:1207-1209. [PubMed] |
| 30. | Sánchez-Tapias JM, Costa J, Mas A, Bruguera M, Rodés J. Influence of hepatitis B virus genotype on the long-term outcome of chronic hepatitis B in western patients. Gastroenterology. 2002;123:1848-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 312] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 31. | Yuen MF, Wong DK, Sablon E, Tse E, Ng IO, Yuan HJ, Siu CW, Sander TJ, Bourne EJ, Hall JG. HBsAg seroclearance in chronic hepatitis B in the Chinese: virological, histological, and clinical aspects. Hepatology. 2004;39:1694-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 194] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 32. | Kao JH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology. 2000;118:554-559. [PubMed] |
| 33. | Chu CM, Liaw YF. Genotype C hepatitis B virus infection is associated with a higher risk of reactivation of hepatitis B and progression to cirrhosis than genotype B: a longitudinal study of hepatitis B e antigen-positive patients with normal aminotransferase levels at baseline. J Hepatol. 2005;43:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 34. | Yang HI, Yeh SH, Chen PJ, Iloeje UH, Jen CL, Su J, Wang LY, Lu SN, You SL, Chen DS. Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:1134-1143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 447] [Cited by in RCA: 483] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 35. | Yuen MF, Tanaka Y, Mizokami M, Yuen JC, Wong DK, Yuan HJ, Sum SM, Chan AO, Wong BC, Lai CL. Role of hepatitis B virus genotypes Ba and C, core promoter and precore mutations on hepatocellular carcinoma: a case control study. Carcinogenesis. 2004;25:1593-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Chan HL, Hui AY, Wong ML, Tse AM, Hung LC, Wong VW, Sung JJ. Genotype C hepatitis B virus infection is associated with an increased risk of hepatocellular carcinoma. Gut. 2004;53:1494-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 387] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 37. | Kao JH, Chen PJ, Lai MY, Chen DS. Basal core promoter mutations of hepatitis B virus increase the risk of hepatocellular carcinoma in hepatitis B carriers. Gastroenterology. 2003;124:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 428] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 38. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [PubMed] |
| 39. | Kao JH, Wu NH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes and the response to interferon therapy. J Hepatol. 2000;33:998-1002. [PubMed] |
| 40. | Wai CT, Chu CJ, Hussain M, Lok AS. HBV genotype B is associated with better response to interferon therapy in HBeAg(+) chronic hepatitis than genotype C. Hepatology. 2002;36:1425-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Erhardt A, Blondin D, Hauck K, Sagir A, Kohnle T, Heintges T, Häussinger D. Response to interferon alfa is hepatitis B virus genotype dependent: genotype A is more sensitive to interferon than genotype D. Gut. 2005;54:1009-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 191] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 42. | Marcellin P, Bonino F, Lau GK, Farci P, Yurdaydin C, Piratvisuth T, Jin R, Gurel S, Lu ZM, Wu J. Sustained response of hepatitis B e antigen-negative patients 3 years after treatment with peginterferon alpha-2a. Gastroenterology. 2009;136:2169-2179.e1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 260] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 43. | Chien RN, Yeh CT, Tsai SL, Chu CM, Liaw YF. Determinants for sustained HBeAg response to lamivudine therapy. Hepatology. 2003;38:1267-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 44. | Kao JH, Liu CJ, Chen DS. Hepatitis B viral genotypes and lamivudine resistance. J Hepatol. 2002;36:303-304. [PubMed] |
| 45. | Yuen MF, Wong DK, Sablon E, Yuan HJ, Sum SM, Hui CK, Chan AO, Wang BC, Lai CL. Hepatitis B virus genotypes B and C do not affect the antiviral response to lamivudine. Antivir Ther. 2003;8:531-534. [PubMed] |
| 46. | Chan HL, Wong ML, Hui AY, Chim AM, Tse AM, Hung LC, Chan FK, Sung JJ. Hepatitis B virus genotype has no impact on hepatitis B e antigen seroconversion after lamivudine treatment. World J Gastroenterol. 2003;9:2695-2697. [PubMed] |
| 47. | Westland C, Delaney W, Yang H, Chen SS, Marcellin P, Hadziyannis S, Gish R, Fry J, Brosgart C, Gibbs C. Hepatitis B virus genotypes and virologic response in 694 patients in phase III studies of adefovir dipivoxil1. Gastroenterology. 2003;125:107-116. [PubMed] |
| 48. | Hou J, Yin YK, Xu D, Tan D, Niu J, Zhou X, Wang Y, Zhu L, He Y, Ren H. Telbivudine versus lamivudine in Chinese patients with chronic hepatitis B: Results at 1 year of a randomized, double-blind trial. Hepatology. 2008;47:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 50. | Soriano V, Mocroft A, Peters L, Rockstroh J, Antunes F, Kirkby N, de Wit S, Monforte Ad, Flisiak R, Lundgren J. Predictors of hepatitis B virus genotype and viraemia in HIV-infected patients with chronic hepatitis B in Europe. J Antimicrob Chemother. 2010;65:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Thibault V, Gaudy-Graffin C, Colson P, Gozlan J, Schnepf N, Trimoulet P, Pallier C, Saune K, Branger M, Coste M. Epidemiological, virological and clinical characteristics of HBV infection in 223 HIV co-infected patients: a French multi-centre collaborative study. Virol J. 2013;10:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Lacombe K, Massari V, Girard PM, Serfaty L, Gozlan J, Pialoux G, Mialhes P, Molina JM, Lascoux-Combe C, Wendum D. Major role of hepatitis B genotypes in liver fibrosis during coinfection with HIV. AIDS. 2006;20:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 53. | Calin R, Guiguet M, Desire N, Imbert-Bismut F, Munteanu M, Poynard T, Valantin MA, Stitou H, Katlama C, Thibault V. Role of genotype G hepatitis B virus mixed infection on the progression of hepatic fibrosis in HIV positive patients over 5 years of follow-up. J Clin Virol. 2013;58:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Sheng WH, Hung CC, Chang SY, Liu CJ, Chen MY, Hsieh SM, Kao JH, Chen PJ, Chang SC. Differential clinical and virologic impact of hepatitis B virus genotypes B and C on HIV-coinfected patients receiving lamivudine-containing highly active antiretroviral therapy. Clin Infect Dis. 2012;54:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Israël N, Hazan U, Alcami J, Munier A, Arenzana-Seisdedos F, Bachelerie F, Israël A, Virelizier JL. Tumor necrosis factor stimulates transcription of HIV-1 in human T lymphocytes, independently and synergistically with mitogens. J Immunol. 1989;143:3956-3960. [PubMed] |
| 56. | Twu JS, Chu K, Robinson WS. Hepatitis B virus X gene activates kappa B-like enhancer sequences in the long terminal repeat of human immunodeficiency virus 1. Proc Natl Acad Sci USA. 1989;86:5168-5172. [PubMed] |
| 57. | Gómez-Gonzalo M, Carretero M, Rullas J, Lara-Pezzi E, Aramburu J, Berkhout B, Alcamí J, López-Cabrera M. The hepatitis B virus X protein induces HIV-1 replication and transcription in synergy with T-cell activation signals: functional roles of NF-kappaB/NF-AT and SP1-binding sites in the HIV-1 long terminal repeat promoter. J Biol Chem. 2001;276:35435-35443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Eskild A, Magnus P, Petersen G, Sohlberg C, Jensen F, Kittelsen P, Skaug K. Hepatitis B antibodies in HIV-infected homosexual men are associated with more rapid progression to AIDS. AIDS. 1992;6:571-574. [PubMed] |
| 59. | Scharschmidt BF, Held MJ, Hollander HH, Read AE, Lavine JE, Veereman G, McGuire RF, Thaler MM. Hepatitis B in patients with HIV infection: relationship to AIDS and patient survival. Ann Intern Med. 1992;117:837-838. [PubMed] |
| 60. | Sinicco A, Raiteri R, Sciandra M, Bertone C, Lingua A, Salassa B, Gioannini P. Coinfection and superinfection of hepatitis B virus in patients infected with human immunodeficiency virus: no evidence of faster progression to AIDS. Scand J Infect Dis. 1997;29:111-115. [PubMed] |
| 61. | Bodsworth NJ, Cooper DA, Donovan B. The influence of human immunodeficiency virus type 1 infection on the development of the hepatitis B virus carrier state. J Infect Dis. 1991;163:1138-1140. [PubMed] |
| 62. | Hadler SC, Judson FN, O’Malley PM, Altman NL, Penley K, Buchbinder S, Schable CA, Coleman PJ, Ostrow DN, Francis DP. Outcome of hepatitis B virus infection in homosexual men and its relation to prior human immunodeficiency virus infection. J Infect Dis. 1991;163:454-459. [PubMed] |
| 63. | Oshitani H, Kasolo FC, Mpabalwani M, Mizuta K, Luo NP, Suzuki H, Numazaki Y. Prevalence of hepatitis B antigens in human immunodeficiency virus type 1 seropositive and seronegative pregnant women in Zambia. Trans R Soc Trop Med Hyg. 1996;90:235-236. [PubMed] |
| 64. | Rouet F, Chaix ML, Inwoley A, Msellati P, Viho I, Combe P, Leroy V, Dabis F, Rouzioux C. HBV and HCV prevalence and viraemia in HIV-positive and HIV-negative pregnant women in Abidjan, Côte d’Ivoire: the ANRS 1236 study. J Med Virol. 2004;74:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 65. | Colin JF, Cazals-Hatem D, Loriot MA, Martinot-Peignoux M, Pham BN, Auperin A, Degott C, Benhamou JP, Erlinger S, Valla D. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology. 1999;29:1306-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 327] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 66. | Sheng WH, Chen MY, Hsieh SM, Hsiao CF, Wang JT, Hung CC, Chang SC. Impact of chronic hepatitis B virus (HBV) infection on outcomes of patients infected with HIV in an area where HBV infection is hyperendemic. Clin Infect Dis. 2004;38:1471-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 67. | Sheng WH, Hung CC, Kao JH, Chang SY, Chen MY, Hsieh SM, Chen PJ, Chang SC. Impact of hepatitis D virus infection on the long-term outcomes of patients with hepatitis B virus and HIV coinfection in the era of highly active antiretroviral therapy: a matched cohort study. Clin Infect Dis. 2007;44:988-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 68. | Hung CC, Wu SM, Lin PH, Sheng WH, Yang ZY, Sun HY, Tsai MS, Lee KY, Huang MS, Chang SF. Increasing incidence of recent hepatitis D virus infection in HIV-infected patients in an area hyperendemic for hepatitis B virus infection. Clin Infect Dis. 2014;58:1625-1633. [PubMed] |
| 69. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2171] [Article Influence: 135.7] [Reference Citation Analysis (0)] |
| 70. | Marcellin P, Boyer N, Colin JF, Martinot-Peignoux M, Lefort V, Matheron S, Erlinger S, Benhamou JP. Recombinant alpha interferon for chronic hepatitis B in anti-HIV positive patients receiving zidovudine. Gut. 1993;34:S106. [PubMed] |
| 71. | Johnson RM, Ristig MB, Overton ET, Lisker-Melman M, Cummings OW, Aberg JA. Safety and tolerability of sequential pegylated IFN-alpha2a and tenofovir for hepatitis B infection in HIV(+) individuals. HIV Clin Trials. 2007;8:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 72. | Benhamou Y, Bochet M, Thibault V, Calvez V, Fievet MH, Vig P, Gibbs CS, Brosgart C, Fry J, Namini H. Safety and efficacy of adefovir dipivoxil in patients co-infected with HIV-1 and lamivudine-resistant hepatitis B virus: an open-label pilot study. Lancet. 2001;358:718-723. [PubMed] |
| 73. | Matthews GV, Seaberg EC, Avihingsanon A, Bowden S, Dore GJ, Lewin SR, Sasadeusz J, Revill PA, Littlejohn M, Hoy JF. Patterns and causes of suboptimal response to tenofovir-based therapy in individuals coinfected with HIV and hepatitis B virus. Clin Infect Dis. 2013;56:e87-e94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 74. | Soriano V, Tuma P, Vispo E, Labarga P, Fernández JV, Medrano J, Barreiro P. Hepatitis B in HIV patients: what is the current treatment and what are the challenges? J HIV Ther. 2009;14:13-18. [PubMed] |
| 75. | Negredo E, Garrabou G, Puig J, Lòpez S, Morén C, Bellido R, Ayen R, Cardellach F, Miró O, Clotet B. Partial immunological and mitochondrial recovery after reducing didanosine doses in patients on didanosine and tenofovir-based regimens. Antivir Ther. 2008;13:231-240. [PubMed] |
| 76. | Taburet AM, Piketty C, Chazallon C, Vincent I, Gérard L, Calvez V, Clavel F, Aboulker JP, Girard PM. Interactions between atazanavir-ritonavir and tenofovir in heavily pretreated human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2004;48:2091-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 170] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 77. | van Maarseveen NM, Wensing AM, de Jong D, Beilhartz GL, Obikhod A, Tao S, Pingen M, Arends JE, Hoepelman AI, Schinazi RF. Telbivudine exerts no antiviral activity against HIV-1 in vitro and in humans. Antivir Ther. 2011;16:1123-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 78. | Peters MG, Andersen J, Lynch P, Liu T, Alston-Smith B, Brosgart CL, Jacobson JM, Johnson VA, Pollard RB, Rooney JF. Randomized controlled study of tenofovir and adefovir in chronic hepatitis B virus and HIV infection: ACTG A5127. Hepatology. 2006;44:1110-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 79. | Ratcliffe L, Beadsworth MB, Pennell A, Phillips M, Vilar FJ. Managing hepatitis B/HIV co-infected: adding entecavir to truvada (tenofovir disoproxil/emtricitabine) experienced patients. AIDS. 2011;25:1051-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 80. | ADHOC International Steering Committee. A randomized placebo-controlled trial of adefovir dipivoxil in advanced HIV infection: the ADHOC trial. HIV Med. 2002;3:229-238. [PubMed] |
| 81. | Milazzo L, Caramma I, Lai A, Violin M, De Maddalena C, Cesari M, Galli M, Balotta C. Telbivudine in the treatment of chronic hepatitis B: experience in HIV type-1-infected patients naive for antiretroviral therapy. Antivir Ther. 2009;14:869-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 82. | (OARAC) OoARAC. DHHS Guidelines for the Use of Anti-retroviral Agents in HIV-1-infected Adults and Adolescents. AIDS info website, 2013. Available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. |
| 83. | EACS European AIDS Clinical Society. EACS Guidelines Version 7.0, 2013. Available from: http://www.eacsociety.org/Portals/0/Guidelines_Online_131014.pdf. |
| 84. | Wilkins E, Nelson M, Agarwal K, Awoyemi D, Barnes E, Bhagani S, Brook G, Brown A, Castelino S, Cooke G. British HIV Association guidelines for the management of hepatitis viruses in adults infected with HIV 2013. HIV Med. 2013;14 Suppl 4:1-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 85. | Dore GJ, Cooper DA, Barrett C, Goh LE, Thakrar B, Atkins M. Dual efficacy of lamivudine treatment in human immunodeficiency virus/hepatitis B virus-coinfected persons in a randomized, controlled study (CAESAR). The CAESAR Coordinating Committee. J Infect Dis. 1999;180:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 102] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 86. | Wolters LM, Niesters HG, Hansen BE, van der Ende ME, Kroon FP, Richter C, Brinkman K, Meenhorst PL, de Man RA. Development of hepatitis B virus resistance for lamivudine in chronic hepatitis B patients co-infected with the human immunodeficiency virus in a Dutch cohort. J Clin Virol. 2002;24:173-181. [PubMed] |
| 87. | Hoff J, Bani-Sadr F, Gassin M, Raffi F. Evaluation of chronic hepatitis B virus (HBV) infection in coinfected patients receiving lamivudine as a component of anti-human immunodeficiency virus regimens. Clin Infect Dis. 2001;32:963-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 88. | Benhamou Y, Bochet M, Thibault V, Di Martino V, Caumes E, Bricaire F, Opolon P, Katlama C, Poynard T. Long-term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology. 1999;30:1302-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 300] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 89. | Chang TT, Lai CL, Chien RN, Guan R, Lim SG, Lee CM, Ng KY, Nicholls GJ, Dent JC, Leung NW. Four years of lamivudine treatment in Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2004;19:1276-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 90. | Matthews GV, Bartholomeusz A, Locarnini S, Ayres A, Sasaduesz J, Seaberg E, Cooper DA, Lewin S, Dore GJ, Thio CL. Characteristics of drug resistant HBV in an international collaborative study of HIV-HBV-infected individuals on extended lamivudine therapy. AIDS. 2006;20:863-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 91. | Cooksley WG, Piratvisuth T, Lee SD, Mahachai V, Chao YC, Tanwandee T, Chutaputti A, Chang WY, Zahm FE, Pluck N. Peginterferon alpha-2a (40 kDa): an advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J Viral Hepat. 2003;10:298-305. [PubMed] |
| 92. | Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, Gane E, Fried MW, Chow WC, Paik SW. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1188] [Cited by in RCA: 1173] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 93. | Neumann A, Polis M, Rozenberg L, Jackson J, Reitano K, McLaughlin M, Koratich C, Dewar R, Masur H, Haagmans B. Differential antiviral effect of PEG-interferon-alpha-2b on HIV and HCV in the treatment of HIV/HCV co-infected patients. AIDS. 2007;21:1855-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 94. | Liu MQ, Zhou DJ, Wang X, Zhou W, Ye L, Li JL, Wang YZ, Ho WZ. IFN-λ3 inhibits HIV infection of macrophages through the JAK-STAT pathway. PLoS One. 2012;7:e35902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 95. | Mauss S, Valenti W, DePamphilis J, Duff F, Cupelli L, Passe S, Solsky J, Torriani FJ, Dieterich D, Larrey D. Risk factors for hepatic decompensation in patients with HIV/HCV coinfection and liver cirrhosis during interferon-based therapy. AIDS. 2004;18:F21-F25. [PubMed] |
| 97. | Borroto-Esoda K, Parkin N, Miller MD. A comparison of the phenotypic susceptibility profiles of emtricitabine and lamivudine. Antivir Chem Chemother. 2007;18:297-300. [PubMed] |
| 98. | Yang H, Qi X, Sabogal A, Miller M, Xiong S, Delaney WE. Cross-resistance testing of next-generation nucleoside and nucleotide analogues against lamivudine-resistant HBV. Antivir Ther. 2005;10:625-633. [PubMed] |
| 99. | Gish RG, Trinh H, Leung N, Chan FK, Fried MW, Wright TL, Wang C, Anderson J, Mondou E, Snow A. Safety and antiviral activity of emtricitabine (FTC) for the treatment of chronic hepatitis B infection: a two-year study. J Hepatol. 2005;43:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 100. | Nüesch R, Ananworanich J, Srasuebkul P, Chetchotisakd P, Prasithsirikul W, Klinbuayam W, Mahanontharit A, Jupimai T, Ruxrungtham K, Hirschel B. Interruptions of tenofovir/emtricitabine-based antiretroviral therapy in patients with HIV/hepatitis B virus co-infection. AIDS. 2008;22:152-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 101. | Bartholomeusz A, Locarnini S. Hepatitis B virus mutations associated with antiviral therapy. J Med Virol. 2006;78 Suppl 1:S52-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 102. | Thio CL, Locarnini S. Treatment of HIV/HBV coinfection: clinical and virologic issues. AIDS Rev. 2007;9:40-53. [PubMed] |
| 103. | Benhamou Y, Thibault V, Vig P, Calvez V, Marcelin AG, Fievet MH, Currie G, Chang CG, Biao L, Xiong S. Safety and efficacy of adefovir dipivoxil in patients infected with lamivudine-resistant hepatitis B and HIV-1. J Hepatol. 2006;44:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 104. | Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, Jeffers L, Goodman Z, Wulfsohn MS, Xiong S. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1060] [Cited by in RCA: 1019] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 105. | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 681] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 106. | Lampertico P, Viganò M, Manenti E, Iavarone M, Sablon E, Colombo M. Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology. 2007;133:1445-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 244] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 107. | Yatsuji H, Suzuki F, Sezaki H, Akuta N, Suzuki Y, Kawamura Y, Hosaka T, Kobayashi M, Saitoh S, Arase Y. Low risk of adefovir resistance in lamivudine-resistant chronic hepatitis B patients treated with adefovir plus lamivudine combination therapy: two-year follow-up. J Hepatol. 2008;48:923-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 108. | Dore GJ, Cooper DA, Pozniak AL, DeJesus E, Zhong L, Miller MD, Lu B, Cheng AK. Efficacy of tenofovir disoproxil fumarate in antiretroviral therapy-naive and -experienced patients coinfected with HIV-1 and hepatitis B virus. J Infect Dis. 2004;189:1185-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 179] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 109. | Nelson M, Portsmouth S, Stebbing J, Atkins M, Barr A, Matthews G, Pillay D, Fisher M, Bower M, Gazzard B. An open-label study of tenofovir in HIV-1 and Hepatitis B virus co-infected individuals. AIDS. 2003;17:F7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 110. | Kosi L, Reiberger T, Payer BA, Grabmeier-Pfistershammer K, Strassl R, Rieger A, Peck-Radosavljevic M. Five-year on-treatment efficacy of lamivudine-, tenofovir- and tenofovir + emtricitabine-based HAART in HBV-HIV-coinfected patients. J Viral Hepat. 2012;19:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 111. | Price H, Dunn D, Pillay D, Bani-Sadr F, de Vries-Sluijs T, Jain MK, Kuzushita N, Mauss S, Núñez M, Nüesch R. Suppression of HBV by tenofovir in HBV/HIV coinfected patients: a systematic review and meta-analysis. PLoS One. 2013;8:e68152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 112. | Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51:496-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 412] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 113. | Pessôa MG, Gazzard B, Huang AK, Brandão-Mello CE, Cassetti I, Mendes-Corrêa MC, Soriano V, Phiri P, Hall A, Brett-Smith H. Efficacy and safety of entecavir for chronic HBV in HIV/HBV coinfected patients receiving lamivudine as part of antiretroviral therapy. AIDS. 2008;22:1779-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 114. | McMahon MA, Jilek BL, Brennan TP, Shen L, Zhou Y, Wind-Rotolo M, Xing S, Bhat S, Hale B, Hegarty R. The HBV drug entecavir - effects on HIV-1 replication and resistance. N Engl J Med. 2007;356:2614-2621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 115. | Tenney DJ, Levine SM, Rose RE, Walsh AW, Weinheimer SP, Discotto L, Plym M, Pokornowski K, Yu CF, Angus P. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to Lamivudine. Antimicrob Agents Chemother. 2004;48:3498-3507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 427] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 116. | Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, Chen Y, Heathcote EJ, Rasenack J, Bzowej N. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357:2576-2588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 602] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 117. | Liaw YF, Gane E, Leung N, Zeuzem S, Wang Y, Lai CL, Heathcote EJ, Manns M, Bzowej N, Niu J. 2-Year GLOBE trial results: telbivudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136:486-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 443] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 118. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2309] [Cited by in RCA: 2365] [Article Influence: 124.5] [Reference Citation Analysis (0)] |
| 119. | Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol. 2010;53:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 349] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 120. | Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 542] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 121. | Núñez M, Pérez-Olmeda M, Díaz B, Ríos P, González-Lahoz J, Soriano V. Activity of tenofovir on hepatitis B virus replication in HIV-co-infected patients failing or partially responding to lamivudine. AIDS. 2002;16:2352-2354. [PubMed] |
| 122. | Schmutz G, Nelson M, Lutz T, Sheldon J, Bruno R, von Boemmel F, Hoffmann C, Rockstroh J, Stoehr A, Wolf E. Combination of tenofovir and lamivudine versus tenofovir after lamivudine failure for therapy of hepatitis B in HIV-coinfection. AIDS. 2006;20:1951-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 123. | Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1304] [Cited by in RCA: 1280] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 124. | Tuma P, Medrano J, Resino S, Vispo E, Madejón A, Sánchez-Piedra C, Rivas P, Labarga P, Martín-Carbonero L, Barreiro P. Incidence of liver cirrhosis in HIV-infected patients with chronic hepatitis B or C in the era of highly active antiretroviral therapy. Antivir Ther. 2010;15:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 125. | Martín-Carbonero L, Teixeira T, Poveda E, Plaza Z, Vispo E, González-Lahoz J, Soriano V. Clinical and virological outcomes in HIV-infected patients with chronic hepatitis B on long-term nucleos(t)ide analogues. AIDS. 2011;25:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 126. | Clifford GM, Rickenbach M, Polesel J, Dal Maso L, Steffen I, Ledergerber B, Rauch A, Probst-Hensch NM, Bouchardy C, Levi F. Influence of HIV-related immunodeficiency on the risk of hepatocellular carcinoma. AIDS. 2008;22:2135-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 127. | Deeken JF, Pantanowitz L, Dezube BJ. Targeted therapies to treat non-AIDS-defining cancers in patients with HIV on HAART therapy: treatment considerations and research outlook. Curr Opin Oncol. 2009;21:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 128. | Chelis L, Ntinos N, Souftas V, Deftereos S, Xenidis N, Chamalidou E, Maltezos E, Kakolyris S. Complete response after sorafenib therapy for hepatocellular carcinoma in an HIV-HBV co infected patient: Possible synergy with HAART ? A case report. Med Oncol. 2011;28 Suppl 1:S165-S168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 129. | Perboni G, Costa P, Fibbia GC, Morandini B, Scalzini A, Tagliani A, Cengarle R, Aitini E. Sorafenib therapy for hepatocellular carcinoma in an HIV-HCV coinfected patient: a case report. Oncologist. 2010;15:142-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 130. | Ozenne V, Gervais A, Peytavin G, Castelnau C, Valla DC, Degos F. Suspected interaction between sorafenib and HAART in an HIV-1 infected patient: a case report. Hepatogastroenterology. 2011;58:161-162. [PubMed] |
| 131. | Coffin CS, Stock PG, Dove LM, Berg CL, Nissen NN, Curry MP, Ragni M, Regenstein FG, Sherman KE, Roland ME. Virologic and clinical outcomes of hepatitis B virus infection in HIV-HBV coinfected transplant recipients. Am J Transplant. 2010;10:1268-1275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 132. | Tateo M, Roque-Afonso AM, Antonini TM, Medja F, Lombes A, Jardel C, Teicher E, Sebagh M, Roche B, Castaing D. Long-term follow-up of liver transplanted HIV/hepatitis B virus coinfected patients: perfect control of hepatitis B virus replication and absence of mitochondrial toxicity. AIDS. 2009;23:1069-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 133. | Solid Organ Transplant in HIV: Multi-Site Study. When to Start the Transplant Referral Process. Available from: http://spitfire.emmes.com/study/htr/. |
| 134. | Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44:S6-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 637] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 135. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1712] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 136. | Lopes VB, Hassing RJ, de Vries-Sluijs TE, El Barzouhi A, Hansen BE, Schutten M, de Man RA, van der Ende ME. Long-term response rates of successful hepatitis B vaccination in HIV-infected patients. Vaccine. 2013;31:1040-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 137. | Paitoonpong L, Suankratay C. Immunological response to hepatitis B vaccination in patients with AIDS and virological response to highly active antiretroviral therapy. Scand J Infect Dis. 2008;40:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 138. | Lemon SM, Thomas DL. Vaccines to prevent viral hepatitis. N Engl J Med. 1997;336:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 197] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 139. | Irungu E, Mugo N, Ngure K, Njuguna R, Celum C, Farquhar C, Dhanireddy S, Baeten JM. Immune response to hepatitis B virus vaccination among HIV-1 infected and uninfected adults in Kenya. J Infect Dis. 2013;207:402-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 140. | Fonseca MO, Pang LW, de Paula Cavalheiro N, Barone AA, Heloisa Lopes M. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine. 2005;23:2902-2908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 176] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 141. | Rey D, Krantz V, Partisani M, Schmitt MP, Meyer P, Libbrecht E, Wendling MJ, Vetter D, Nicolle M, Kempf-Durepaire G. Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. Effects on HIV-1 viral load. Vaccine. 2000;18:1161-1165. [PubMed] |
| 142. | Overton ET, Sungkanuparph S, Powderly WG, Seyfried W, Groger RK, Aberg JA. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin Infect Dis. 2005;41:1045-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 143. | Tedaldi EM, Baker RK, Moorman AC, Wood KC, Fuhrer J, McCabe RE, Holmberg SD. Hepatitis A and B vaccination practices for ambulatory patients infected with HIV. Clin Infect Dis. 2004;38:1478-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 144. | Kim HN, Harrington RD, Van Rompaey SE, Kitahata MM. Independent clinical predictors of impaired response to hepatitis B vaccination in HIV-infected persons. Int J STD AIDS. 2008;19:600-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 145. | Potsch DV, Oliveira ML, Ginuíno C, Miguel JC, Oliveira SA, Silva EF, Moreira RB, Cruz GV, Oliveira AL, Camacho LA. High rates of serological response to a modified hepatitis B vaccination schedule in HIV-infected adults subjects. Vaccine. 2010;28:1447-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 146. | Launay O, van der Vliet D, Rosenberg AR, Michel ML, Piroth L, Rey D, Colin de Verdière N, Slama L, Martin K, Lortholary O. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA. 2011;305:1432-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 147. | Cornejo-Juárez P, Volkow-Fernández P, Escobedo-López K, Vilar-Compte D, Ruiz-Palacios G, Soto-Ramírez LE. Randomized controlled trial of Hepatitis B virus vaccine in HIV-1-infected patients comparing two different doses. AIDS Res Ther. 2006;3:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 148. | Ungulkraiwit P, Jongjirawisan Y, Atamasirikul K, Sungkanuparph S. Factors for predicting successful immune response to hepatitis B vaccination in HIV-1 infected patients. Southeast Asian J Trop Med Public Health. 2007;38:680-685. [PubMed] |
| 149. | Chedid MG, Deulofeut H, Yunis DE, Lara-Marquez ML, Salazar M, Deulofeut R, Awdeh Z, Alper CA, Yunis EJ. Defect in Th1-like cells of nonresponders to hepatitis B vaccine. Hum Immunol. 1997;58:42-51. [PubMed] |
| 150. | Shokrgozar MA, Shokri F. Enumeration of hepatitis B surface antigen-specific B lymphocytes in responder and non-responder normal individuals vaccinated with recombinant hepatitis B surface antigen. Immunology. 2001;104:75-79. [PubMed] |
| 151. | Wang C, Tang J, Song W, Lobashevsky E, Wilson CM, Kaslow RA. HLA and cytokine gene polymorphisms are independently associated with responses to hepatitis B vaccination. Hepatology. 2004;39:978-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 152. | Bruguera M, Cremades M, Salinas R, Costa J, Grau M, Sans J. Impaired response to recombinant hepatitis B vaccine in HIV-infected persons. J Clin Gastroenterol. 1992;14:27-30. [PubMed] |
| 153. | Veiga AP, Casseb J, Duarte AJ. Humoral response to hepatitis B vaccination and its relationship with T CD45RA+ (naïve) and CD45RO+ (memory) subsets in HIV-1-infected subjects. Vaccine. 2006;24:7124-7128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 154. | Gandhi RT, Wurcel A, McGovern B, Lee H, Shopis J, Corcoran CP, Toner S, Giachetti C, Dockter J, Sax PE. Low prevalence of ongoing hepatitis B viremia in HIV-positive individuals with isolated antibody to hepatitis B core antigen. J Acquir Immune Defic Syndr. 2003;34:439-441. [PubMed] |
| 155. | Shire NJ, Rouster SD, Rajicic N, Sherman KE. Occult hepatitis B in HIV-infected patients. J Acquir Immune Defic Syndr. 2004;36:869-875. [PubMed] |
| 156. | Moir S, Fauci AS. Pathogenic mechanisms of B-lymphocyte dysfunction in HIV disease. J Allergy Clin Immunol. 2008;122:12-9; quiz 20-1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 157. | Bailey CL, Smith V, Sands M. Hepatitis B vaccine: a seven-year study of adherence to the immunization guidelines and efficacy in HIV-1-positive adults. Int J Infect Dis. 2008;12:e77-e83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 158. | Landrum ML, Huppler Hullsiek K, Ganesan A, Weintrob AC, Crum-Cianflone NF, Barthel RV, Peel S, Agan BK. Hepatitis B vaccine responses in a large U.S. military cohort of HIV-infected individuals: another benefit of HAART in those with preserved CD4 count. Vaccine. 2009;27:4731-4738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 159. | Winnock M, Neau D, Castera L, Viot J, Lacoste D, Pellegrin JL, Dupon M, Jutand MA, Colombani F, Dabis F. Hepatitis B vaccination in HIV-infected patients: a survey of physicians and patients participating in the Aquitaine cohort. Gastroenterol Clin Biol. 2006;30:189-195. [PubMed] |
| 160. | Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep. 2001;50:1-43. [PubMed] |
| 161. | Poland GA, Jacobson RM. Clinical practice: prevention of hepatitis B with the hepatitis B vaccine. N Engl J Med. 2004;351:2832-2838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 162. | Connor BA, Patron DJ. Use of an accelerated immunization schedule for combined hepatitis A and B protection in the corporate traveler. J Occup Environ Med. 2008;50:945-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 163. | Marchou B, Excler JL, Bourderioux C, Salaun J, Picot N, Yvonnet B, Cerisier JE, Salomon H, Auvergnat JC. A 3-week hepatitis B vaccination schedule provides rapid and persistent protective immunity: a multicenter, randomized trial comparing accelerated and classic vaccination schedules. J Infect Dis. 1995;172:258-260. [PubMed] |
| 164. | de Vries-Sluijs TE, Hansen BE, van Doornum GJ, Kauffmann RH, Leyten EM, Mudrikova T, Brinkman K, den Hollander JG, Kroon FP, Janssen HL. A randomized controlled study of accelerated versus standard hepatitis B vaccination in HIV-positive patients. J Infect Dis. 2011;203:984-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 165. | Potsch DV, Camacho LA, Tuboi S, Villar LM, Miguel JC, Ginuíno C, Silva EF, Mendonça RM, Moreira RB, Barroso PF. Vaccination against hepatitis B with 4-double doses increases response rates and antibodies titers in HIV-infected adults. Vaccine. 2012;30:5973-5977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 166. | Fabrizi F, Dixit V, Magnini M, Elli A, Martin P. Meta-analysis: intradermal vs. intramuscular vaccination against hepatitis B virus in patients with chronic kidney disease. Aliment Pharmacol Ther. 2006;24:497-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 167. | Overton ET, Kang M, Peters MG, Umbleja T, Alston-Smith BL, Bastow B, Demarco-Shaw D, Koziel MJ, Mong-Kryspin L, Sprenger HL. Immune response to hepatitis B vaccine in HIV-infected subjects using granulocyte-macrophage colony-stimulating factor (GM-CSF) as a vaccine adjuvant: ACTG study 5220. Vaccine. 2010;28:5597-5604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 168. | Overton ET, Sungkanuparph S, Klebert M, Royal M, Demarco-Shaw D, Powderly WG, Aberg JA. GM-CSF Fails to Improve Immune Responses to Booster Hepatitis B Vaccination in HIV-Infected Individuals. Open Virol J. 2011;5:109-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 169. | Sasaki Md, Foccacia R, de Messias-Reason IJ. Efficacy of granulocyte-macrophage colony-stimulating factor (GM-CSF) as a vaccine adjuvant for hepatitis B virus in patients with HIV infection. Vaccine. 2003;21:4545-4549. [PubMed] |
| 170. | Cooper CL, Angel JB, Seguin I, Davis HL, Cameron DW. CPG 7909 adjuvant plus hepatitis B virus vaccination in HIV-infected adults achieves long-term seroprotection for up to 5 years. Clin Infect Dis. 2008;46:1310-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 171. | Gatanaga H, Hayashida T, Tanuma J, Oka S. Prophylactic effect of antiretroviral therapy on hepatitis B virus infection. Clin Infect Dis. 2013;56:1812-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 172. | Sheng WH, Chuang YC, Sun HY, Tsai MS, Chang SY, Hung CC, Chang SC. Prophylactic effect of lamivudine-based antiretroviral therapy on incident hepatitis B virus infection among HIV-infected patients. Clin Infect Dis. 2013;57:1504-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |