Published online Jan 28, 2014. doi: 10.3748/wjg.v20.i4.1107
Revised: October 26, 2013
Accepted: November 12, 2013
Published online: January 28, 2014
Processing time: 123 Days and 13.2 Hours
AIM: To conduct a meta-analysis to evaluate the prognostic role of hypoxia inducible factor-1α (HIF-1α) expression in gastric cancer.
METHODS: The PubMed, EMBASE, and Web of Science databases were searched systematically for all articles published in English before August, 2013. Pooled effect was calculated from the available data to evaluate the association between HIF-1α expression and 5-year overall survival and tumor clinicopathological features in gastric cancer patients. Pooled odds ratios (ORs) with 95%CIs were calculated using either a fixed-effects or a random-effects model.
RESULTS: Nine studies matched the selection criteria, which reported on 1103 subjects, 548 of whom had HIF-1α positive expression (50%). This meta-analysis indicated that HIF-1α positive expression in gastric cancer correlated with lower 5-year overall survival (OR = 0.36; 95%CI: 0.21-0.64), worse tumor differentiation (OR = 0.38; 95%CI: 0.23-0.64), deeper invasion (OR = 0.42; 95%CI: 0.32-0.57), higher rates of lymph node metastasis (OR = 2.23; 95%CI: 1.46-3.40), lymphatic invasion (OR = 2.50; 95%CI: 1.46-4.28), and vascular invasion (OR = 1.80; 95%CI: 1.29-2.51), and higher TNM stage (III + IV) (OR = 0.31; 95%CI: 0.15-0.60).
CONCLUSION: HIF-1α positive expression indicates a poor prognosis for patients with gastric cancer. Further studies are required to confirm these results.
Core tip: We conducted a meta-analysis to evaluate the correlation between hypoxia inducible factor-1α (HIF-1α) expression and clinical outcome in gastric cancer patients, and reported that HIF-1 positive expression indicates a poor prognosis for patients with gastric cancer. This is the first comprehensive and detailed meta-analysis to assess the association of HIF-1α positive expression with 5-year overall survival and tumor clinicopathological features for gastric cancer patients. We believed that the results will provide useful information for clinical decision-making regarding gastric cancer.
- Citation: Lin S, Ma R, Zheng XY, Yu H, Liang X, Lin H, Cai XJ. Meta-analysis of immunohistochemical expression of hypoxia inducible factor-1α as a prognostic role in gastric cancer. World J Gastroenterol 2014; 20(4): 1107-1113
- URL: https://www.wjgnet.com/1007-9327/full/v20/i4/1107.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i4.1107
Gastric cancer is one of the most common cancers worldwide. Although the prognosis of gastric cancer has improved due to early diagnosis, radical operation, and the development of adjuvant therapy, patients with gastric cancer still have a poor prognosis[1,2]. The main prognostic factors for gastric cancer are clinicopathological features of the disease, including tumor differentiation, depth of invasion, lymph node metastasis and stage. However, the prognostic factors do not fully predict individual clinical outcome. As a result, there is great interest in finding better markers to identify patients with a poor prognosis at the time of diagnosis[3,4]. Hypoxia inducible factor-1 (HIF-1) is a basic helix-loop-helix transcription factor composed of HIF-1α and HIF-1β subunits; and HIF-1α determines HIF-1 activity[5]. Increased evidence has revealed that HIF-1α positive expression was associated with an unfavorable prognosis in many kinds of cancer[6,7].
One published meta-analysis has reported that HIF-1α in Asian patients was associated with poor overall survival (OS), but not with disease free survival (DFS)[8]. However, there have been no data about tumor clinicopathological features, which were known to provide useful information for tumor prognosis. Since that meta-analysis only included several studies of low quality, the data reported were not sufficient to derive conclusions with regards to the OS and DFS. Given that several high-quality studies have been published recently, we reviewed the currently available evidence in the medical literature to determine the association between HIF-1α positive expression and 5-year overall survival of gastric cancer as well as common clinicopathological features, and to assess the significance of HIF-1α positive expression in the prediction of clinical outcome of gastric cancer.
The PubMed, EMBASE, and Web of Science databases were searched systematically for all articles published in English before August, 2013. The terms used for the search were: “HIF-1α” or “hypoxia-inducible factor-1α” and “Gastric Cancer” or “Gastric Neoplasm” or “Stomach Neoplasm”.
Reference lists of all retrieved articles were also manually searched for additional studies. Two reviewers independently extracted the data from each study. All relevant text, tables, and figures were reviewed for data extraction. Discrepancies between the two reviewers were resolved by discussion and consensus.
Only studies in the English language were considered for inclusion. In addition, each study had to fulfill the following criteria: (1) patients with gastric cancer diagnosed by pathology; (2) studies that examined the relationship between HIF-1α and survival of gastric cancer; (3) studies that utilized immunohistochemistry to determine the expression of HIF-1α in paraffin-embedded surgical specimens; and (4) the most informative article when multiple articles were published by the same authors or groups.
Abstracts, letters, editorials and expert opinions, reviews without original data, case reports, and studies lacking a control group were excluded. The studies or data were also excluded for: (1) overlapping articles or duplicate data; (2) articles about cell lines or animals; (3) being impossible to extract the appropriate data from the published results; (4) conference records; (5) studies lacking information on survival; or (6) patients who had previous chemotherapy or radiotherapy.
We mainly aimed at evaluating the prognostic value of HIF-1α positive expression in gastric cancer patients regarding 5-year overall survival. Our second aim was to assess the association of HIF-1α positive expression with tumor clinicopathological features, such as tumor differentiation, depth of tumor invasion, lymph node metastasis, lymphatic invasion, vascular invasion, and tumor node metastasis (TNM) stage. Overall survival was measured from the date of medical resection to either the day of death or the day of the last follow-up visit.
Two reviewers independently extracted the following parameters from each study: (1) first author and year of publication; (2) study population characteristics; (3) number of subjects who were included in studies; and (4) 5-year overall survival and clinicopathological features.
Quality assessment was performed with the Newcastle-Ottawa quality assessment scale (NOS).
The meta-analysis was performed using the Review Man-ager (RevMan) software, (version 5.2; Cochrane collaboration, http://ims.cochrane.org/revman/download). We analyzed dichotomous variables using estimation of odds ratio (OR) with 95%CI. The pooled effect was calculated using either a fixed-effects or a random-effects model. Heterogeneity between studies was evaluated using the χ2 and I2 tests, and we considered heterogeneity present if the I2 statistic was ≥ 50%. P < 0.05 was considered significant. Assessment of publication bias for each of the pooled study groups was performed using a funnel plot.
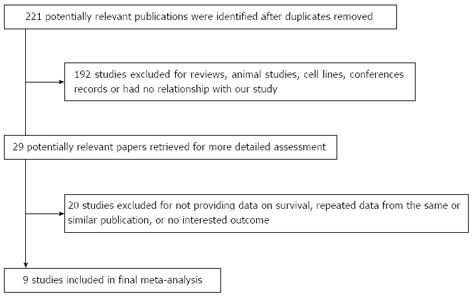
The initial search strategy retrieved 221 publications. After screening all titles, abstracts, and full texts, nine studies[9-17] met our entry criteria and were retrieved for more detailed evaluation (Figure 1). All nine studies were retrospectively analyzed, and their characteristics are summarized in Table 1. Sample sizes ranged from 51 to 216, and the total number was 1103, 548 of whom had HIF-1α positive expression (50%). Of nine included studies, five provided data on 5-year overall survival. The studies were conducted in four countries (China, Japan, South Korea and Turkey).
| Ref. | Country of origin | Sample size (M/F, n) | Mean/median age (yr) | Study quality (points) | 5-yr OS rate analysis | Expression rate |
| Zhan et al[9] | China | 60 (38/22) | 56.5 | 6/9 | Reported | 58.3% |
| Lu et al[10] | China | 68 (43/25) | 49.86 | 5/9 | NR | 52.9% |
| Isobe et al[11] | Japan | 128 (91/37) | 67.3 | 6/9 | Reported | 65.6% |
| Qiu et al[12] | China | 188 (127/61) | 57 | 6/9 | Reported | 54.6% |
| Oh et al[13] | South Korea | 114 (67/47) | 59 | 5/9 | NR | 15.8% |
| Kolev et al[14] | Japan | 152 (110/42) | 59.5 | 6/9 | Reported | 62.5% |
| Cabuk et al[15] | Turkey | 51 (30/21) | 63 | 4/9 | NR | 71% |
| Sumiyoshi et al[16] | Japan | 216 (148/68) | 65.2 | 5/9 | NR | 39.4%/85 |
| Mizokami et al[17] | Japan | 126 (83/41) | 65.3 | 6/9 | Reported | 38.9% |
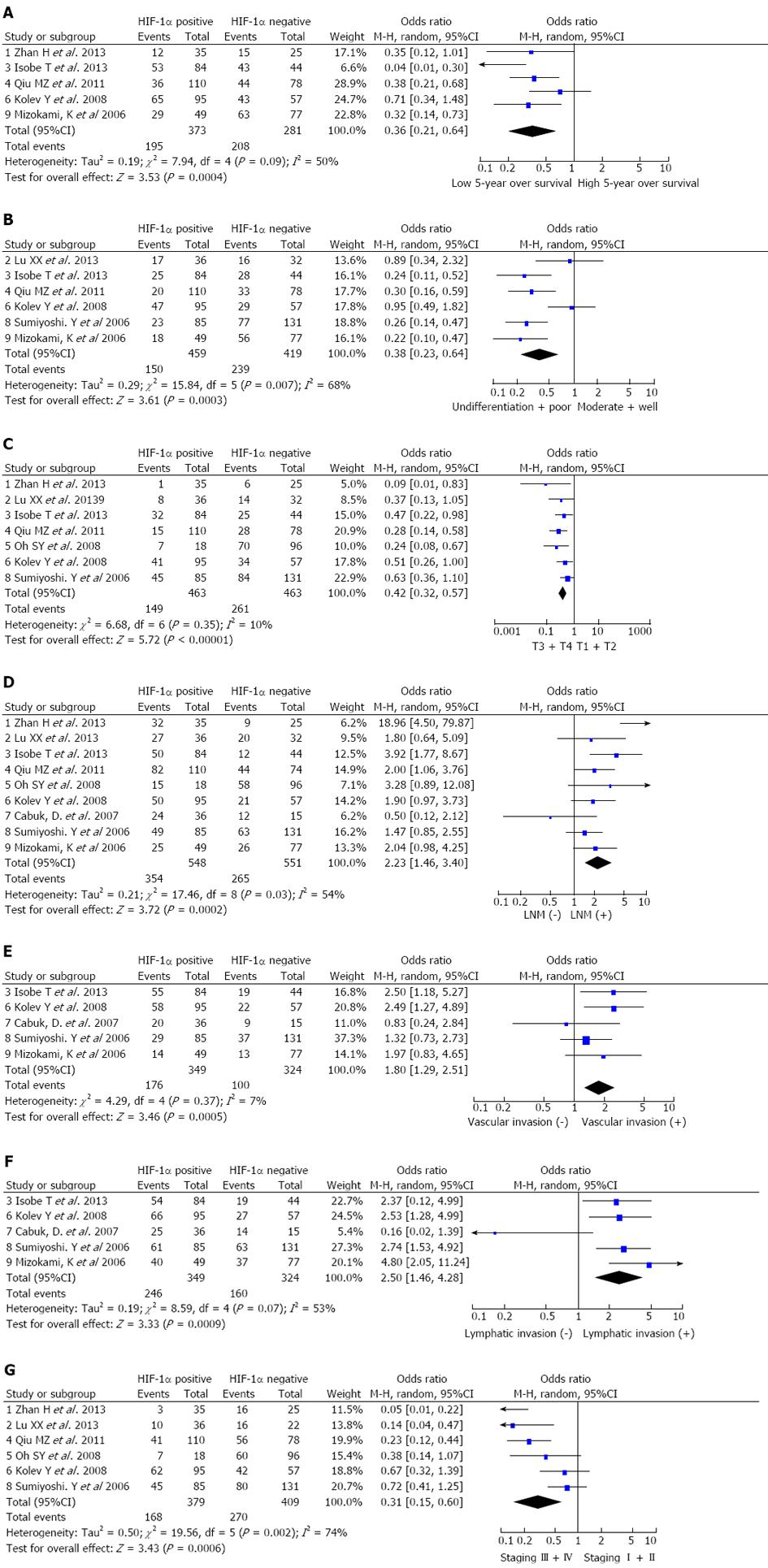
The 5-year overall survival was extracted from five studies. Meta-analysis indicated that patients with HIF-1α positive expression suffered with a lower 5-year overall survival (OR = 0.36; 95%CI: 0.21-0.64). The random effects model was used because of the heterogeneity (I2 = 50.0%) (Figure 2A).
Analysis of the pooled data showed that HIF-1α positive expression in gastric cancer was associated with biologically aggressive phenotypes such as tumor differentiation (OR = 0.38; 95%CI: 0.23-0.64; random effects model) (Figure 2B), depth of invasion (OR = 0.42; 95%CI: 0.32-0.57; fixed effects model) (Figure 2C), lymph node metastasis (OR = 2.23; 95%CI: 1.46-3.40; random effects model) (Figure 2D), lymphatic invasion (OR = 2.50; 95%CI: 1.46-4.28; random effects model) (Figure 2E), vascular invasion (OR = 1.80; 95%CI: 1.29-2.51; fixed effects model) (Figure 2F) and TNM stages III + IV (OR = 0.31; 95%CI: 0.15-0.60; random effects model) (Figure 2G). In other words, the incidence of HIF-1α positive expression was significantly higher in the poorly differentiated and undifferentiated gastric cancer than in well and moderately differentiated types, and significantly lower in carcinomas in stages I + II than in stages III + IV. HIF-1α positive expression was correlated with higher proportions of depth of invasion, lymphatic invasion, vascular invasion and lymph node metastasis.
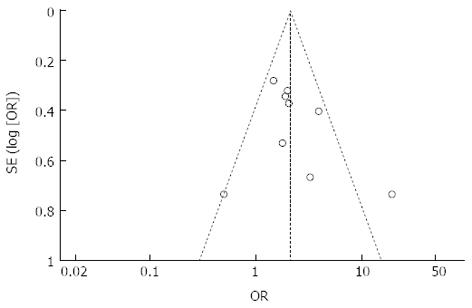
We used the inverted funnel plot to assess publication bias for all comparisons, and inspected its asymmetry visually. The shapes of the funnel plots showed a low potential for publication bias (Figure 3). Moreover, we used an influence analysis to evaluate the influence of a single study on the summary effect. The meta-analysis was not dominated by any individual study, and removing any study at a time made no difference.
Meta-analysis has been traditionally applied and was mostly confined to randomized controlled trials (RCTs), and meta-analytical techniques using non-randomized controlled trials (NRCTs) might be a good method for use in some clinical settings in which either the number or the sample size of the RCTs is insufficient[18,19]. To our best knowledge, our study is the first comprehensive and detailed meta-analysis to assess the association of HIF-1α positive expression with 5-year overall survival and tumor clinicopathological features in gastric cancer patients. We believe that our results will provide useful information for clinical decision-making regarding gastric cancer.
Nowadays, many studies about the role of HIF-1α in tumors have already been conducted and the relationship between HIF-1α and tumors has been confirmed. HIF-1α plays a role in the tumor formation, progression and metastasis by activating genes which are related to regulation of angiogenesis, cell survival and metabolism[20-22]. Not all gastric cancers express HIF-1α and 548 (50%) of 1103 gastric cancer patients had HIF-1α positive expression in this meta-analysis. However, once gastric cancer cells acquire HIF-1α expression, they transform to have more aggressive and metastatic behavior. The meta-analysis about prognostic significance of HIF-1α has been studied in several cancers such as non-small cell lung cancer and hepatocellular carcinoma[5,23], and HIF-1α positive expression indicates a poor prognosis. In this study, we found that the 5-year overall survival in the HIF-1α positive group was significantly lower than that in the HIF-1α negative group. Thus, HIF-1α was a poor prognosis factor for gastric cancer patients.
Our result also demonstrated that HIF-1α positive expression was correlated with increased vascular invasion and lymphatic invasion. The presence of vascular invasion and lymphatic invasion may indicate increased biological aggressiveness and a greater possibility of systemic diffusion. As shown in previous studies, vascular invasion and lymphatic invasion were the main risk factors for tumor occurrence and had the close relation with tumor invasiveness[24,25]. Moreover, we analyzed the relationship between the expression of HIF-1α and clinicopathologic features of gastric cancer, and found that the expression of HIF-1α was related to higher proportions of poor tumor differentiation, deep invasion, lymph node metastasis and TNM stages III + IV. This indicates that HIF-1α positive expression is closely related to the poor biological behavior of gastric cancer.
There are several limitations to this meta-analysis, and consequently, the results should be interpreted with caution. First, the data came from NRCTs, and the overall level of clinical evidence was low. Abraham et al[26] had found that meta-analyses carried out on well designed NRCTs of surgical procedures were probably as accurate as those carried out on RCTs. Second, there was heterogeneity across studies. We applied a random-effects model to take variation between studies into consideration, and we believe that the heterogeneity would have had very limited influence. Third, reports in languages other than English were excluded. The risk of language bias had to be considered, but it may not result in any notable bias in the assessment of interventional effectiveness. Finally, publication bias was present in our analysis. The reason was that investigative groups might be more likely to report positive results, and that studies with significant outcomes are more likely to be published.
In conclusion, the results of this meta-analysis of 1103 patients showed that HIF-1α positive expression was associated with poor 5-year overall survival and clinicopathological features in patients with gastric cancer. Moreover, HIF-1α positive expression could be a useful prognostic marker for gastric cancer. Further studies are required to confirm these results.
The authors would like to thank all of the patients and clinical investigators who were involved in the studies selected for this meta-analysis.
Although the prognosis of gastric cancer has improved, patients with gastric cancer still have a poor prognosis. The present prognostic factors do not fully predict individual clinical outcome. As a result, there is great interest in finding better markers to identify patients with a poor prognosis at the time of diagnosis.
Meta-analysis was used to evaluate the prognostic role of hypoxia inducible factor-1α (HIF-1α) expression in gastric cancer in this study.
To the best knowledge, this is the first comprehensive and detailed meta-analysis to assess the association of HIF-1α expression with 5-year overall survival and tumor clinicopathological features in gastric cancer.
This study reported that the HIF-1α positive expression was associated with poor 5-year overall survival and clinicopathological features in patients with gastric cancer. In addition, HIF-1α positive expression could be a useful prognostic marker for gastric cancer.
This manuscript describes an interesting meta-analysis of the HIF-1α positive expression associated with poor 5-year overall survival and clinicopathological features in patients with gastric cancer. In addition, HIF-1α positive expression could be a useful prognostic marker for gastric cancer. The manuscript was very well prepared and written and can be accepted for publication.
P- Reviewers: Gurzu S, Munoz M, Yamakawa M S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg. 2005;241:27-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 505] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 2. | Zhang H, Sun LL, Meng YL, Song GY, Hu JJ, Lu P, Ji B. Survival trends in gastric cancer patients of Northeast China. World J Gastroenterol. 2011;17:3257-3262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 3. | Liu JL, Gao W, Kang QM, Zhang XJ, Yang SG. Prognostic value of survivin in patients with gastric cancer: a systematic review with meta-analysis. PLoS One. 2013;8:e71930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Lim JY, Yoon SO, Hong SW, Kim JW, Choi SH, Cho JY. Thioredoxin and thioredoxin-interacting protein as prognostic markers for gastric cancer recurrence. World J Gastroenterol. 2012;18:5581-5588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3808] [Cited by in RCA: 3968] [Article Influence: 172.5] [Reference Citation Analysis (0)] |
| 6. | Zheng SS, Chen XH, Yin X, Zhang BH. Prognostic significance of HIF-1α expression in hepatocellular carcinoma: a meta-analysis. PLoS One. 2013;8:e65753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Marton I, Knezevic F, Ramic S, Milosevic M, Tomas D. Immunohistochemical expression and prognostic significance of HIF-1α and VEGF-C in neuroendocrine breast cancer. Anticancer Res. 2012;32:5227-5232. [PubMed] |
| 8. | Zhang ZG, Zhang QN, Wang XH, Tian JH. Hypoxia-inducible factor 1 alpha (HIF-1α) as a prognostic indicator in patients with gastric tumors: a meta-analysis. Asian Pac J Cancer Prev. 2013;14:4195-4198. [PubMed] |
| 9. | Zhan H, Liang H, Liu X, Deng J, Wang B, Hao X. Expression of Rac1, HIF-1α, and VEGF in gastric carcinoma: correlation with angiogenesis and prognosis. Onkologie. 2013;36:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Lu XX, Chen YT, Feng B, Mao XB, Yu B, Chu XY. Expression and clinical significance of CD73 and hypoxia-inducible factor-1α in gastric carcinoma. World J Gastroenterol. 2013;19:1912-1918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 11. | Isobe T, Aoyagi K, Koufuji K, Shirouzu K, Kawahara A, Taira T, Kage M. Clinicopathological significance of hypoxia-inducible factor-1 alpha (HIF-1α) expression in gastric cancer. Int J Clin Oncol. 2013;18:293-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Qiu MZ, Han B, Luo HY, Zhou ZW, Wang ZQ, Wang FH, Li YH, Xu RH. Expressions of hypoxia-inducible factor-1α and hexokinase-II in gastric adenocarcinoma: the impact on prognosis and correlation to clinicopathologic features. Tumour Biol. 2011;32:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Oh SY, Kwon HC, Kim SH, Jang JS, Kim MC, Kim KH, Han JY, Kim CO, Kim SJ, Jeong JS. Clinicopathologic significance of HIF-1alpha, p53, and VEGF expression and preoperative serum VEGF level in gastric cancer. BMC Cancer. 2008;8:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Kolev Y, Uetake H, Takagi Y, Sugihara K. Lactate dehydrogenase-5 (LDH-5) expression in human gastric cancer: association with hypoxia-inducible factor (HIF-1alpha) pathway, angiogenic factors production and poor prognosis. Ann Surg Oncol. 2008;15:2336-2344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Cabuk D, Basaran G, Celikel C, Dane F, Yumuk PF, Iyikesici MS, Ekenel M, Turhal NS. Vascular endothelial growth factor, hypoxia-inducible factor 1 alpha and CD34 expressions in early-stage gastric tumors: relationship with pathological factors and prognostic impact on survival. Oncology. 2007;72:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Sumiyoshi Y, Kakeji Y, Egashira A, Mizokami K, Orita H, Maehara Y. Overexpression of hypoxia-inducible factor 1alpha and p53 is a marker for an unfavorable prognosis in gastric cancer. Clin Cancer Res. 2006;12:5112-5117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Mizokami K, Kakeji Y, Oda S, Irie K, Yonemura T, Konishi F, Maehara Y. Clinicopathologic significance of hypoxia-inducible factor 1alpha overexpression in gastric carcinomas. J Surg Oncol. 2006;94:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Lin S, Chen ZH, Jiang HG, Yu JR. Robotic thyroidectomy versus endoscopic thyroidectomy: a meta-analysis. World J Surg Oncol. 2012;10:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Lin S, Jiang HG, Chen ZH, Zhou SY, Liu XS, Yu JR. Meta-analysis of robotic and laparoscopic surgery for treatment of rectal cancer. World J Gastroenterol. 2011;17:5214-5220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Matsumoto K, Arao T, Tanaka K, Kaneda H, Kudo K, Fujita Y, Tamura D, Aomatsu K, Tamura T, Yamada Y. mTOR signal and hypoxia-inducible factor-1 alpha regulate CD133 expression in cancer cells. Cancer Res. 2009;69:7160-7164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Lin MT, Kuo IH, Chang CC, Chu CY, Chen HY, Lin BR, Sureshbabu M, Shih HJ, Kuo ML. Involvement of hypoxia-inducing factor-1alpha-dependent plasminogen activator inhibitor-1 up-regulation in Cyr61/CCN1-induced gastric cancer cell invasion. J Biol Chem. 2008;283:15807-15815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Xu LF, Ni JY, Sun HL, Chen YT, Wu YD. Effects of hypoxia-inducible factor-1α silencing on the proliferation of CBRH-7919 hepatoma cells. World J Gastroenterol. 2013;19:1749-1759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Li C, Lu HJ, Na FF, Deng L, Xue JX, Wang JW, Wang YQ, Li QL, Lu Y. Prognostic role of hypoxic inducible factor expression in non-small cell lung cancer: a meta-analysis. Asian Pac J Cancer Prev. 2013;14:3607-3612. [PubMed] |
| 24. | Yang Y, Sun M, Wang L, Jiao B. HIFs, angiogenesis, and cancer. J Cell Biochem. 2013;114:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 25. | Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967-975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1126] [Cited by in RCA: 1055] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 26. | Abraham NS, Byrne CJ, Young JM, Solomon MJ. Meta-analysis of well-designed nonrandomized comparative studies of surgical procedures is as good as randomized controlled trials. J Clin Epidemiol. 2010;63:238-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |