Published online Jan 28, 2014. doi: 10.3748/wjg.v20.i4.1067
Revised: November 14, 2013
Accepted: December 12, 2013
Published online: January 28, 2014
Processing time: 121 Days and 18.9 Hours
AIM: To determine the maximum tolerated dose (MTD) and dose-limiting toxicity (DLT) of capecitabine combined with postoperative radiotherapy for gastric cancer.
METHODS: We enrolled patients with any T stage and node-positive gastroesophageal or gastric adenocarcinoma after complete resection with negative margins (R0) or microscopic (R1) or macroscopic (R2) resection. Intensity modulated radiotherapy (IMRT) using a five-to-seven-field, coplanar, sliding window technique was delivered to the tumor bed (T4b), anastomosis site, duodenal stump and regional lymph nodes (LNs) to a total dose of 45 Gy (1.8 Gy/fraction, 5 d/wk). Patients with R1 or R2 resection received 10.8 Gy as a boost. Capecitabine was administered twice daily on every radiotherapy treatment day in a dose-escalation schedule (mg/m2) of 625 (level I, n = 6), 700 (level II, n = 6), 800 (level III, n = 6), 900 (level IV, n = 0) and 1000 (level V, n = 0). DLT was defined as grade 4 leukopenia or neutropenia, grade 3-4 thrombocytopenia or anemia and grade 3-4 non-hematological toxicity.
RESULTS: Between October 2007 and August 2009, 18 patients (12 men, 6 women; median age, 54 years) were enrolled in the study. The median number of positive LNs was 6, and total number of resected LNs was 19. Twelve patients underwent R0 resection (66.7%). Fifteen patients received adjuvant chemotherapy under the leucovorin, fluorouracil and oxaliplatin (FOLFOX4) regimen. Six patients each were enrolled at dose levels I, II and III. Grade 1-3 leukopenia (16 patients, 88.9%), anorexia (15, 83.3%) and nausea (15, 83.3%) were the most common toxicities. Grade 3 anorexia/nausea and grade 4 vomiting occurred in one level-I patient. Grade 3 anorexia and nausea occurred in one level-II patient. One level-III patient developed grade 4 neutropenia, while another developed grade 3 radiation esophagitis. No abnormal liver or renal function examinations were observed. Three patients did not finish chemoradiotherapy because of DLTs and two without DLTs received sequential boosts (total dose, 55.8 Gy).
CONCLUSION: The MTD of capecitabine was 800 mg/m2 twice daily concurrent with IMRT for gastric cancer after surgery. The DLTs were anorexia/nausea, vomiting, neutropenia and radiation esophagitis.
Core tip: Postoperative chemoradiotherapy is a good option for patients with locally advanced, gastric cancer who have undergone R0 and D0-1 lymphadenectomy. To avoid acute side effects and make the drug safer, a combination of the use of advanced techniques such as intensity modulated radiotherapy and mature chemotherapy regimens with capecitabine is highly recommended, especially in China which accounts for 40% of the world’s gastric cancer patients. The aim of this single-institution, phase I, clinical trial was to assess the feasibility and toxicity of a postoperative regimen involving dose escalation of capecitabine combined with IMRT for locally advanced gastric cancer.
- Citation: Wang X, Jin J, Li YX, Ren H, Fang H, Wang SL, Liu YP, Wang WH, Yu ZH, Song YW, Liu XF. Phase I study of postoperative radiotherapy combined with capecitabine for gastric cancer. World J Gastroenterol 2014; 20(4): 1067-1073
- URL: https://www.wjgnet.com/1007-9327/full/v20/i4/1067.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i4.1067
Gastric cancer is the fourth most common cancer worldwide[1]. In China, gastric cancer has the third highest mortality rate among all cancers according to the latest Chinese Cancer Registry Annual Report[2]. A complete resection with negative margins (R0) remains the cornerstone of treatment for resectable gastric cancer. Nonetheless, less than 50% of patients will have an R0 resection of their primary tumor[3]. Therefore, long-term survival is poor, especially in patients with stage III or IV gastric cancer. Based on the results of Intergroup study (INT) 0116, concurrent chemoradiotherapy (CRT) has been considered as the gold standard of treatment for patients with locally advanced gastric cancer who have undergone radical surgery (R0) and less than a D2 lymphadenectomy has been achieved[4]. In addition, postoperative radiotherapy (RT) with concurrent fluoropyrimidine is the main treatment for patients with residual disease after a microscopic (R1) or macroscopic (R2) resection, according to the National Comprehensive Cancer Network (NCCN) guidelines for gastric cancer[5,6].
The 5-fluorouracil (5FU) analog, capecitabine, has been widely used for gastric cancer treatment, either in chemotherapy regimens or in concurrent CRT. The results using capecitabine were found to be comparable to 5FU, and this drug carries a considerably safer side-effects profile and a more convenient oral route of administration[7-11].
According to INT 0116, the significant toxicities of capecitabine were of great concern when this drug was applied in routine clinical practice. However, conventional RT with anteroposterior opposing fields (AP-PA) to the upper abdomen contributed to the observed severe acute toxicities. The recently developed RT techniques, three dimensional conformal therapy (3DCRT) and intensity modulated radiotherapy (IMRT), are greatly superior to conventional RT because they spare more normal tissue and critical organs outside the radiation field[12-14]. Although some studies have evaluated conformal RT combined with 5FU infusion or conventional RT combined with capecitabine as postoperative treatments for gastric cancer, a new regimen of IMRT with concomitant capecitabine has not yet been investigated[9,15,16].
The aim of this single-institution, phase I, clinical trial was to assess the feasibility and toxicity of a postoperative regimen involving the dose escalation of capecitabine combined with IMRT for locally advanced gastric cancer.
The eligibility criteria included the following: (1) pathologically confirmed adenocarcinoma; (2) postoperative classification of anyTN + M0 according to the 7th edition of the American Joint Committee on Cancer TNM Classification[17]; (3) World Health Organization performance status of ≤ 1 and age ≤ 70 years; (4) no prior or concurrent malignancy (except non-melanoma skin cancers or in situ carcinoma of the cervix); (5) no history of abdominal radiation; and (6) hemoglobin level ≥ 10.0 g/L, leukocyte count ≥ 3.5 × 109/L, neutrophil count ≥ 1.5 × 109/L, platelet count ≥ 100 × 109/L and normal liver and kidney function.
The pretreatment workup consisted of physical examination, chest X-ray, abdominal and pelvic computed tomography (CT) scans (chest CT was included for proximal lesions), and a complete blood count and biochemical profile. Patients with heart disease that required medication, other severe comorbidities or psychiatric history which rendered them incapable of complying with the treatment regimen were excluded.
After being informed and having given their written consent, all patients who underwent R0/R1/R2 resection with pathologically proven, locally advanced gastric adenocarcinoma (anyTN + M0) were enrolled in this study. Patients who were administered any adjuvant chemotherapy before or after CRT were included in the study.
IMRT was selected because of its superior protocol design, which potentially reduces toxicities by reducing radiation exposure to adjacent normal structures. Patients were required to be fasted for 4 h before the CT simulation and take an oral positive contrast (300 mL) 30 min before CT simulation to make the small intestine visible. To decrease variability in distention due to gastric filling, a standard meal (300 mL of ready-to-eat canned porridge) was given to the patients 15 min before CT scanning and before each daily treatment. Intravenous administration of contrast was added for the IMRT; the patients were placed in a supine position with thermoplastic immobilization during IMRT with a 6-MV photon beam.
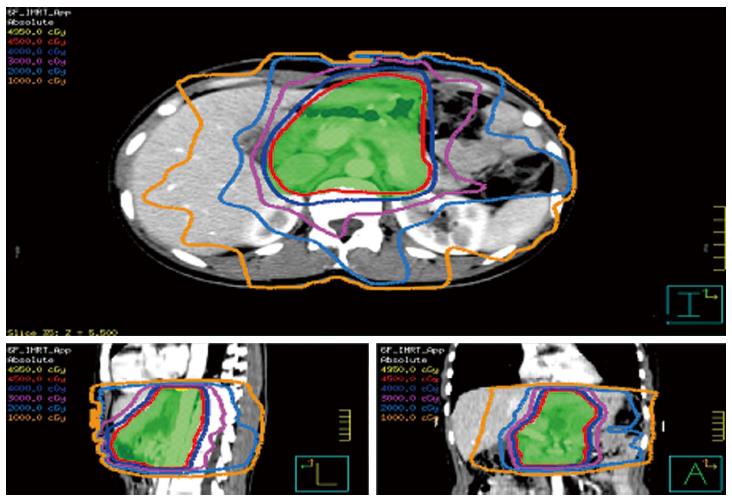
The gross tumor volume (GTV) encompassed either the visible, residual primary tumor or lymph nodes (LNs) based on the CT and/or positron-emission tomography-CT findings in patients who had undergone R2 resection or the confirmable microscopic area in patients who had a R1 resection. The delineation of the clinical target volume (CTV) for each patient depended on the extension and location of the primary tumor and the guidelines for the involved LN region issued by the Japanese Gastric Cancer Association[18]. Generally, the CTV included the GTV (if present), anastomoses, duodenal stump, tumor bed (only for stage T4b, if present) and regional LNs (Table 1). The remnant stomach was not routinely included within the radiation field. The planning target volume (PTV) consisted of the CTV with a 0.5-0.7 cm margin in the radial direction and a 1 cm margin in the superior-inferior direction. For R1 or R2 resection, the GTV (if visible) plus a 0.5-0.7 cm three-dimensional extension formed a boost planning GTV. Dose constraints for organ at risk (OAR) were as follows: V30 (volume receiving a dose of 30 Gy or more) < 40% for the liver, V20 < 30% for both kidneys or a mean dose of < 20 Gy, V30 < 30% for the heart and the maximal dose for the spinal cord planning OAR volume was 40 Gy. With regards to the small bowel and colon, the maximal dose was less than the prescribed dose, and V50 < 10% was used for patients receiving an additional boost. An experienced physicist designed the IMRT plans using a five-to-seven-field, coplanar, sliding window technique on the Pinnacle system, version 3.0 (Figure 1).
A total irradiation dose of 45 Gy was delivered in daily 1.8-Gy fractions (5 d a week over 5 wk) to R0 patients, and a sequential 10.8-Gy boost was delivered in six fractions to either the visible residual tumor (R2 resection) or the confirmed microscopic area (R1 resection).
Capecitabine was administered twice daily (after breakfast and after dinner) from the beginning to the end of the duration of RT, in a dose-escalation schedule of 625 mg/m2 (level I), 700 mg/m2 (level II), 800 mg/m2 (level III), 900 mg/m2 (level IV) and 1000 mg/m2 (level V).
If a dose-limiting toxicity (DLT) occurred in one of the first three patients, three additional patients were assigned to receive the same dose level. If none of the first three patients initially receiving a given dose level developed a DLT, or if only one of six patients had DLT, the dose was increased to the next level. If a second patient experienced a DLT at the same level, then the escalation was stopped, and the maximum tolerated dose was defined as the level at which the DLT occurred in this protocol.
Adverse events were coded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. DLTs were defined as follows: grade 4 leukopenia or neutropenia, grade 3-4 thrombocytopenia or anemia and grade 3-4 non-hematological toxicity.
During treatment, patients were observed daily by their radiation oncologist and underwent weekly physical examinations as well as assessments of weight. Blood was tested for routine analysis at least once weekly, while liver and renal function were assessed every 2 wk. Antacid and gastric mucosa protectants were administered on a prophylactic basis. Anti-emetics and antidiarrheal agents were prescribed when needed.
The trial was designed using a conventional dose-escalation schema with the primary endpoint of defining the MTD of capecitabine when combined with IMRT. The second endpoint was about the calculation of overall survival (OS), which was defined from the date of surgery to the date of death or last follow-up. Locoregional recurrence (LRR) was defined as any recurrence in the tumor bed, anastomoses, stumps, gastric remnant or a recurrence in the regional lymphatics and locoregional control (LRC) was calculated accordingly. Survival curves were calculated with the Kaplan-Meier method by means of the SPSS for Windows program, version 15.0 (SPSS, Chicago, Illinois, United States).
Between October 2007 and August 2009, 18 patients (12 men, 6 women) were enrolled in the study. The patient characteristics are presented in Table 2. The median age was 54 years (range, 29-66 years). All patients had metastatic LNs; the median number of positive LNs was 6 (range, 1-15), and the total number of resected LNs was 19 (range, 5-35). Twelve patients underwent R0 resection (66.7%). Fifteen patients received adjuvant chemotherapy under the leucovorin, fluorouracil and oxaliplatin (FOLFOX4) regimen, with a median number of 6 cycles (range, 3-11); of these 15 patients, seven received FOLFOX4 therapy before CRT, and the rest received it after CRT.
| Characteristic | Value |
| Median (range) | 54 (29-66) |
| Men | 12 (66.7) |
| Location of primary tumor | |
| Upper 1/3 | 4 (22.2) |
| Middle 1/3 | 2 (11.1) |
| Lower 1/3 | 12 (66.7) |
| Surgery type | |
| Proximal partial gastrectomy | 4 (22.2) |
| Distal partial gastrectomy | 12 (66.7) |
| Total gastrectomy | 2 (11.1) |
| Extent of dissection | |
| R0 (D1, D2) | 12 (9, 3) (66.7) |
| R1 | 2 (11.1) |
| R2 | 4 (22.2) |
| Tumor differentiation | |
| Well | 1 (5.6) |
| Moderately | 3 (16.7) |
| Poorly | 14 (77.8) |
| Signet ring cell | |
| Yes | 9 (50.0) |
| No | 9 (50.0) |
| Adjuvant chemotherapy | |
| Yes | 15 (83.3) |
| No | 3 (16.7) |
| Stage (AJCC 7th) | |
| II | 3 (16.7) |
| III | 15 (83.3) |
Six patients each were enrolled at dose levels I, II and III. Grade 3 anorexia and nausea and grade 4 vomiting were observed in one of six patients at the first level after only three fractions of radiation had been performed. At level II, one of the first three patients encountered grade 3 anorexia and nausea. After upgrading to level III, one patient developed grade 4 neutropenia, and another patient of the subsequent three patients developed grade 3 radiation esophagitis. The trial was then ended, and no patient was upgraded to levels IV and V. Therefore, level III (capecitabine, 800 mg/m2, twice daily) was determined to be the MTD. The DLTs met at levels I, II and III (Table 3) were grade 3 anorexia and nausea (levels I and II), grade 4 vomiting (level I), grade 4 neutropenia and grade 3 radiation esophagitis (level III).
| Level | Capecitabine (mg/m2, bid) | n | No. patients with the DLT | DLT (G3/4) | RT dose when DLT occurred (Gy) |
| I | 625 | 6 | 1 | Nausea, vomiting, anorexia | 5.4 |
| II | 700 | 6 | 1 | Nausea, anorexia | 45 |
| III | 800 | 6 | 1 | Neutropenia | 36 |
| 1 | Radiation esophagitis | 43.2 |
Grade 1-3 leukopenia (16 patients, 88.9%), anorexia (15, 83.3%) and nausea (15, 83.3%) were the most common toxicities. No renal or liver toxicity occurred in any patient. The detected grade 1-4 toxicities have been shown in Table 4.
| Toxicity | Grade 1-2 | Grade 3-4 |
| Nausea | 13 (72.2) | 2 (11.1) |
| Vomiting | 5 (27.8) | 1 (5.6) |
| Diarrhea | 2 (11.1) | 0 |
| Stomatitis | 3 (16.7) | 0 |
| Anorexia | 13 (72.2) | 2 (11.1) |
| Fatigue | 13 (72.2) | 0 |
| Weight loss | 7 (38.9) | 0 |
| HFS | 3 (16.7) | 0 |
| Esophagitis | 0 | 1 (5.6) |
| Leukopenia | 14 (77.8) | 2 (11.1) |
| Neutropenia | 3 (16.7) | 1 (5.6) |
| Anemia | 3 (16.7) | 0 |
| Thrombocytopenia | 5 (27.8) | 0 |
| ALT/AST | 0 | 0 |
| Renal | 0 | 0 |
Of the 12 patients with R0 resection, 45 Gy was delivered to nine patients as planned, including one patient who developed a DLT at level II but finally completed the entire treatment protocol. The remaining three patients completed CRT with 5.4, 36 and 43.2 Gy, owing to the occurrence of DLTs. Of the six patients with R1/R2 resection, two received sequential boosts for a total dose of 55.8 Gy without any DLTs. The remaining patients did not receive boosts because of difficulty in contouring the location of the residual tumor area without the placement of clips during surgery.
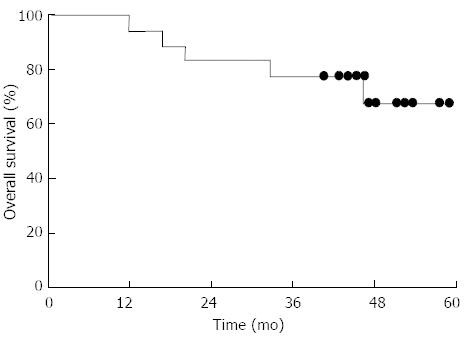
During a median follow-up of 45 mo (range, 5-58 mo), five patients died: four of progression of gastric cancer and one of pulmonary embolism. The 4-year LRC and OS rates were 93.8% and 68.1%, respectively (Figure 2).
Our results suggest that postoperative CRT with oral capecitabine and 45 Gy IMRT was well tolerated in locally advanced, gastric cancer patients after a partial or total gastrectomy. The most frequent adverse events were leukopenia, anorexia and nausea, although most of these were at grade 1 or 2. DLTs included grade 3 nausea, anorexia and radiation esophagitis and grade 4 neutropenia and vomiting. The MTD of oral capecitabine was determined to be 800 mg/m2 twice daily.
The benefit of postoperative CRT for locally advanced gastric cancer remains controversial. The benefits or drawbacks of this treatment mainly depend on whether a D2 or D0-1 lymphadenectomy has been performed. Differing results regarding D1 or D2 lymphadenectomy have been reported from both Western and Eastern studies[19-21]. Two large, randomized, clinical trials, INT 0116 and the ARTIST study, have provided compelling evidence on this topic. Since INT 0116 was published in 2001, the application of concurrent CRT has become widespread. This was the first study to provide evidence demonstrating that combined CRT following R0 resection and D0/D1 lymphadenectomy improves disease-free survival (DFS) and OS. Even after 10 years of follow-up, updated aanalysis of the INT 0116 study still show a strong, persistent benefit of adjuvant CRT in terms of DFS and OS, because the reduction of LRR may reduce the overall relapse in the majority of patients[22]. Recently, a large, prospective, randomized trial (ARTIST) from Korea indicated a 3-year DFS benefit for postoperative, concurrent CRT in patients with positive LNs after R0 resection and D2 lymphadenectomy compared with those who received chemotherapy alone[7]. Although this result was obtained via a subgroup analysis, further studies that focus on LN-positive patients with R0 resection and D2 lymphadenectomy are warranted. Owing to its remarkable control of LRR, fluoropyrimidine-based postoperative CRT following R1 or R2 resection is unquestionably a recommended and effective treatment for gastric cancer according to NCCN guidelines[6].
In our specialized cancer hospital, most surgeons perform D1 or D2 lymphadenectomy, which accounts for the high 5-year LRR rate of 27.6%[23]. This result is not comparable to those of studies from Japan and Korea due to the inferior performance of the D2 lymphadenectomy[7,11,24]; therefore, postoperative CRT should be introduced in our hospital as a standard of care. Unfortunately, this regimen is still not well accepted or routinely performed. One of the reasons for this might be the concern about the high rate of side effects of postoperative CRT based on the INT 0116 report.
In the INT 0116 study, 54% and 32% patients developed grade 3/4 hematological and gastrointestinal toxicities, respectively. Three patients (1%) suffered toxic deaths, and 31% did not complete the treatment due to toxicities[4]. However, these outcomes were obtained in the era of two-dimensional RT with a large AP-PA field. We currently utilize two methods to avoid these outcomes. Firstly, 3DCRT or IMRT provides excellent coverage of the target volume while avoiding normal tissue. Leong et al[14] reported that 3DCRT provides more adequate coverage of the target volume with 99% PTV receiving 95% of the prescribed dose, compared to 93% PTV using AP-PA fields. The doses to the kidneys and spinal cord were much lower with the conformal technique. Furthermore, IMRT could deliver more efficient doses to the target volume while reducing the dose to the kidneys when compared with the conventional technique[25].
Secondly, the exclusion of the remnant stomach from the radiation field could significantly reduce the acute side effects without compromising long-term survival rates (DFS, with remnant stomach irradiated vs without, 70.4% vs 71.0%, P = NS; OS, 72.3% vs 72.9%, P = NS)[26]. Our analysis also revealed that only 4.7% out of 297 patients with locally advanced gastric cancer developed a recurrence in the remnant stomach after a D1 or D2 lymphadenectomy[23].
More importantly, capecitabine was demonstrated to be a safer and more effective regimen than 5FU, which was used as part of the concurrent chemotherapy regimen in INT 0116. In the past 10 years, capecitabine has been implemented extensively in the treatment of colorectal, breast and gastric cancer, either as a single agent or combined with other chemotherapeutic and targeted agents. Oral capecitabine has shown comparable results to those of 5FU infusion, with a much safer side effects profile and without the need for an invasive delivery route. Therefore, capecitabine has been recognized as a standard of care for the treatment of advanced gastric cancer worldwide[6,27,28]. Moreover, a German, randomized, non-inferiority, phase III trial of 392 rectal cancer patients concluded that capecitabine could replace 5FU in adjuvant or neoadjuvant CRT regimens for patients with locally advanced rectal cancer, with a non-inferior OS (P = 0.0004), a significantly lower distant metastasis rate (P = 0.04) and better DFS rate (P = 0.07)[28].
The combined current literature indicates that postoperative CRT is a good option for patients with locally advanced gastric cancer who have undergone R0 and D0-1 lymphadenectomy. To avoid acute side effects and make the drug safer, a combination of an appropriate irradiation field, and the use of advanced techniques such as 3DCRT or IMRT and mature chemotherapy regimens with capecitabine is highly recommended.
In the present study, we considered that postoperative CRT with 800 mg/m2 oral capecitabine twice daily combined with IMRT with a dose of at least 45 Gy in 25 fractions was feasible and safe. The recommended dose of capecitabine was similar to that used in the RT phase of the CRT group in the ARTIST trial (capecitabine, 825 mg/m2 twice daily during RT treatment)[7]. Although the number of patients in our study was limited, the results of a 4-year follow-up show LRC and OS rates as high as 93.8% and 68.1%, respectively, which are very promising. A phase II study is ongoing, and its results are eagerly awaited.
Gastric cancer has the third highest mortality rate of all cancers in China. The optimal treatment is a R0 resection, but this can be offered to < 50% patients because of advanced disease. Chemoradiotherapy can be offered to these patients, but side effects can be severe.
The 5-fluorouracil (5FU) analog, capecitabine, has been widely used for gastric cancer treatment and is comparable to the widely used drug, 5FU. The use of intensity modulated radiotherapy (IMRT) is also known to be superior to standard radiotherapy, but the combined use of both capecitabine and IMRT for gastric cancer has not been investigated.
Postoperative capecitabine (800 mg/m2) with IMRT of at least 45 Gy for 25 fractions was found to be safe and feasible. Although the number of patients in their study (n = 18) was limited, the results of a 4-year follow-up show locoregional control and overall survival rates as high as 93.8% and 68.1%, respectively, which are very promising.
Further work is required to establish the range of side effects with the use of capecitabine in combination with IMRT. The dose limiting toxicities observed in this study were anorexia, nausea and vomiting, neutropenia and radiation esophagitis. A Phase II study will then be established to determine the treatment efficacy of this regimen.
IMRT is a method of radiotherapy that is delivering radiation to precise tissue areas that is greatly superior to conventional radiotherapy as it spares more normal tissue outside the radiation field.
This is a very interesting phase I trial about capecitabine combined with IMRT for locally advanced gastric cancer. Although the small number of patients involved, the trial was well done and the conclusions are according to another trial published and are correct. This is an interesting issue because utilize IMRT in the treatment for locally advanced gastric cancer and it will be very important to do a phase II trial.
P- Reviewers: Araujo A, Cihan YB, Tunio MA S- Editor: Gou SX L- Editor: A E- Editor: Wu HL
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 2. | He J, Chen WQ. Chinese Cancer Registry Annual Report, 2012. Beijing: Military Medical Science Press 2012; 28-30. |
| 3. | Zhan YQ, Li W, Sun XW, Chen YB, Xu L, Chen G, Guan YX, Li YF, Xu DZ, Sun XF. [Long-term results of surgical treatment of stomach cancer: clinical experience of forty years from Sun Yat-sen University Cancer Center]. Zhonghua Waike Zazhi. 2005;43:1109-1113. [PubMed] |
| 4. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2465] [Cited by in RCA: 2437] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 5. | Solomon NL, Cheung MC, Byrne MM, Zhuge Y, Franceschi D, Livingstone AS, Koniaris LG. Does chemoradiotherapy improve outcomes for surgically resected adenocarcinoma of the stomach or esophagus? Ann Surg Oncol. 2010;17:98-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | NCCN Guidelines Panel. National Comprehensive Cancer Network Guidelines in Oncology (NCCN Guidelines). Gastric cancer. 2013. Version 2, 2013-04-25; GAST-3: 11 screens. Available from: http://www.nccn.org/index.asp. |
| 7. | Lee J, Lim do H, Kim S, Park SH, Park JO, Park YS, Lim HY, Choi MG, Sohn TS, Noh JH. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 580] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 8. | Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, Lichinitser M, Guan Z, Khasanov R, Zheng L. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 591] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 9. | Jansen EP, Boot H, Dubbelman R, Bartelink H, Cats A, Verheij M. Postoperative chemoradiotherapy in gastric cancer -- a Phase I/II dose-finding study of radiotherapy with dose escalation of cisplatin and capecitabine chemotherapy. Br J Cancer. 2007;97:712-716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Hong YS, Song SY, Lee SI, Chung HC, Choi SH, Noh SH, Park JN, Han JY, Kang JH, Lee KS. A phase II trial of capecitabine in previously untreated patients with advanced and/or metastatic gastric cancer. Ann Oncol. 2004;15:1344-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1291] [Article Influence: 99.3] [Reference Citation Analysis (0)] |
| 12. | Ringash J, Perkins G, Brierley J, Lockwood G, Islam M, Catton P, Cummings B, Kim J, Wong R, Dawson L. IMRT for adjuvant radiation in gastric cancer: a preferred plan? Int J Radiat Oncol Biol Phys. 2005;63:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Lohr F, Dobler B, Mai S, Hermann B, Tiefenbacher U, Wieland P, Steil V, Wenz F. Optimization of dose distributions for adjuvant locoregional radiotherapy of gastric cancer by IMRT. Strahlenther Onkol. 2003;179:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Leong T, Willis D, Joon DL, Condron S, Hui A, Ngan SY. 3D conformal radiotherapy for gastric cancer--results of a comparative planning study. Radiother Oncol. 2005;74:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Kassam Z, Lockwood G, O’brien C, Brierley J, Swallow C, Oza A, Siu L, Knox JJ, Wong R, Cummings B. Conformal radiotherapy in the adjuvant treatment of gastric cancer: Review of 82 cases. Int J Radiat Oncol Biol Phys. 2006;65:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Jansen EP, Boot H, Saunders MP, Crosby TD, Dubbelman R, Bartelink H, Verheij M, Cats A. A phase I-II study of postoperative capecitabine-based chemoradiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys. 2007;69:1424-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors . AJCC cancer staging manual (7th edition). New York: Springer 2010; 145-152. |
| 18. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. [PubMed] |
| 19. | Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, Lui WY, Whang-Peng J. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006;7:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 472] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 20. | Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 754] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 21. | Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, Meyer S, Plukker JT, Van Elk P, Obertop H. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1069] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 22. | Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, Gunderson LL, Goldman B, Martenson JA, Jessup JM. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30:2327-2333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 627] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 23. | Wang X, Jin J, Li YX, Wang SL, Wang WH, Song YW, Liu YP, Liu XF, Yu ZH. Analysis of recurrence for locally advanced gastric or gastroesophageal cancer patients after receiving curative gastrectomy (>D1) and its indication for adjuvant chemoradiotherapy. Zhonghua Fangshe Zhongliuxue Zazhi. 2011;20:133-141. |
| 24. | Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 1943] [Article Influence: 107.9] [Reference Citation Analysis (0)] |
| 25. | Wieland P, Dobler B, Mai S, Hermann B, Tiefenbacher U, Steil V, Wenz F, Lohr F. IMRT for postoperative treatment of gastric cancer: covering large target volumes in the upper abdomen: a comparison of a step-and-shoot and an arc therapy approach. Int J Radiat Oncol Biol Phys. 2004;59:1236-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Nam H, Lim do H, Kim S, Kang WK, Sohn TS, Noh JH, Kim YI, Park CH, Park CK, Ahn YC. A new suggestion for the radiation target volume after a subtotal gastrectomy in patients with stomach cancer. Int J Radiat Oncol Biol Phys. 2008;71:448-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | NCCN Guidelines Panel. National Comprehensive Cancer Network Guidelines in Oncology (Chinese version). Gastric cancer. 2011. Version 1, GAST-2: 9 screens. Available from: http: //www.nccnchina.org/nccn-guidelines-china.aspx. |
| 28. | Hofheinz RD, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT, Müller L, Link H, Moehler M, Kettner E. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012;13:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 350] [Article Influence: 26.9] [Reference Citation Analysis (0)] |