Published online Jan 28, 2014. doi: 10.3748/wjg.v20.i4.1021
Revised: July 23, 2013
Accepted: September 4, 2013
Published online: January 28, 2014
Processing time: 297 Days and 20.6 Hours
AIM: To investigate the impact of portal inflow on liver remnants in a stable pig model of small-for-size syndrome.
METHODS: Twenty pigs underwent mesocaval shunt (MCS) surgery followed by 85%-90% hepatectomy. The control group had no shunt placement; the S1 group had portal flow maintained at an average of 2.0 times the baseline values; and the S2 group had portal flow maintained at an average of 3.2 times the baseline flow. The effect of portal functional competition on the liver remnant was investigated for 48 h postoperatively. Data were presented as mean ± SD. Statistical significance was determined using Student’s t test (SPSS, Chicago, IL, United States). Values of P < 0.05 were considered statistically significant.
RESULTS: At 24 h after hepatectomy, biochemical and histological changes were not significantly different between the S1 and S2 groups, but changes in both sets of variables were significantly less than in the control group. At 48 h, biochemical and histological changes were significantly less in the S2 group than in the S1 or control group. The regeneration index was significantly higher in the S2 group than in the S1 group, and was similar to that in the control group. Apoptosis index, serum lipopolysaccharide, and bacterial DNA levels were significantly lower in the S2 group than in the other two groups.
CONCLUSION: Diversion of portal inflow using MCS reduces portal overflow injury. Excessive diversion of portal inflow inhibits liver regeneration following major hepatectomy. Maintaining portal inflow at an average of 3.2 times above baseline helps promote hypertrophy of the liver remnant and reduce apoptosis.
Core tip: We established a model of small-for-size syndrome in pigs undergoing 85%-90% hepatectomy with mesocaval shunt (MCS) placement to define the optimal portal inflow required to preserve liver regeneration. Our findings indicate that diversion of portal inflow by MCS reduces injury from portal overflow following major hepatectomy, whereas excessive diversion of portal flow can retard liver regeneration. Preservation of portal inflow to at least 3.2 times above baseline levels appeared to promote hepatocyte hypertrophy and reduce apoptosis.
- Citation: Wang XQ, Xu YF, Tan JW, Lv WP, Liu Z, Zeng JP, Dong JH. Portal inflow preservation during portal diversion in small-for-size syndrome. World J Gastroenterol 2014; 20(4): 1021-1029
- URL: https://www.wjgnet.com/1007-9327/full/v20/i4/1021.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i4.1021
Major hepatectomy with partial graft transplantation causes simultaneous death and regeneration of hepatocytes. Small-for-size syndrome (SFSS) develops following this procedure if the functional liver mass is inadequate to maintain a balance between regeneration and metabolic demands[1-4]. Portal venous hypoperfusion of an extremely small residual liver or partial liver allograft is considered to be one of the most important factors leading to dysfunction following hepatectomy[5]. Portal diversion to the vena cava, using a mesocaval shunt (MCS) or portocaval shunt (PCS), is used to relieve portal hypoperfusion in both experimental and clinical settings[6-10]. However, the functional competition that occurs between the portal vein and systemic circulation, and its impact on the liver remnant have yet to be investigated.
In this study, we established a model of SFSS in pigs undergoing 85%-90% hepatectomy with MCS placement. Portal vein inflow (PVF) was regulated by modulating the size of the MCS. The study was undertaken to define the optimal portal inflow required to preserve liver regeneration.
Twenty-five male Bama miniature pigs (15-20 kg), aged 4-6 mo were obtained from the Pig and Poultry Production Institute (Guangxi Province, China). The pigs were raised from a closed herd and kept under strict quarantine. All experiments were conducted in accordance with Chinese legislation on protection of animals and complied with the Principles of Laboratory Animal Care (NIH publication No. 85-23, revised 1985). The study was approved by the Animal Care and Use Committee and the Ethics Committee of the Chinese People’s Liberation Army General Hospital. Every effort was made to minimize any suffering of the animals used in this study.
The pigs were deprived of food for 8 h before the operation. Initial sedation was achieved with a deep intramuscular injection of ketamine (15-20 mg/kg) and chlorpromazine (6-8 mg/kg), which were administered 15 min after atropine (0.01 mg/kg). Oxygen saturation and heart rate were monitored throughout the operation, and anesthesia was maintained using 1.5% halothane in oxygen titrated to provide anesthesia.
Central venous access was established using a catheter in the right femoral vein. Normal saline (1 L) and 5% dextrose (500 mL) were administered intravenously during the surgical procedure. No attempt was made to lower central venous pressure.
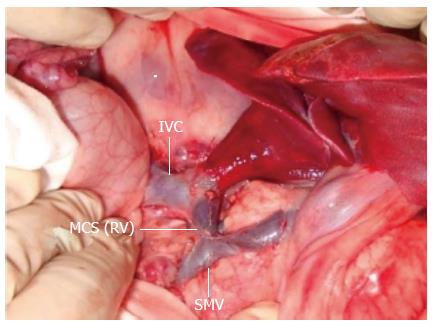
An upper-midline incision was made, and a 16-gauge catheter was inserted into the main portal vein via the gastroduodenal vein to measure portal vein pressure (PVP). Two ultrasonic probes (TS420; Transonic Systems, Ithaca, NY, United States) were used to assist the laparotomy. A 9-mm diameter probe was placed around the main portal vein (downstream of the gastroduodenal vein), and a 3.5-mm probe was placed around the hepatic artery near its origin from the celiac artery. The origin of the hepatic artery was isolated by ligation of the right gastric and gastroduodenal arteries. MCSs with different anastomotic diameters (5-10 mm) were implanted. Left trilobectomy was performed, together with partial right-posterior-lobe resection, without hepatic pedicle occlusion[11]. Parts of the right posterior and caudate lobes were retained to leave a residual hepatic volume of 10%-15% of the normal liver volume.
The mesenteric venous inflow was diverted through an MCS constructed using the prepared left renal vein with the PVF partly occluded (Figure 1). MCS, PVF and hepatic artery flow (HAF) were measured before and 30 min after MCS implantation. If the portal vein inflow was > 3.5 times higher than baseline or if shunt occlusion occurred, the shunt was closed, and the pigs were assigned to the control group (n = 6). Measurement of portal flow was repeated 10 min later. If the portal flow was < 1.8 times the baseline value, the shunt was adjusted to increase the portal flow to 1.8-2.3 or 3.0-3.5 times the baseline value. If necessary, an empty balloon with a catheter was placed around the shunt so that blood flow could be regulated by expanding the balloon. Animals with a portal flow 1.8-2.3 times the baseline value were assigned to the S1 group (n = 7). Animals with a portal flow 3.0-3.5 times the baseline value were assigned to the S2 group (n = 7). Five animals were excluded from the study because of shunt obliteration or other surgical complications during the observation period.
Forty-eight hours after hepatectomy, the animals were reopened. PVP, PVF and HAF were recorded and blood and tissue samples were obtained. Local anesthetic (50 mg marcaine in 20 mL) was administered subcutaneously to the abdominal wound. Halothane was discontinued postoperatively and a single dose of 375 mg penicillin was given intramuscularly to all pigs. Normal saline (500 mL) and 10% glucose solution (500 mL) were administered during recovery and daily thereafter.
The pigs were monitored until 48 h after hepatectomy, when they were anesthetized and reopened before euthanasia. Injury to the sinusoidal endothelial cells, dynamic PVF and HAF, injury and regeneration of the liver remnant, serum endotoxin levels, and bacterial translocation were compared between the three groups. At the end of the experiments, the pigs were sacrificed by an overdose of potassium chloride.
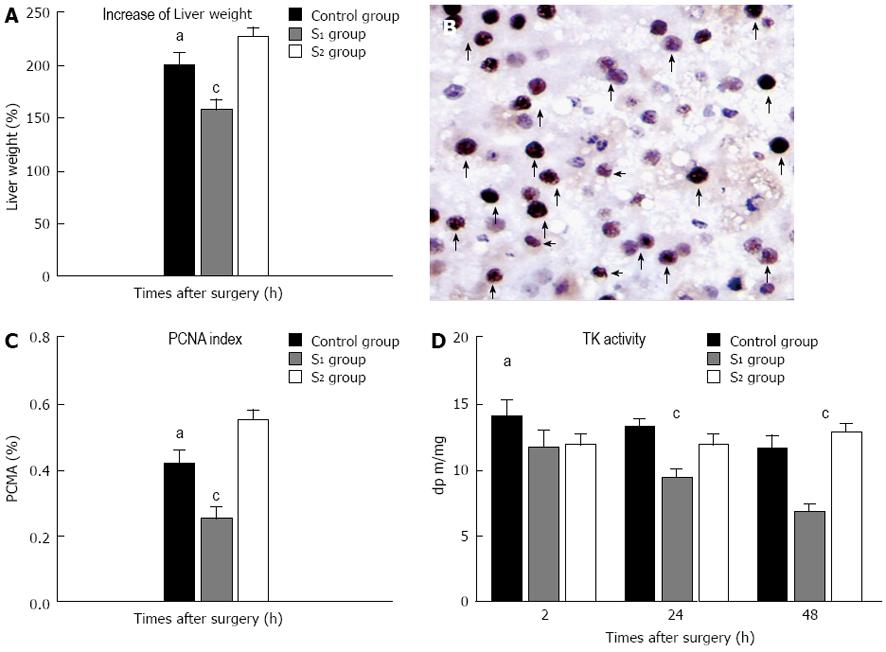
Serial serum samples were collected during the follow-up period. Blood sampling was performed preoperatively, at 2 h after hepatectomy, then daily until euthanasia. Serum levels of alanine aminotransferase (ALT), total bilirubin (TB), international normalized ratio (INR), hyaluronic acid (HA), and thymidine kinase (TK) activity were determined. HA levels were monitored to reflect the degree of sinusoidal endothelial damage[12,13]. Values were determined using the Pharmacia HA radiometric assay kit (Shanghai Yi Hua Scientific, Inc., China). TK activity was used as an index of hepatic regeneration[14] and was measured in serial serum samples using the Liaison TK assay. Results were expressed as dpm/mL protein (Jingmei Biotech Co. Ltd., Shenzhen, China).
Hepatic tissue was sampled in the three groups at 48 h after hepatectomy. Each biopsy sample was divided into two sections. One was immediately cut into 1-mm cubes and fixed in 2.5% glutaraldehyde in cacodylate buffer (0.1 mol/L sodium cacodylate-HCl, pH 7.4) overnight at 4 °C prior to sectioning for transmission electron microscopy (TEM). The other section was preserved in 10% formaldehyde prior to embedding in paraffin. The tissue samples were sectioned and stained with hematoxylin and eosin (HE) using standard histological techniques.
The pigs were sacrificed at 48 h after hepatectomy, and the patency of the MCS was verified surgically. The liver was excised, weighed and processed. Hepatic tissue was sampled for proliferating cell nuclear antigen (PCNA) staining and in situ terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL).
For histology and morphometry, 4-μm-thick sections, prepared from formalin-fixed, paraffin-embedded liver tissues, were stained with hematoxylin-phloxin-saffron and periodic acid Schiff staining. PCNA expression was detected by immunostaining using a monoclonal anti-PCNA-antibody kit (Jingmei Biotech). In addition, 3-μm sections were stained in situ with TUNEL using an apoptosis in situ detection kit (Jingmei Biotech Co., Ltd, Shenzhen, China) according to the manufacturer’s instructions.
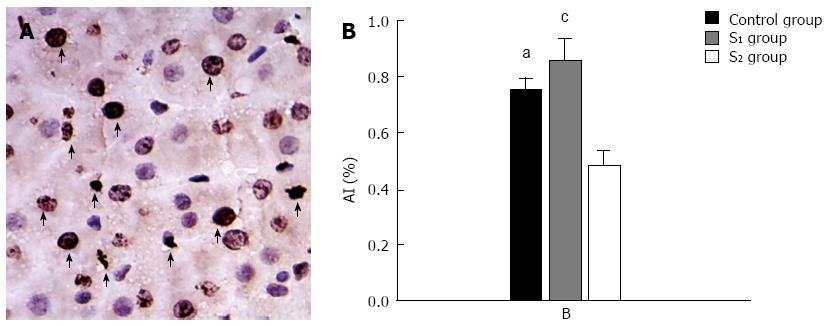
Increases in liver volume and PCNA index (PI) were used to quantify hepatic regeneration. The rate of increase in liver volume after hepatectomy was evaluated as: regenerated liver volume at sacrifice/estimated remnant liver volume at operation × 100%. PCNA data were expressed as the percentage of PCNA-stained hepatocytes per total number of hepatocytes (PI). The percentage of TUNEL-positive cells relative to the total cell count was used to estimate the apoptosis index (AI). Counts were made in 10 high-power fields for each of the three groups.
Lipopolysaccharide (LPS) levels were quantitated using the limulus amebocyte lysate (LAL) assay, which is based on the methods introduced by Iwanaga and colleagues[15]. The assay was performed using a commercially available chromogenic LAL endpoint QCL 1000 Kit (Yihua BioScience, Shanghai, China) following the manufacturer’s instructions. Standards and samples were analyzed in duplicate.
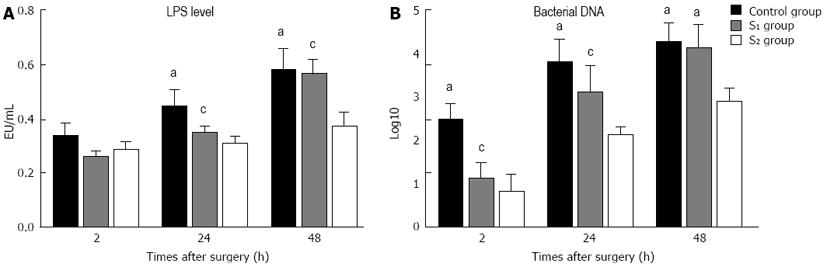
DNA was extracted from blood using the Fast DNA Spin Kit (Qiagen, Valencia, CA, United States) according to the manufacturer’s instructions. Total bacterial quantification was performed using 16S rRNA-gene-targeted primers. The universal primers were 5’-TTCCGGTTGATCCTGCCGGA-3’ forward, 5’-GGTTACCTTGTTACGACTT-3’ reverse[16,17].
Real-time polymerase chain reaction (PCR) was performed on an iCycler IQ real-time detection system coupled to iCycler optical system interface version 2.3 software (Bio-Rad, Veenendaal, Netherlands). Serially diluted genomic DNA from selected bacterial isolates was used as a real-time PCR control for total bacterial quantification. PCR bacterial counts were expressed as log10 cells/g tissue ± SE.
Data were presented as mean ± SD. Statistical significance was determined using Student’s t test (SPSS, Chicago, IL, United States). Values of P < 0.05 were considered statistically significant.
The operative characteristics are shown in Table 1. There were no significant differences among the three groups (P > 0.05).
| Control group | S1 group | S2 group | Pvalue1 | Pvalue2 | |
| Body weight (kg) | 17.8 ± 3.1 | 18.1 ± 2.9 | 18.5 ± 3.9 | 0.85 | 0.87 |
| Left trilobes (g) | 351.2 ± 14.9 | 357.5 ± 17.2 | 365.5 ± 15.8 | 0.89 | 0.91 |
| ETL (g) | 443.1 ± 18.8 | 446.9 ± 21.5 | 457.0 ± 19.8 | 0.77 | 0.86 |
| WRL (g) | 391.8 ± 19.4 | 389.8 ± 17.4 | 400.8 ± 21.4 | 0.95 | 0.92 |
| ERL(g) | 51.3 ± 6.8 | 57.1 ± 8.5 | 56.2 ± 7.1 | 0.89 | 0.84 |
| Proportion of ERL | 11.8% ± 2.3% | 12.8% ± 3.3% | 12.2% ± 3.5% | 0.87 | 0.83 |
Systemic arterial pressure was monitored throughout the study. Serial changes in PVF and HAF are shown in Table 2. At baseline, PVF, HAF and PVP in the three groups did not differ significantly. However, at 24 or 48 h after hepatectomy, PVF and portal-to-arterial flow ratio in the S2 group were significantly lower than in the control group, and significantly higher than in the S1 group. HAF in the S2 group was significantly higher than in the control group, and did not differ significantly from that in the S1 group. PVP in the S2 group was significantly lower than in the control group, and did not differ significantly compared with the S1 group.
| Control group | S1 group | S2 group | Pvalue1 | Pvalue2 | |
| PVF, L/min per 100 g | |||||
| BAS | 59.4 ± 11.4 | 62.1 ± 11.4 | 67.4 ± 11.6 | 0.840 | 0.780 |
| PH | 451.8 ± 31.1 | 146.8 ± 21.1 | 218.8 ± 29.3 | 0.000 | 0.001 |
| EUT | 220.3 ± 41.3 | 69.8 ± 18.6 | 125.3 ± 31.6 | 0.000 | 0.000 |
| HAF, mL/min per 100 g | |||||
| BAS | 19.4 ± 4.5 | 18.3 ± 3.4 | 19.9 ± 4.1 | 0.920 | 0.910 |
| PH | 6.1 ± 2.5 | 12.1 ± 3.5 | 14.9 ± 2.5 | 0.001 | 0.061 |
| EUT | 5.5 ± 2.1 | 11.1 ± 3.4 | 13.2 ± 4.2 | 0.000 | 0.052 |
| P/A | |||||
| BAS | 3.1 ± 0.2 | 3.4 ± 0.3 | 3.4 ± 0.2 | 0.780 | 0.940 |
| PH | 74.0 ± 8.1 | 12.1 ± 2.8 | 14.8 ± 3.1 | 0.001 | 0.040 |
| EUT | 40.8 ± 6.6 | 6.3 ± 1.2 | 9.5 ± 1.8 | 0.000 | 0.001 |
| PVP | |||||
| BAS | 6.4 ± 1.8 | 6.9 ± 1.3 | 6.0 ± 0.8 | 0.930 | 0.750 |
| PH | 13.8 ± 2.6 | 7.6 ± 1.6 | 8.7 ± 1.4 | 0.022 | 0.061 |
| EUT | 15.9 ± 2.5 | 8.9 ± 1.2 | 9.6 ± 1.5 | 0.001 | 0.042 |
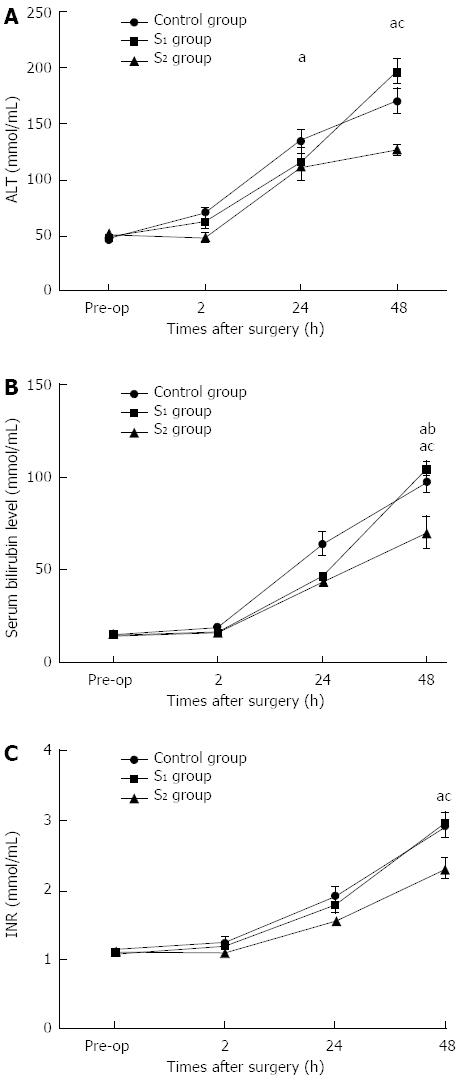
Preoperative and serial postoperative measurements of serum ALT, TB, and INR are shown in Figure 2. During the first 24 h after hepatectomy, all parameters except ALT were significantly higher in the control group than in the S1 group. There were no significant differences in TB or INR between the groups. However, at 48 h after hepatectomy, serum ALT, TB and INR were significantly lower in the S2 group than in the S1 and control groups.
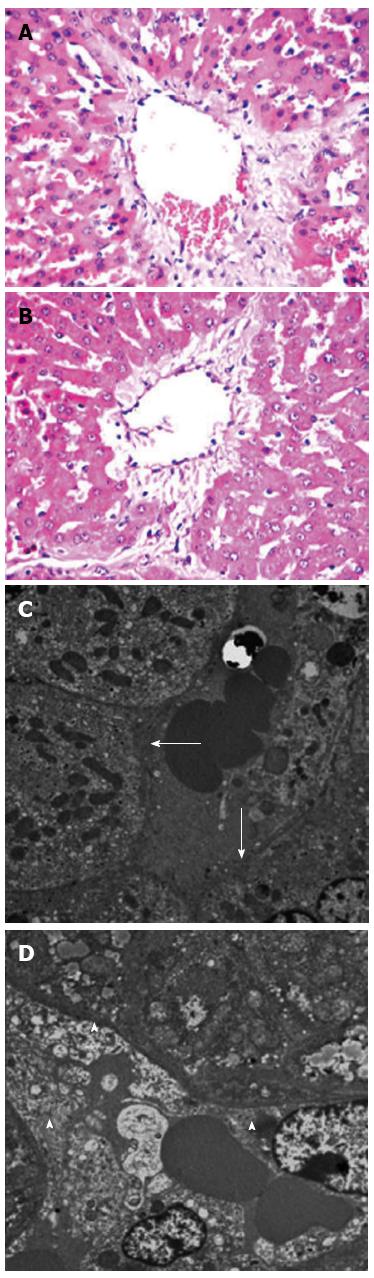
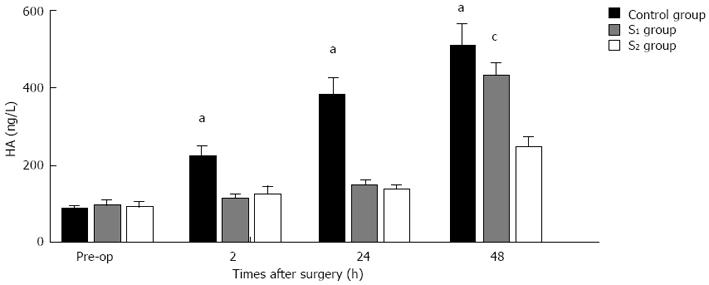
In the control group, there was no portal diversion. Both HE and TEM examination showed significant endothelial injury, accompanied by sinusoidal dilation, hydropic changes in hepatocytes and hemorrhage in the hepatic parenchyma (Figure 3A and C). In the S1 and S2 groups there was only mild sinusoidal injury to the hepatic microarchitecture and no intraparenchymal hemorrhage was seen (Figure 3B and D). Serial changes in HA levels in the three groups are shown in Figure 4. Following 85%-90% hepatectomy, serum HA levels increased in all three groups. At 2 h after hepatectomy, HA levels were significantly higher in the control group than in the S1 or S2 groups.
The rate of increase in the weight of the liver remnants was significantly higher in the S2 group than in the control or S1 groups. The rate of increase was lower in the S1 group than in the control group (Figure 5A). There were also differences between the three groups with respect to the estimated PI in PCNA-stained tissue at 48 h PH (Figure 5B and C).
At 2 h after hepatectomy, TK activity was significantly higher in the control group than in the S1 or S2 groups (Figure 5D). TK levels in the S2 group remained stable, and at 48 h after hepatectomy, they were significantly higher than in the S1 group and comparable to those in the control group.
At 48 h after hepatectomy, there were high numbers of TUNEL-positive cells in the liver remnant (Figure 6A). The AI at 48 h after hepatectomy was significantly lower in the S2 group than in the control group (Figure 6B).
Animal experiments have shown that hepatectomy decreases the size of the hepatic vascular bed and has the potential to increase portal pressure and vascular resistance, resulting in excessive portal flow and hemodynamic instability[3,4,18]. Similar findings have been reported in clinical practice[7,8,19,20] and contribute to high postoperative morbidity and mortality rates[3,6,8]. Furthermore, severe damage to the sinusoidal endothelial cells of the remnant liver at 3 h postoperatively has been reported as one of the main factors responsible for the high mortality rates in dogs undergoing massive hepatectomy[21].
Many studies have shown that diversion of portal inflow, using PCS, or MCS and splenectomy, can relieve overflow injury and improve survival and prognosis[3,4,21-24]. Despite these encouraging results, the use of PCS is associated with a marked delay in liver regeneration[25,26]. This is thought to be the result of over-reduction of vascular shear stress in the portal vein, possibly accompanied by diversion of hepatotrophic factors into the systemic circulation. This technique may also lead to loss of portal flow between the liver remnant and systemic shunt. The problem is exacerbated as portal systemic pressure increases in the regenerating liver. To overcome these difficulties associated with MCS or PCS, sufficient portal inflow and pressure needs to be preserved to promote liver regeneration without injuring the sinusoidal endothelium.
The optimum portal inflow required to stimulate liver regeneration with minimal or no overflow injury to the liver remnant remains unknown. This is because opinions regarding the manageable upper limit of portal pressure differ between transplant centers. Workers in Japan[27] set the appropriate PVP at < 20 mmHg, whereas another study[24] recommended PVP < 15 mmHg for living donor liver transplantation (LDLT). Another group[28] reported that small left-lobe grafts with < 40% graft volume/standard liver volume can be used safely with a portal flow < 25 mmHg. In two other studies of LDLT[20,23], suitable cutoff values for portal inflow were reported to be 250 and 260 mL/min/100 g tissue.
A previous study in pigs[25] showed that it was necessary to maintain portal vein flow at approximately two times the baseline value in order to produce a favorable outcome. However, this study provided no information about the effects of portal functional competition on optimum portal inflow for the liver remnant.
In our study, we demonstrated that using an MCS in the S1 and S2 groups relieved sinusoidal endothelial injury relative to that seen in the control group with no shunt. Liver regeneration (determined by rate of growth and PI) in the S2 group using a median portal inflow 3.2 times above baseline, was similar to that in the control group at 48 h after hepatectomy, and was significantly higher than in the S1 group with a median portal inflow of 2.0 times baseline. The AI in the S2 group was significantly lower than in the S1 and control groups, indicating the portal inflow regimen used in the S2 group supported liver regeneration and reduced apoptosis.
LPS levels indicated that the inflammatory response at 48 h after hepatectomy was less marked in the S2 group than in the S1 and control groups, further supporting the rationale for preserving > 3 times the baseline portal flow per unit tissue volume.
It has previously been demonstrated that competition between the portal vein and systemic circulation begins after a functional MCS has been established[20,23]. In our study the PVF per unit volume was lower in the S1 and S2 groups than in the control group. In these groups, hypertrophy of the liver remnant resulted in an increase in vascular resistance per unit volume.
In the S1 group, the PVF per unit volume decreased to the baseline value at 48 h after hepatectomy, whereas in the S2 group, portal inflow remained twice that at baseline at the same time point (Table 2). These results indicate that preserving portal flow at twice the baseline level was insufficient to sustain hypertrophy of the liver remnant. However, preserving approximately 3.2 times the baseline portal flow resulted in a high growth rate and a PI similar to that in the control group.
Portal overflow injury, LPS/bacterial translocation, and inflammatory responses represent an important mechanism of pathogenesis. The liver contains reticuloendothelial cells (Kupffer cells), and it has been shown that function of the reticuloendothelial system decreases significantly after major hepatectomy[29,30]. Innate immunity is also significantly impaired following major liver resection[26,31], and portal hypertension has been shown to increase LPS absorption and bacterial translocation and cause severe inflammation[31,32]. In our study the marked LPS/bacterial translocation and inflammation responses seen in the control and S1 groups delayed liver regeneration and aggravated apoptosis and injury to the liver remnant (Figure 7). These responses were far less marked in the S2 group.
Taken together our findings indicate that diversion of portal inflow by MCS reduces injury from portal overflow following major hepatectomy, whereas excessive diversion of portal flow can retard liver regeneration. Preservation of portal inflow to at least 3.2 times above baseline levels appeared to promote hepatocyte hypertrophy and reduce apoptosis.
Excessive diversion of portal inflow associated with mesocaval shunts (MCS) in ‘small-for-size’ syndrome (SFSS) has the potential to retard liver regeneration. However, it is unclear the optimal portal inflow is required to preserved liver regeneration. This study investigated the impact of portal inflow on liver remnants in a stable pig model of SFSS.
Portal diversion to the vena cava, using a MCS or portocaval shunt, is used to relieve portal hypoperfusion in both experimental and clinical settings. The functional competition between the portal vein and systemic circulation occurred, which may have an impact on the liver remnant.
This is the first study focusing on the impact of portal inflow on liver remnants in a stable pig model of SFSS. The authors demonstrated that diversion of portal inflow using MCS reduces portal overflow injury. Excessive diversion of portal inflow inhibits liver regeneration following major hepatectomy. Maintenance of portal inflow to at an average of 3.2 times above baseline levels appeared to promote hepatocyte hypertrophy and reduce apoptosis.
The results of this study provide some evidence that regulation of portal inflow is useful for patient following major hepatectomy to avoid SFSS.
This study demonstrated that maintenance of portal inflow to at an average of 3.2 times above baseline levels appeared to promote hepatocyte hypertrophy and reduce apoptosis in a stable pig model of SFSS. Therefore, measures should be considered to modulate the portal inflow when the risk of SFSS in liver transplantation or extended hepatectomy is high.
P- Reviewer: Riehle KJ S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
| 1. | Lee SS, Hadengue A, Girod C, Braillon A, Lebrec D. Reduction of intrahepatic vascular space in the pathogenesis of portal hypertension. In vitro and in vivo studies in the rat. Gastroenterology. 1987;93:157-161. [PubMed] |
| 2. | Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005;5:2605-2610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 472] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 3. | Kanematsu T, Takenaka K, Furuta T, Ezaki T, Sugimachi K, Inokuchi K. Acute portal hypertension associated with liver resection. Analysis of early postoperative death. Arch Surg. 1985;120:1303-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Kiuchi T, Kasahara M, Uryuhara K, Inomata Y, Uemoto S, Asonuma K, Egawa H, Fujita S, Hayashi M, Tanaka K. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation. 1999;67:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 722] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 5. | Fondevila C, Hessheimer AJ, Taurá P, Sánchez O, Calatayud D, de Riva N, Muñoz J, Fuster J, Rimola A, García-Valdecasas JC. Portal hyperperfusion: mechanism of injury and stimulus for regeneration in porcine small-for-size transplantation. Liver Transpl. 2010;16:364-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Wang H, Ohkohchi N, Enomoto Y, Usuda M, Miyagi S, Masuoka H, Sekiguchi S, Kawagishi N, Fujimori K, Sato A. Effect of portocaval shunt on residual extreme small liver after extended hepatectomy in porcine. World J Surg. 2006;30:2014-2022; discussion 2023-2024. [PubMed] |
| 7. | Lan AK, Luk HN, Goto S, Chen SM, Eng HL, Chen YS, de Villa VH, Wang CC, Cheng YF, Chen CL. Stress response to hepatectomy in patients with a healthy or a diseased liver. World J Surg. 2003;27:761-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Troisi R, Ricciardi S, Smeets P, Petrovic M, Van Maele G, Colle I, Van Vlierberghe H, de Hemptinne B. Effects of hemi-portocaval shunts for inflow modulation on the outcome of small-for-size grafts in living donor liver transplantation. Am J Transplant. 2005;5:1397-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 207] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Suehiro T, Shimada M, Kishikawa K, Shimura T, Soejima Y, Yoshizumi T, Hashimoto K, Mochida Y, Hashimoto S, Maehara Y. Effect of intraportal infusion to improve small for size graft injury in living donor adult liver transplantation. Transpl Int. 2005;18:923-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Yamada T, Tanaka K, Uryuhara K, Ito K, Takada Y, Uemoto S. Selective hemi-portocaval shunt based on portal vein pressure for small-for-size graft in adult living donor liver transplantation. Am J Transplant. 2008;8:847-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Court FG, Wemyss-Holden SA, Morrison CP, Teague BD, Laws PE, Kew J, Dennison AR, Maddern GJ. Segmental nature of the porcine liver and its potential as a model for experimental partial hepatectomy. Br J Surg. 2003;90:440-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Itasaka H, Suehiro T, Wakiyama S, Yanaga K, Shimada M, Sugimachi K. Significance of hyaluronic acid for evaluation of hepatic endothelial cell damage after cold preservation/reperfusion. J Surg Res. 1995;59:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Eriksson S, Fraser JR, Laurent TC, Pertoft H, Smedsrød B. Endothelial cells are a site of uptake and degradation of hyaluronic acid in the liver. Exp Cell Res. 1983;144:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 214] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Kahn D, Stadler J, Terblanche J, van Hoorn-Hickman R. Thymidine kinase: an inexpensive index of liver regeneration in a large animal model. Gastroenterology. 1980;79:907-911. [PubMed] |
| 15. | Iwanaga S, Morita T, Harada T, Nakamura S, Niwa M, Takada K, Kimura T, Sakakibara S. Chromogenic substrates for horseshoe crab clotting enzyme. Its application for the assay of bacterial endotoxins. Haemostasis. 1978;7:183-188. [PubMed] |
| 16. | Ludwig W. Nucleic acid techniques in bacterial systematics and identification. Int J Food Microbiol. 2007;120:225-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143-169. [PubMed] |
| 18. | Oliver RH, Sutton PM. The effects of partial hepatectomy on portal pressure in rats. Br J Surg. 1966;53:138-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Gruttadauria S, Mandala’ L, Miraglia R, Caruso S, Minervini MI, Biondo D, Volpes R, Vizzini G, Marsh JW, Luca A. Successful treatment of small-for-size syndrome in adult-to-adult living-related liver transplantation: single center series. Clin Transplant. 2007;21:761-766. [PubMed] |
| 20. | Shimamura T, Taniguchi M, Jin MB, Suzuki T, Matsushita M, Furukawa H, Todo S. Excessive portal venous inflow as a cause of allograft dysfunction in small-for-size living donor liver transplantation. Transplant Proc. 2001;33:1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Ueno S, Kobayashi Y, Kurita K, Tanabe G, Aikou T. Effect of prior portosystemic shunt on early hepatic hemodynamics and sinusoids following 84% hepatectomy in dogs. Res Exp Med (Berl). 1995;195:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Campos BD, Botha JF. Strategies to optimize donor safety with smaller grafts for adult-to-adult living donor liver transplantation. Curr Opin Organ Transplant. 2012;17:230-234. [PubMed] |
| 23. | Troisi R, Cammu G, Militerno G, De Baerdemaeker L, Decruyenaere J, Hoste E, Smeets P, Colle I, Van Vlierberghe H, Petrovic M. Modulation of portal graft inflow: a necessity in adult living-donor liver transplantation? Ann Surg. 2003;237:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Chan SC, Fan ST, Lo CM, Liu CL. Effect of side and size of graft on surgical outcomes of adult-to-adult live donor liver transplantation. Liver Transpl. 2007;13:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Hessheimer AJ, Fondevila C, Taurá P, Muñoz J, Sánchez O, Fuster J, Rimola A, García-Valdecasas JC. Decompression of the portal bed and twice-baseline portal inflow are necessary for the functional recovery of a “small-for-size” graft. Ann Surg. 2011;253:1201-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 416] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 27. | Ito T, Kiuchi T, Yamamoto H, Oike F, Ogura Y, Fujimoto Y, Hirohashi K, Tanaka AK. Changes in portal venous pressure in the early phase after living donor liver transplantation: pathogenesis and clinical implications. Transplantation. 2003;75:1313-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 209] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 28. | Yagi S, Iida T, Hori T, Taniguchi K, Yamamoto C, Yamagiwa K, Uemoto S. Optimal portal venous circulation for liver graft function after living-donor liver transplantation. Transplantation. 2006;81:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Asakura T, Ohkohchi N, Orii T, Koyamada N, Tsukamoto S, Sato M, Enomoto Y, Usuda M, Satomi S. Portal vein pressure is the key for successful liver transplantation of an extremely small graft in the pig model. Transpl Int. 2003;16:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Botha JF, Campos BD, Johanning J, Mercer D, Grant W, Langnas A. Endovascular closure of a hemiportocaval shunt after small-for-size adult-to-adult left lobe living donor liver transplantation. Liver Transpl. 2009;15:1671-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Medzhitov R, Janeway C. Innate immunity. N Engl J Med. 2000;343:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1517] [Cited by in RCA: 1450] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 32. | Yagi S, Iida T, Hori T, Taniguchi K, Nagahama M, Isaji S, Uemoto S. Effect of portal haemodynamics on liver graft and intestinal mucosa after small-for-size liver transplantation in swine. Eur Surg Res. 2012;48:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |