INTRODUCTION
Since Heald published on the “Holy Plane” of rectal cancer surgery[1], local recurrence and overall survival have improved greatly[2-4]. Traditionally, the overall survival for rectal cancer has been significantly worse than for colon cancer. However, since total mesorectal excision has been more broadly adopted, these figures are now reversed in some countries[5,6].
In 2007, Hohenberger published his sentinel paper on complete mesocolic excision (CME) with central vascular ligation for colon cancer[7] which was then published in English in 2009[8]. CME surgery follows similar oncological principles as total mesorectal excision does for rectal cancer. Since CME became standard of care in Honenberger’s unit, their 5 year local recurrence rate nearly halved from 6.5% to 3.6% and 5 year cancer related survival improved from 82.1% to 89.1%.
CME in the West is arguably a new concept[9] as traditionally oncological principles of surgery amongst Western surgeons, deemed that local control of disease dictated survival and lymphadenectomy was performed for pathological staging purposes and prognostication, rather than actual survival benefits[10].
In a number of countries in the East however, oncological principles of surgery are slightly different with more emphasis being placed on lymphadenectomy. Eastern teaching feels that leaving draining lymph nodes behind potentially leaves behind residual disease which has implications for local control and thus survival. Much of our literature on lymph node mapping of disease has come from Asia[11-14]. As such, a D3 extended lymphadenectomy has become the standard of care for many oncological operations especially in Japan, Korea and a number of other Asian countries. This extends to colonic cancer with a D3 lymphadenectomy considered the standard of care for clinical stage II and III disease[15].
The concept of CME does not appear to have generated as much interest in the West as TME has done previously. One of the reasons for this might be a notion that there is a lack of evidence to convince units to retrain and change the surgical tradition already present. Maybe the west should look east to broaden their horizon.
PRINCIPLES OF CME
The aim of a CME is to resect the affected colon with its associated lymphovascular supply by taking the colon and mesocolon in an intact envelope of visceral peritoneum. This envelope contains potentially involved lymph nodes and by keeping it intact, aims to minimise the risk of spillage of cancer cells into the peritoneal cavity. An intact visceral peritoneal layer is best achieved through sharp dissection. To improve lymph node harvest, central vascular ligation of the relevant blood supply flush with its feeding vessel is performed[7,8].
When comparing CME to current standard surgery, for left sided resections, there realistically is not much difference between standard surgery and CME as most colorectal units now follow the TME principles which aims for an intact mesocolic/mesorectal fascia and includes central vascular ligation of the inferior mesenteric artery flush with the aorta. However, for right sided resections, there can be quite radical differences. Even though a high vascular tie has always been recommended, there has never been clear guidelines as to exactly how high the vascular tie should be. Thus it has been at the surgeons’ discretion as to where the vascular ligation occurs and as such, it often is where it is anatomically convenient. This is usually at the mid mesenteric level or equivalent to taking intermediate nodes[16,17] (Figure 1). We have described our CME right hemicolectomy in previously published papers[18,19] and this is similar to other descriptions[20]. For a true central vascular ligation, mobilisation of the mesocolon needs to be more radical than a standard resection, fully exposing the head of the pancreas, the anterior surface of the SMV and SMA. This allows accurate identification of the origins of the ileocolic artery and vein and middle colic artery and vein (Figure 2).
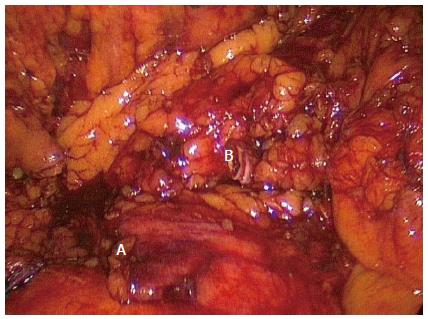
Figure 1 Standard right hemicolectomy.
Completed standard dissection of a right hemicolectomy with ligated ileocolic pedicle (A) and right branch of the middle colic artery (B). Note no dissection over the superior mesenteric vein or artery.
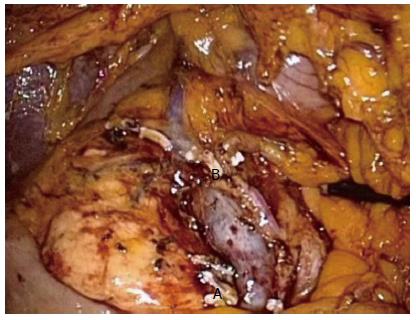
Figure 2 Complete mesocolic excision right hemicolectomy.
complete mesocolic excision dissection of a right hemicolectomy showing the superior mesenteric vein and artery and ligated Ileocolic (A) and right branch of the middle colic (B) pedicles.
GRADING OF A CME
The most commonly used grading system is the one devised for the CLASSIC trial by the medical research council[21,22] and is based on the grading system used for TME. Surgery is classified as being in the muscularis propria plane (“poor”) if the plane of dissection gives little bulk to the mesocolon and contains disruptions extending down to the muscularis propria. The intramesocolic plane (“moderate” plane of surgery) occurs if the plane of dissection gives moderate bulk to the mesocolon and breaches in the mesocolon fascia do not reach the muscularis propria. The mesocolic plane (“good” plane of surgery) occurs when there is an intact mesocolon with a smooth, peritoneal lined surface.
WHY PERFORM A CME?
Hohenberger proposes that a CME is a more oncologically sound procedure than a standard colonic resection as it includes a more radical lymph node dissection within an intact fascial envelope to reduce risk of tumour spread. Higher numbers of lymph nodes more accurately stage a patient and may improve survival in its own right[8].
Many studies have shown with a CME technique, an improved lymph node yield can be attained. The median number of lymph nodes across a number of studies on CME range from 18-46[22-31]. Traditionally, many western surgeons believe that the number of lymph nodes harvested determines prognosis but does not affect survival. However, there are a number of studies that now show the lymph node count regardless of positivity correlates with survival in stage II and III disease[12,32,33]. Hohenberger also showed that a lymph node count of ≥ 28 was independently associated with an improved 5 year cancer related survival (96.3% vs 90.7%; P = 0.018) in node negative patients. Even in node positive patients, this trend continued with 5 year cancer related survival improving from 64.6% to 71.7% if ≥ 28 nodes were harvested however with the smaller numbers in this group, but did not reach statistical significance (P = 0.088). This paper suggested that lymph node count was a good surrogate marker for surgical quality[8].
Other studies have also shown an improved overall survival, disease free survival and local recurrence when CME has been compared to standard controls[33,34] with further studies showing similarly impressive survival and local recurrence figures[19,31].
Stage migration is also thought to play a role in the improved survival of patients undergoing CME[35]. The hypothesis is that a higher lymph node yield achieved by CME may more accurately stage disease. This is particularly true for colon cancer as it has been shown that lymph node metastases may not occur in a step wise fashion (i.e., from paracolic to intermediate to apical nodes) in up to 18% of patients[12,16]. More importantly in these studies, the apical node was involved in up to 5.1% of patients who had no other nodal disease. Thus with standard surgery, these patients may not have received appropriate adjuvant therapy.
Excising the mesocolon within an intact fascial envelope is also associated with improved survival in its own right. West reported a 15% improved overall survival if the mesocolic plane was intact in a comparison to dissection in the muscularis plane. This survival advantage increased to 27% in stage III disease[23]. Similarly, another study by Bokey et al[36] showed a 15.6% improvement in overall 5 year survival when a standardized technique of mobilisation along embryological planes was introduced.
WHY NOT PERFORM A CME?
The issue with CME is that performing the procedure can be more traumatic for the patient (and maybe for the surgeon) than a standard operation. In the original description of a CME, surgery was performed via a laparotomy[7,8] where many centres are preferentially performing laparoscopic surgery with its associated benefits[37-39] as standard for colonic cancer. Operation time on average is much longer for a CME compared with standard surgery with reported average operation times ranging from 150-220 min[26-30]. Longer operation times may or may not translate to increased morbidity but it does affect the efficiency of an operating theatre and thus health economics in a time of increasingly tight health budgets.
There is also the risk of complications. Being a longer and more technically challenging operation, one may assume a higher complication rate and also the possibility of catastrophic complications that might occur less frequently in a standard procedure. However, the literature does not seem to support these concerns, with equivalent major morbidity rates being reported when CME is compared to standard surgery[24,40,41] or acceptable morbidity rates in other case series published[19,27,29,30,41,42]. One series reported exceptionally high rates of genitourinary dysfunction in patients undergoing CME for rectosigmoid carcinomas. When genitourinary function was formally tested, voiding dysfunction occurred in 75.5% of patients with 14.8% of these being permanent dysfunction. Sexual dysfunction was even higher with 91.7% with ejaculatory difficulty[25]. Some more unusual complications have been reported including chyle leakage, duodenal injury and significant vascular injury[29,42]. Our unit has also reported cases of chyle leak post CME[19]. In our experience, chyle leakage does not require intervention and spontaneously resolves in a matter of days.
Sceptics may also argue that there might not be as good a survival advantage as proposed by specialised units. Most studies use surrogate markers for survival including lymph node count and distance from the apical node to the tumour. In a systematic review of 22 papers on CME, there was an overall survival (58.7% vs 53.5%), disease free survival (77.4% vs 66.7%) and local recurrence advantage (4.5% vs 7.8%) in the CME patients, however, the external validity of Hohenbergers paper was questioned and when this data was excluded, survival was similar to current survival data with standard surgery[40].
IS THE CONCEPT OF CME NEW?
Complete mesocolic excision has been called “the new kid on the block”[9] and in western literature appears to be a current controversy in colonic surgery[43]. Meanwhile, in eastern countries such as Korea, a D3 lymphadenectomy for clinical stage II and III patients is highly recommended for colon cancers across the country and has been the standard of care in our institution for over 10 years.
A D3 lymphadenectomy has been recommended by the Japanese since their first publication of the General rules for Clinical and Pathological studies on Cancer of the colon, rectum and anus was published in 1977[44]. They defined a D3 lymphadenectomy as resection of the paracolic nodes, intermediate nodes and central lymph nodes. The Chinese have also published similar recommendations in the “Chinese Standards” where lymph node dissection must cover three groups: paracolic lymph nodes, intermediate lymph nodes and nodes at the mesenteric root[45]. D3 lymphadenectomy resections for colon cancer have now been widely published by the Japanese, Chinese, Korean and Taiwanese[26-29,46,47].
In these publications, the principles of surgery follows the same oncological principles as Hohenberger has suggested for their CME, that is, excision of the mesocolon within an intact fascial envelope with central vascular ligation. In Japan, a D3 lymphadenectomy has remained the standard of care for clinical stage II and III colon and rectal cancer surgery through all editions of their General Rules[15,44].
However, is a D3 lymphadenectomy equivalent to a CME? Theoretically, they should be equivalent procedures with the principles of both procedures being the same. In a pathological comparison study between specimens from Hohenberger’s unit in Erlangen and two Japanese centres, the length from tumour to high tie was equivalent. Rates for intact mesocolic plane were both high as were lymph node yield but both were significantly greater in the Erlangen specimens, however, lymph node positivity rates was equivalent. It was postulated that the differences between these specimens were likely related to the technique adopted by each country. The Japanese have previously shown that positive lymph node spread rarely occurs beyond 10 cm from the tumour and as such, the Japanese rarely resect more than 10 cm from the tumour. The Erlangen specimens on the other hand, were significantly longer and hence the resulting larger mesocolic surface area and lymph node counts. Survival and local recurrence data are similarly impressive between these countries and as such, a D3 lymphadenectomy should be considered equivalent to CME[23].
LAPAROSCOPIC CME
The original description of a CME by Hohenberger used an open technique and as a result, this has been adopted by other centres[31,35]. However, this might be a factor as to why many centres have not adopted CME as a laparoscopic approach is the preferred approach in their institutions. There have been a number of studies looking at the feasibility of performing a CME laparoscopically, especially for right sided cancers, all of which have shown feasibility and safety of a laparoscopic procedure with acceptable operation times, morbidity and oncological outcomes[26,28,30,42,47]. There have also been 4 comparison studies looking at laparoscopic versus open CME.
In a small Japanese study[1] 31 laparoscopic cases were compared to 118 open cases. The laparoscopic cases for right and left sided tumours yielded a similar number of lymph nodes compared to the open cases (R median 24 vs 24; P = 0.81, Left median 19 vs 16 P = 0.257). There was no difference in the achievement of an intact mesocolic plane in the laparoscopic group vs the open group (R 82% vs 76%; P = 0.681, L 85% vs 73%; P = 0.257). Interestingly, the laparoscopic group showed a greater distance from tumour to high tie and nearest bowel wall to high tie in both right and left sided resections (Right: 121 vs 100 mm; P = 0.018, 113 vs 89 mm; P = 0.18, Left: 136 vs 122 mm; P = 0.013, 126 vs 110; P = 0.018). However, the laparoscopic left sided resections, the specimen length was significantly shorter than the open comparison group (106 vs 154 mm; P < 0.001). Transverse colon tumours were not performed laparoscopically. However, the median BMI of the entire group was 22 and the question is to be raised if this is applicable to a western population.
In a comparison study from Greece[25], open vs laparoscopic proximal right, hepatic flexure and transverse colon and left sided resection were compared. The median BMI of this population was 27.7. This study also showed equivalence between laparoscopic and open groups in terms of lymph node harvest, rate of achievement of an intact mesocolic plane and distance from high tie to tumour and high tie to nearest bowel wall in proximal right and left sided resections. However, for hepatic flexure and transverse colon tumours, the open group achieved superior outcomes in distance from tumour to high tie and nearest bowel wall to high tie compared to the laparoscopic group (11.7 cm vs 8.72 cm; P = 0.049, 9.1 cm vs 6.5 cm; P = 0.015). There was also a correspondingly lower lymph node yield for this group [median LN yield 48 (32-56) vs 39 (32-46); P = 0.04].
In the only comparison study with long term outcomes[41], 123 open CME were compared to 128 laparoscopic CME. The median BMI was 25.6. Transverse colon tumours were included in the analysis but there was no subgroup analysis. Pathologically, lymph node harvest was equivalent [17.5 (open) vs 15.8 (lap) P = 0.092] although specimen length was significantly longer in the open group (29.4 cm vs 22.8 cm; P < 0.001). There was no mention of rates of intact mesocolic plane. Morbidity was significantly higher in the open group including major morbidity (11.4% vs 5.4%; P < 0.001). 3 year overall survival (80.4% vs 88.2%; P = 0.152) and disease free survival (74.8% vs 80.0%; P = 0.405) were equivalent.
In a Danish article[24], laparoscopic resections pre and post the introduction of the CME as a standard of care were compared. The mean BMI of this group was 26.7. Again, hepatic flexure and proximal transverse colon tumours were not performed laparoscopically. They were able to show that for proximal right sided cancers, the introduction of a laparoscopic CME increased the lymph node yield [26.8 (11-58) in the CME group vs 23.6 (9-43) in the standard group (P = 0.010)] and distance from tumour to high tie [9.4 cm (4-16) vs 7.7 cm (1-12) P = 0.0018] compared to their standard laparoscopic operation. There was also an increase in length from the tumour to high tie in the left sided resections [9.8 cm (3-17) vs 6.7 cm (3.5-10) P < 0.001], but no significant increase in the number of lymph nodes harvested was noted [25.7 (11-57) vs 22.8 (11-41) P = 0.076].
Thus, CME can be performed laparoscopically for proximal right and left sided tumours safely with a good oncological profile. The evidence for hepatic flexure and transverse colon tumours however still remains lacking.
Interestingly, there has not been much published recently on the feasibility of a laparoscopic CME from Asia and yet, in many parts of Asia, a resection with an equivalent D3 lymphadenectomy is performed laparoscopically or robotically as standard of care. Certainly, in Korea, our institution has performed such a procedure for more than 10 years. It can only be deduced that as a D3 lymphadenectomy has always been the standard of care in these countries open or laparoscopically, one would assume that it has not been felt to be necessary to publish specifically on the feasibility of performing a CME laparoscopically as performing general colonic surgery laparoscopically had already been published.
DISCUSSION
Complete mesocolic excision for colon cancer follows the same principles as total mesorectal excision does for rectal cancer. Sharp dissection along embryological planes to keep an intact fascial envelope to reduce tumour seeding combined with central vascular ligation to maximise the lymph node yield made a marked difference to rectal cancer and it appears to make a similar difference to colon cancer. However the international uptake of this concept has been slow. This may be related to a number of factors. A CME is a technically challenging operation that usually takes longer than the standard operation. A re-education process is necessary and this takes time to propagate. In Australia, most colorectal units already advocate dissection in the embryological plane with a high tie on the inferior mesenteric artery flush with the aorta recommended for most left sided resections. As such, many units are already doing a CME for left sided resections and as a result, some units felt the CME concept was not a new concept and that it was already being achieved for all resections. However, in most units, this is not true for right sided resections. Sharp dissection in the embryological plane is being achieved, but central vascular ligation of the ileocolic artery - flush with the superior mesenteric artery and right branch of the middle colic artery at its bifurcation is often less radical. Even in left sided resections, some Asian units perform a much more extensive paraaortic dissection compared to that being performed in Australia. The offset of this, however, is a higher pelvic nerve dysfunction rate[12]. In Asia, surgeons are prepared to accept this higher risk as a D3 lymphadenectomy is considered necessary for good oncology and a risk of pelvic nerve dysfunction is accepted by the patients and surgeons as a part of doing an adequate operation. In the West, however, this is not the case and nerve preservation is often considered a marker of “good surgery”. As such, patients and surgeons will preferentially preserve nerve function rather than perform a more radical operation unless there is a very clear indication to do so.
It is generally well known that a D3 lymphadenectomy is essentially the standard of care in Asia but there is also a general feeling amongst Western surgeons that this is because a more radical dissection is much easier in the lower BMI, Asian patient. Many Western surgeons feel that Asian data cannot be translated to the generally more overweight/obese Western patients. However, Hohenberger[8] and other units have shown that these differences in BMI between regions does not affect feasibility for achieving CME. Indeed, a laparoscopic CME is still possible even in larger patients[24,25,41].
The problem may well be propagation of the technique. Learning quite a different technique is best achieved by visiting a unit that is performing such resections. In Australia, very few units are well known to be doing CME for right sided resections and as such, very few surgeons are learning the technique. Internationally, language may also be a barrier as many of the units advocating CME are not English speaking and this may be a disincentive for Westerners to visit these units.
Many may feel that this increased complexity of the procedure, time of surgery and the perceived increased risk to the patients is not worth the gain made by doing a more radical lymphadenectomy as the gains are small compared to those achieved by TME for rectal cancer[48]. However, sufficient data now exists to show that a CME can be done safely, in a timely fashion, laparoscopically with little or no increased morbidity to the patients[19,25,26,28-30,42,47,48].
Local recurrence in colon cancer has traditionally been less of a problem than in rectal cancer. Prior to TME resections, local recurrence in rectal cancer was such a problem that surgeons searched for a solution[1,3,4,48]. This has not been the case for colon cancer and as such many surgeons have not felt the need to search for a better option.
From a teaching perspective, if a CME becomes the standard right hemicolectomy, there is a loss of the basic right hemicolectomy which has traditionally been the first bowel resection taught to junior trainees. However, this fact is more of a problem in Eastern countries where there is very little benign colorectal disease[49]. In the West, benign colorectal disease is more prevalent[50] and as such, there will always be the need for a standard right hemicolectomy.
CONCLUSION
Complete mesocolic excision is considered a new concept in the west. Centres adopting CME have shown great improvements in local recurrence and overall survival similar to the improved outcomes TME had for rectal cancer. Many centres, however, have not taken up CME in the west and it remains a rarity for a unit to perform CME routinely. The equivalent D3 lymphadenectomy has been performed in the East much more routinely for much longer and is considered standard of care in many countries. Maybe if the West looked East, more units might give CME more consideration.
P- Reviewer: Lang BH, Ogiso S, Richardson WS S- Editor: Qi Y L- Editor: A E- Editor: Liu XM