Published online Oct 14, 2014. doi: 10.3748/wjg.v20.i38.14051
Revised: June 20, 2014
Accepted: July 16, 2014
Published online: October 14, 2014
Processing time: 171 Days and 17.2 Hours
AIM: To assess the efficacy and safety of bone marrow-derived mesenchymal stem cell (BM-MSC) in the treatment of decompensated liver cirrhosis.
METHODS: The search terms “bone marrow stem cell”“chronic liver disease”“transfusion” and “injection” were used in the Cochrane Library, Med-Line (Pub-Med) and Embase without any limitations with respect to publication date or language. Journals were also hand-searched and experts in the field were contacted. The studies which used BM-MSC in the treatment of any chronic liver disease were included. Comprehensive Review Manager and Meta-Analyst software were used for statistical analysis. Publication bias was evaluated using Begg’s test.
RESULTS: Out of 78 studies identified, five studies were included in the final analysis. The studies were conducted in China, Iran, Egypt and Brazil. Analysis of pooled data of two controlled studies by Review Manager showed that the mean decline in scores for the model for end-stage liver disease (MELD) was -1.23 [95%CI: -2.45-(-0.01)], -1.87 [95%CI: -3.16-(-0.58)], -2.01 [95%CI: -3.35-(-0.68)] at 2, 4 and 24 wk, respectively after transfusion. Meta-analysis of the 5 studies showed that the mean improvement in albumin levels was -0.28, 2.60, 5.28, 4.39 g/L at the end of 8, 16, 24, and 48 wk, respectively, after transfusion. MELD scores, alanine aminotransferase, total bilirubin levels and prothrombin times improved to some extent. BM-MSC injections resulted in no serious adverse events or complications.
CONCLUSION: BM-MSC infusion in the treatment of decompensated liver cirrhosis improved liver function. At the end of year 1, there were no serious side effects or complications.
Core tip: Out of 78 studies identified, five studies were included in the final analysis, which showed that the mean decline in scores for the model for end-stage liver disease was -1.23 [95%CI: -2.45-(-0.01)], -1.87 [95%CI: -3.16-(-0.58)], -2.01 [95%CI: -3.35-(-0.68)] at 2, 4 and 24 wk, respectively. The mean improvement in albumin levels was -0.28, 2.60, 5.28, 4.39 g/L at the end of 8, 16, 24, and 48 wk, respectively. Alanine aminotransferase, total bilirubin levels and prothrombin times improved to some extent. Bone marrow-derived mesenchymal stem cell injections resulted in no serious adverse events or complications.
- Citation: Pan XN, Zheng LQ, Lai XH. Bone marrow-derived mesenchymal stem cell therapy for decompensated liver cirrhosis: A meta-analysis. World J Gastroenterol 2014; 20(38): 14051-14057
- URL: https://www.wjgnet.com/1007-9327/full/v20/i38/14051.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i38.14051
Liver fibrosis is the main cause of morbidity in patients with chronic liver disease (CLD). The most common causes of CLD are hepatitis B virus (HBV), alcohol, hepatitis C virus (HCV), autoimmune liver disease and primary biliary cirrhosis (PBC). CLD frequently progresses to liver cirrhosis (LC)[1]. LC usually progresses irreversibly to a decompensated stage which is characterized by liver functional impairment and portal hypertension. Many patients die from one or more clinical complications of decompensated liver cirrhosis (DLC)[2]. Although DLC can be treated conventionally, liver transplantation is the only option that can improve the survival rate of these patients. However, because of the shortage of donor livers, high costs, and potential serious complications, the availability of liver transplantation is limited worldwide[3-5]. Therefore, alternative strategies are under intense investigation.
Mesenchymal stem cells (MSC) originate from the many mesenchymal and connective tissues and have the potential to differentiate into various lineages[6,7]. Petersen et al[8] suggested that bone marrow can differentiate into mature hepatocytes. Furthermore, bone-marrow stem cells are thought to contribute to liver regeneration[9-11], and this aspect has been studied in the treatment of some liver diseases[12,13]. However, the effectiveness of bone marrow-MSC (BM-MSC) in the treatment of DLC has been inconclusive. Some studies on animal models have shown that BM-MSC infusions ameliorated liver fibrosis, and reversed fulminant hepatic failure[14-17]. Clinical trials have shown that BM-MSC transfusion can quickly improve liver function without significant side effects[18-22]. Use of BM-MSCs decreased ascites and fatigue as well as improving survival rates[18]. Some studies have suggested that BM-MSCs improved cases of liver fibrosis and hepatocellular carcinoma[23]. In contrast, other studies have reported that treatment using BM-MSC did not improve liver function or survival, and even aggravated liver fibrosis[24]. The aim of the current study was to determine the efficacy and safety of BM-MSC in the treatment of DLC by meta-analysis.
We searched the Cochrane Library, Pub-Med and EM-BASE for BM-MSC infusions in the treatment of CLD using key words, “bone marrow stem cell”, “chronic liver disease”, “liver cirrhosis”, “transfusion”, and “injection”. No language limitation was imposed. Major journals were hand-searched and experts in this field were contacted to identify potentially eligible clinical studies, published, and unpublished.
Adults between the ages of 18 and 74 years were enrolled with advanced CLD of various etiologies. These included chronic hepatic failure, evidence of ultrasonographic cirrhosis and portal hypertension with abnormal serum albumin (ALB) and/or total bilirubin (TBIL) levels and/or prothrombin times (PT), model for end-stage liver disease (MELD) scores less than 25, and platelet counts ≥ 30000/mm3. Exclusion criteria were the presence of liver tumors, human immunodeficiency virus infection, kidney or heart failure, portal vein thrombosis, and pregnancy or lactation.
In order to avoid systematic error in this meta-analysis, two reviewers, Zheng LQ and Pan XN, independently assessed all the studies to ensure conformity in the application of the inclusion and exclusion criteria. Disagreements were resolved by discussion with a third author until a consensus was reached.
The data were analyzed using Meta-Analyst (version 3.13 Beta), and Review Manager (version 5.1) software was used to extract and pool data for summary estimates. Results for continuous outcomes were expressed as weighted mean differences and variances by using Review Manager. The data of mean differences before and after treatment were calculated by Forest plot by using meta-analysis in one arm. Statistical heterogeneity of the results was evaluated using Cochrane Q-test and the I2 statistic with significance set at P < 0.10. We used a fixed-effect model for non-heterogeneous data using 95%CIs. For data with significant heterogeneity, a random-effects statistical model was used. Publication bias was assessed using the Begg-test.
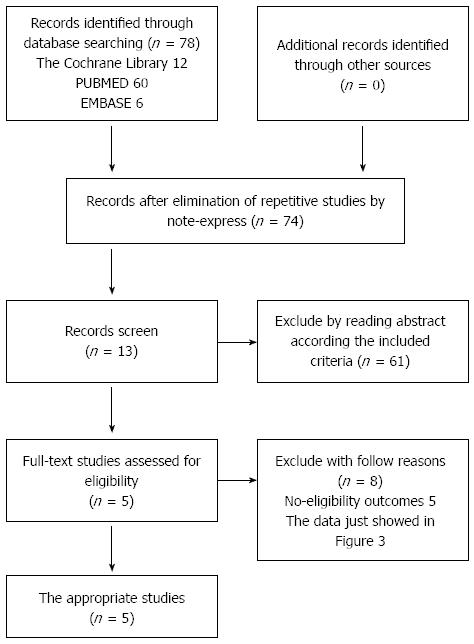
We identified 78 potentially eligible studies and excluded 73 studies for the following reasons: they were either animal studies, review articles, meeting reports, or there was a lack of proper data. Finally, 5 appropriate studies, including a total of 80 patients were selected for analysis (Figure 1). The characteristics of the 5 studies are shown in Table 1. These studies were published between 2007 and 2011, and used injections of between 106 to 108 cells in the treatment groups. The studies were from four countries (China, Iran, Egypt, and Brazil). In a study by Peng et al[19], reduced glutathione, glycyrrhizin, ademetionine, polyene phosphatidylcholine, alprostadil, and human serum albumin were administered to both the BM-MSC and the control group[18]. None of the other trials used this extra treatment.
| Ref. | Year | Country | Assessment of purity | Type of infused cells and volume | Infused cells (n) | Patients and disease etiology (n) | Frequency of stem cell transfusions | The route of transfusion |
| Peng et al[19] | 2011 | China | Flow cytometry | BMNCs (MSCs) from iliac crest (120 mL) | NA | 6 HBV + 15ctrl. after 48 wk follow-up | Once | Hepatic artery |
| Mohamadnejad et al[20] | 2007 | Iran | Fluorescence-assisted cell sorting flowcytometry | Cultured bone marrow-derived MSCs (80-100 mL) | 31.7 × 106 (mean) | 3 Cryptogenic, 1 AIH | Once | Cubital vein of the arm over 30 min |
| Mohamadnejad et al[21] | 2007 | Iran | FACS Calibur flow cytometer | Bone marrow-derived CD34+ Cells from iliac crest (200 mL) | 5.25 × 106 (mean) | 1 HBV, 1 PBC, 1 AIH1, 1 cryptogenic | Once | Hepatic artery |
| Amer et al[18] | 2010 | Egypt | Immunophenotyping | Cultured bone-marrow derived MSCs stimulated to hepatic lineage using HGF-containing medium (95 ± 25 mL) | 2 × 108 | 20 HCV + 20 ctrl | Once | Intrasplenic or intrahepatic |
| Lyra et al[22] | 2007 | Brazil | NA | BMNCs (MSCs) from iliac crest (maximum 50 mL) | 1 × 108 | 10 NA | Once | Hepatic artery |
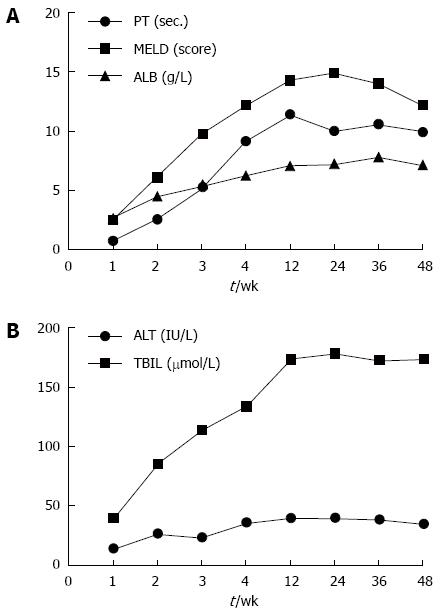
Long-term follow-up studies were performed by Peng et al[19] in which 6 cases of end-stage liver disease were reported. After injection of BM-MSC, the liver function improved significantly. The minimum MELD score occurred at 6 mo, and minimum PT occurred at 3-mo after infusion. Values increased slowly thereafter. The maximum decline in TBIL levels occurred at 3 mo while the peak ALB level occurred at 1 mo post-infusion. However, the TBIL levels were maintained at low levels during the follow-up period. The ALB levels remained at about 7 g/L higher than baseline after 1 year of follow-up (Figures 1 and 2). After 36 wk, there were no significant differences compared with the control group (P = 0.132)[19].
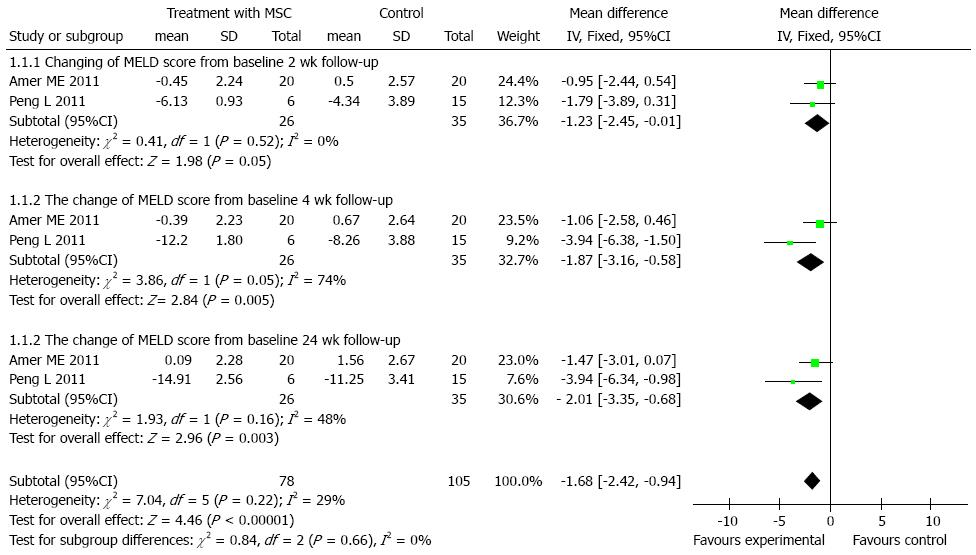
We found 2 control trials[18,19] (61 participants) which reported declines of MELD scores. There was no heterogeneity at the 2 and 24 wk time points among the studies included, using the fixed-effects model [total of mean difference was 1.68, 95%CI: -2.42-(-0.94), P < 0.001]. The outcomes of the subgroup at 2, 4 and 24 wk were -1.23 [95%CI: -2.45-(-0.01), P = 0.05], -1.87 [95%CI: -3.16-(-0.58), P = 0.005], -2.01 [95%CI: -3.35-(-0.68), P = 0.003], respectively. As time progressed, the magnitude of the mean differences became more obvious as shown in Figure 3.
Five studies[18-22] reported changes in levels of alanine aminotransferase (ALT), TBIL, ALB, PT-value and MELD scores. We extracted and pooled differences in the means before and after BM-MSC infusions in treatment group using Meta Analyst software (one arm). According to the heterogeneity evaluation, there was significant heterogeneity between these studies (Table 2). Therefore, a random effects model was used. The trend in ALB levels gradually increased after 8 wk, which was significantly better than before transfusion. After BM-MSC treatment, levels of ALT and TBIL increased while PT and MELD scores decreased during 1 year follow-up. The results are shown in Table 2. None of the studies described the changes in liver histology before and after the transfusion.
| Time point | Studies (n) | Cases (n) | Type of model | I2 | P value | Estimate | 95%CI |
| Change from baseline of albumin levels (g/L) | |||||||
| 8 wk | 2 | 9 | RE | 0.000 | 0.969 | -0.28 | -3.31-2.85 |
| 16 wk | 2 | 13 | RE | 0.000 | 0.995 | 2.60 | -0.35-5.56 |
| 24 wk | 3 | 13 | RE | 0.413 | 0.185 | 5.28 | 1.81-8.75 |
| 48 wk | 2 | 14 | RE | 0.831 | 0.015 | 4.39 | -1.62-10.40 |
| Change from baseline of ALT levels (IU/L) | |||||||
| 4 wk | 2 | 16 | RE | 0.667 | 0.083 | -16.33 | -50.71-18.06 |
| 24 wk | 3 | 13 | RE | 0.647 | 0.059 | -7.04 | -33.99-19.91 |
| 48 wk | 2 | 10 | RE | 0.333 | 0.221 | -18.18 | -44.03-7.06 |
| Change from baseline of TBIL levels | |||||||
| 4 wk | 2 | 16 | RE | 0.948 | 0.000 | -69.34 | -191.12-53.45 |
| 24 wk | 3 | 13 | RE | 0.945 | 0.000 | -47.21 | -130.18-35.76 |
| 48 wk | 2 | 10 | RE | 0.966 | 0.000 | -86.17 | -254.53-82.19 |
| Change from baseline of PT values (s) | |||||||
| 24 wk | 3 | 13 | RE | 0.843 | 0.002 | -4.77 | -9.22--0.33 |
| 48 wk | 2 | 10 | RE | 0.931 | 0.000 | -5.88 | -13.42-1.65 |
| Change from baseline MELD scores | |||||||
| 2 wk | 2 | 26 | RE | 0.977 | 0.000 | -3.27 | -8.84-2.30 |
| 4 wk | 2 | 26 | RE | 0.994 | 0.000 | -6.28 | -17.86-5.29 |
| 8 wk | 2 | 23 | RE | 0.606 | 0.111 | -1.43 | -4.92-2.06 |
| 16 wk | 2 | 23 | RE | 0.000 | 0.644 | 0.31 | -0.67-1.30 |
| 24 wk | 4 | 33 | RE | 0.982 | 0.000 | -4.28 | -13.39-4.83 |
| 48 wk | 2 | 10 | RE | 0.948 | 0.000 | -7.62 | -16.86-1.63 |
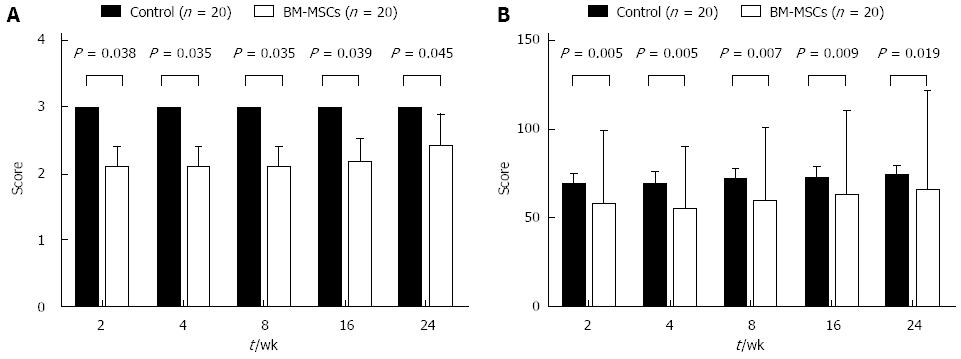
A control trial reported by Amer et al[18] in which 40 HCV patients were treated with BM-MSC or control revealed that fatigue scores were significantly lower, and the performance status significantly better in the BM-MSC group than the control group (Figure 4). The amount of ascites in the treatment group was less than that in the control group at 2 wk (χ2 = 0.01, P = 0.00-0.04), but this was not maintained beyond 6 mo (χ2 = 1.03, P = 0.311). Other studies[20,25] demonstrated that after injecting BM-MSC, the liver volume increased compared to that before treatment.
There were no significance adverse effects after the infusions. Peng et al[19] reported that in 53 cases there were no serious side effects or complications observed in short- and long-term follow up. However, in the study by Mohamadnejad et al[20] which included 4 patients, one of them appeared to develop some degree of renal failure after 5 mo follow-up. Another patient developed progressive renal failure and died of liver failure. The authors speculate that radio-contrast nephropathy may have contributed to the acute renal failure in that case. Clinical studies by Lyra et al[22] in Brazil, which included 10 patients, reported that two patients experienced mild pain at the sites of bone marrow puncture, but no other complications or specific side effects related to the infusion. In a report by Amer et al[18] which included 40 patients, fever was observed within 24 h. This responded to antipyretic therapy. In summary, there were no significant side effects in the treatment of chronic liver disease using BM-MSCs.
Peng et al[19] showed that improvements of liver function and MELD score were not maintained after 36 wk, and there were no significant differences in the incidence of HCC or survival rates between the two groups during 192 wk of follow-up. The animal studies of Zheng et al[15] showed that the number of human BM-MSCs which were injected into the liver by the intraportal route decreased significantly by week 15. However, 13 of 15 pigs achieved long-term survival in the intraportal transplantation group while all of the animals that received peripheral vein transplantations and the animals in the control group died within 96 h. Some recent studies considered that these preliminary outcomes suggested that transient benefit was most likely to occur in persons with acute liver diseases.
A pilot randomized controlled study by Lyra et al[25] showed that MELD scores stabilized in the cell therapy group, but increased in the control group. Peng et al[19] showed that liver function and MELD scores gradually declined from 4 to 24 wk (Figure 2). Similarly, in the current studies, MELD scores declined from -1.23 to -2.01 during the first 24 wk, and were significantly different from those of the control group (P < 0.05) (Figure 3). In one arm analysis, the MELD scores were also decreased at 4, 8, 24, and 48 wk, but were stable at 16 wk. This phenomenon may be due to renal failure at 16 wk in some studies. Some trials reported that the improvement of liver function began at 1 wk, but was not maintained after 36 wk. In studies by Kharaziha et al[26] which included 8 patients, ALB and TBIL levels were improved and remained up to 24 wk. In the current studies, ALB increased after 8 wk, and remained stable during the 1 year follow-up. Since the half-life of ALB is about 21 d, the improvement observed in the first few weeks may have been due to an injection of blood product in some studies. As seen in previous studies, the levels of ALT and TBIL increased, while PT and MELD scores decreased after transplantation. These levels were maintained at low levels during the 1 year follow-up (Table 2). There were no serious side effects or complications.
There are some limitations to this meta-analysis. The population, purity, method of assessment, type of infused cells and volume of cells in the included studies were not consistent. For example, in the studies of Peng et al[19], the BM-MSCs were isolated from the iliac crest, and grown to a density of 1.0 × 106 cells/mL. Cells were detected by flow cytometry (FACScan; BD Biosciences) using mouse isotype immunoglobulin G1 as a control. Mohamadnejad et al[20] extracted about 80-100 mL bone marrow from the iliac crest, harvested and cultured MSCs at density at 1.0 × 106 cells/cm2. Typical surface marker proteins were analyzed using florescence-assisted cell sorting (FACS) flow cytometry. In another study by Mohamadnejad et al[21], a total of 200 mL of bone marrow was aspirated from four different sites of the iliac crest. The cells were counted and assessed for viability using trypan blue dye exclusion. Purity was determined using a FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA, United States). In a study by Amer et al[18], 120 ml of bone marrow was aspirated from three different sites in the iliac crest, and immunophenotyping was done on the mononuclear cell population using CD34, CD133, CD90, CD105, CD73, and CD44 markers. In a study by Lyra et al[22], approximately 50 mL of bone marrow was aspirated from the iliac crest, centrifuged, and about 108 mononuclear- enriched BMC was suspended in 20 mL of saline. Assessing these methodologies, it appears possible that the LC preparations were not the same throughout the trials, and it is therefore likely that some variations in the infusions may have affected the results.
The methodology of the studies was generally poor. Due to ethical and other issues, we did not include many studies, and these studies included only two controlled trials, neither of which were randomized or blinded. This might have generated high performance bias and measurement bias. In the studies by Peng et al[19], HBV load, genotype, and E antigen status for subjects and controls were not matched. In order to reduce the effect from baseline, we calculated the mean relative changes from baseline. Although this method can reduce some selection bias, it may also have affected the stability of the outcome. We did not perform the Begg’s test for publication bias because there were less than 5 studies in each subgroup. Some included studies which did not mention the application and effects of the antiviral and anti-fibrosis treatment that could have influenced the stability of the results.
The findings of this meta-analysis indicate that BM-MSCs may be beneficial in improving liver function in the treatment of LC. There were few symptoms and no serious side effects or complications after 1 year follow-up. BM-MSC therapy may potentially improve fibrosis and reduce ascites. However, this improvement was not maintained in long term follow-up analyses. Further studies using multicenter, randomized, prospective trials to control for the primary disease, number of transfusions, and routes of injection are needed to substantiate the findings of this meta-analysis.
Many patients die from one or more clinical complications of decompensated liver cirrhosis (DLC), and conventional treatment is limited worldwide. Mesenchymal stem cells (MSC) originate from the many mesenchymal and connective tissues that can differentiate into mature hepatocytes. However, the effectiveness of bone marrow (BM)-MSC in the treatment of DLC has been inconclusive.
After the research by Petersen suggested that bone marrow can differentiate into mature hepatocytes, many studies have been performed to demonstrate the safety and effectiveness of BM-MSC in the treatment of DLC. Moreover, several systematic reviews were also recently performed to investigate these results. However, these reviews were methodologically insufficient and thus could not achieve a comprehensive conclusion.
Based on this meta-analysis, the mean decline in scores for the model for end-stage liver disease (MELD) was -1.23, -1.87 and -2.01 at 2, 4 and 24 wk, respectively, and the mean improvement in albumin levels was -0.28, 2.60, 5.28, 4.39 g/L at the end of 8, 16, 24, and 48 wk, respectively. BM-MSC injections resulted in no serious adverse events or complications. These findings were not presented clearly in previous systematic reviews.
BM-MSCs can improve the liver function of DLC, and there were no serious side effects or complications. Base on these, BM-MSCs may become a new method of therapy for DLC.
Alanine aminotransferase is the indicator that reacted to liver inflammation. Flow cytometry is conducted for cell analysis and sorting automatically. It can quickly measure, store, display cells that are suspended in a liquid dispersion in a series of important characteristic parameters, and separate the cells from the liquid. Trypan blue dye exclusion is a way to detect survival rates of the cells.
The authors describe the role of transfusion of autologous bone marrow-derived mesenchymal stem cells in the treatment of decompensated cirrhosis based on meta-analysis. The issue presented is noteworthy and the result showed that BM-MSCs can improve the liver fuction and MELD scores during the first 1 year, with no serious side effect and complications. This paper is well-written and has interesting and important findings.
P- Reviewer: Cichoz-Lach H, Moussa MM, Saito T, Skrypnyk IN S- Editor: Gou SX L- Editor: O’Neill M E- Editor: Ma S
| 1. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1712] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 2. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4120] [Article Influence: 206.0] [Reference Citation Analysis (3)] |
| 3. | Lee DS, Gil WH, Lee HH, Lee KW, Lee SK, Kim SJ, Choi SH, Heo JS, Hyon WS, Kim GS. Factors affecting graft survival after living donor liver transplantation. Transplant Proc. 2004;36:2255-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Dai LJ, Li HY, Guan LX, Ritchie G, Zhou JX. The therapeutic potential of bone marrow-derived mesenchymal stem cells on hepatic cirrhosis. Stem Cell Res. 2009;2:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Terai S, Takami T, Yamamoto N, Fujisawa K, Ishikawa T, Urata Y, Tanimoto H, Iwamoto T, Mizunaga Y, Matsuda T. Status and prospects of liver cirrhosis treatment by using bone marrow-derived cells and mesenchymal cells. Tissue Eng Part B Rev. 2014;20:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 6. | Hang HL, Xia Q. Role of BMSCs in liver regeneration and metastasis after hepatectomy. World J Gastroenterol. 2014;20:126-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Lee JH, Park HJ, Jang IK, Kim HE, Lee DH, Park JK, Lee SK, Yoon HH. In vitro differentiation of human liver-derived stem cells with mesenchymal characteristics into immature hepatocyte-like cells. Transplant Proc. 2014;46:1633-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. [PubMed] |
| 9. | Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 416] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 10. | Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y, Urata Y, Yokoyama Y, Uchida K, Yamasaki T, Fujii Y. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24:2292-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 349] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 11. | Kamiya A, Inagaki Y. Stem and progenitor cell systems in liver development and regeneration. Hepatol Res. 2014;Apr 28; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Liao X, AnCheng JY, Zhou QJ, Liao C. Therapeutic effect of autologous bone marrow-derived liver stem cells transplantation in hepatitis B virus-induced liver cirrhosis. Hepatogastroenterology. 2013;60:406-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Zhao W, Li JJ, Cao DY, Li X, Zhang LY, He Y, Yue SQ, Wang DS, Dou KF. Intravenous injection of mesenchymal stem cells is effective in treating liver fibrosis. World J Gastroenterol. 2012;18:1048-1058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Li J, Zhang L, Xin J, Jiang L, Li J, Zhang T, Jin L, Li J, Zhou P, Hao S. Immediate intraportal transplantation of human bone marrow mesenchymal stem cells prevents death from fulminant hepatic failure in pigs. Hepatology. 2012;56:1044-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Zheng L, Chu J, Shi Y, Zhou X, Tan L, Li Q, Cui L, Han Z, Han Y, Fan D. Bone marrow-derived stem cells ameliorate hepatic fibrosis by down-regulating interleukin-17. Cell Biosci. 2013;3:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Shao CH, Chen SL, Dong TF, Chai H, Yu Y, Deng L, Wang Y, Cheng F. Transplantation of bone marrow-derived mesenchymal stem cells after regional hepatic irradiation ameliorates thioacetamide-induced liver fibrosis in rats. J Surg Res. 2014;186:408-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Yuan SF, Jiang T, Sun LH, Zheng RJ, Cao GQ, Ahat NZ, Zhang YX. Use of bone mesenchymal stem cells to treat rats with acute liver failure. Genet Mol Res. 2014;13:6962-6980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Amer ME, El-Sayed SZ, El-Kheir WA, Gabr H, Gomaa AA, El-Noomani N, Hegazy M. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur J Gastroenterol Hepatol. 2011;23:936-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C, Zheng YB, Gao ZL. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54:820-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 20. | Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, Bagheri M, Bashtar M, Ghanaati H, Baharvand H, Ghavamzadeh A, Malekzadeh R. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007;10:459-466. [PubMed] |
| 21. | Mohamadnejad M, Namiri M, Bagheri M, Hashemi SM, Ghanaati H, Zare Mehrjardi N, Kazemi Ashtiani S, Malekzadeh R, Baharvand H. Phase 1 human trial of autologous bone marrow-hematopoietic stem cell transplantation in patients with decompensated cirrhosis. World J Gastroenterol. 2007;13:3359-3363. [PubMed] |
| 22. | Lyra AC, Soares MB, da Silva LF, Fortes MF, Silva AG, Mota AC, Oliveira SA, Braga EL, de Carvalho WA, Genser B. Feasibility and safety of autologous bone marrow mononuclear cell transplantation in patients with advanced chronic liver disease. World J Gastroenterol. 2007;13:1067-1073. [PubMed] |
| 23. | Maeda M, Takami T, Terai S, Sakaida I. Autologous bone marrow cell infusions suppress tumor initiation in hepatocarcinogenic mice with liver cirrhosis. J Gastroenterol Hepatol. 2012;27 Suppl 2:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Wu XZ, Chen D. Helicobacter pylori and hepatocellular carcinoma: correlated or uncorrelated? J Gastroenterol Hepatol. 2006;21:345-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Lyra AC, Soares MB, da Silva LF, Braga EL, Oliveira SA, Fortes MF, Silva AG, Brustolim D, Genser B, Dos Santos RR. Infusion of autologous bone marrow mononuclear cells through hepatic artery results in a short-term improvement of liver function in patients with chronic liver disease: a pilot randomized controlled study. Eur J Gastroenterol Hepatol. 2010;22:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 26. | Kharaziha P, Hellström PM, Noorinayer B, Farzaneh F, Aghajani K, Jafari F, Telkabadi M, Atashi A, Honardoost M, Zali MR. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009;21:1199-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 322] [Article Influence: 20.1] [Reference Citation Analysis (0)] |