Published online Oct 14, 2014. doi: 10.3748/wjg.v20.i38.13930
Revised: May 13, 2014
Accepted: July 15, 2014
Published online: October 14, 2014
Processing time: 196 Days and 16.8 Hours
AIM: To assess the impact of bacterial infections on cancer-specific survival in patients with colorectal cancer.
METHODS: This was a retrospective cohort study of colorectal cancer patients treated at the A.C. Camargo Cancer Center between January 2006 and April 2010. The presence of bacterial infection during cancer treatment, or up to one year after, was confirmed by laboratory tests or by the physician. Infections of the urinary, respiratory or digestive tracts, bloodstream, skin or surgical site were defined by testing within a single laboratory. Criteria for exclusion from the study were: chronically immunosuppressed patients; transplant patients (due to chronic immunosuppression); human immunodeficiency virus carriers; chronic use of corticosteroids or other immunosuppressive drugs; patients with autoimmune disease or primary immunodeficiency; known viral or parasitic infections. Patients with infections that did not require hospitalization were not included in the study because of the difficulty of collecting and tracking data related to infectious processes. In addition, patients hospitalized for pulmonary thromboembolism, stroke, acute myocardial infarction, uncontrolled diabetes, malignant hypercalcemia or other serious non-infectious complications not directly related to infection were also excluded. Survival curves were plotted using the Kaplan-Meier method, and log-rank tests (univariate analysis) and a Cox test assuming a proportional hazards model (multivariate analysis) were performed to examine associations between clinical history and characteristics of infection with cancer-specific survival.
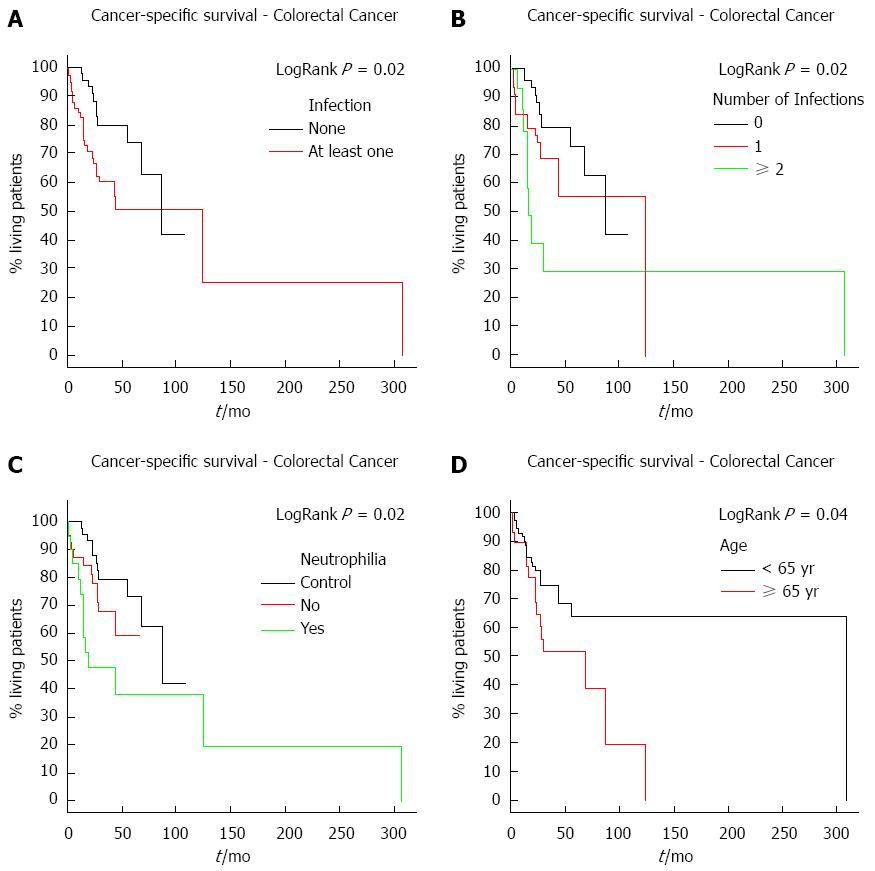
RESULTS: One-hundred and six patients with colorectal cancer were divided into two groups based on the presence or absence of bacterial infection. Patient ages ranged from 23 to 91 years, with a median of 55 years. The majority of patients were male (57/106, 53.77%) with stage III colorectal cancer (45/106, 44.11%). A total of 86 bacteriologic events were recorded. Results indicate that the presence and number of infections during or after the end of treatment were associated with poorer-cancer specific survivals (P = 0.02). Elevated neutrophil counts were also associated with poorer cancer-specific survival (P = 0.02). Analysis of patient age revealed that patients > 65 years of age had a poorer cancer-specific survival (P = 0.04). A multivariate analysis demonstrated that infection was an independent predictor of poor survival (HR = 2.62, 95%CI: 1.26-5.45; P = 0.01) along with advanced clinical staging (HR = 2.63, 95%CI: 1.08-6.39; P = 0.03).
CONCLUSION: Infection and high neutrophil counts are associated with a poorer cancer-specific survival in colorectal cancer patients.
Core tip: Previous works show evidence of both antitumoral- and tumor tolerance-directed effects of bacterial infections. The development of immunotherapies has commenced despite the lack in understanding of the underlying mechanism, or of the cancer-specific survival effects, of bacterial infections. To examine the effect of bacterial infections on cancer-specific survival, a retrospective study of colorectal cancer patients was performed. The results indicate that bacterial infections, as well as the accompanying increase in neutrophil counts, are associated with poor cancer survival. As a result, greater attention should be paid to treatment of infections incurred during or after cancer treatment.
- Citation: Attiê R, Chinen LTD, Yoshioka EM, Silva MCF, de Lima VCC. Acute bacterial infection negatively impacts cancer specific survival of colorectal cancer patients. World J Gastroenterol 2014; 20(38): 13930-13935
- URL: https://www.wjgnet.com/1007-9327/full/v20/i38/13930.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i38.13930
Chronic infections contributed to approximately 2 million of the 12.7 million new cases of cancer in 2008[1]. Furthermore, infections are associated with a higher rate of cancer mortality, such as with the human papillomavirus in cervical cancer and with Helicobacter pylori (H. pylori) in stomach cancer. Inflammation, one of the principal components of chronic infections, was first linked to cancer by Rudolf Virchow in 1863, a link that has been supported by further understanding of inflammatory mechanisms in malignant tissue[2]. However, beneficial effects of inflammation have been implicated, as well. Busch et al had treated cancer patients with bacterial extracts of Streptococcus pyogenes, presuming that the resultant high fever would lead to the production of exotoxins, which can function as superantigens and trigger polyclonal expansion of T-cells[3]. There is evidence that tumor infiltration by T-lymphocytes is associated with a better prognosis, though the composition and architecture of the cellular infiltrate may influence the clinical outcome[4].
Since the first reports of bacteria use in cancer treatments in the 19th century, therapeutic effects of bacterial infections have been observed in neoplasias[5]. The capacity of these pathogens to overcome tumor-induced immune tolerance has led to the development of a variety of immunotherapies, including the use of viruses and bacterial vectors, such as Bacillus Calmette-Guérin, Salmonella, Listeria monocytogenes and Streptococcus[6]. Despite the evidence that bacterial infections can contribute to the resolution of neoplasms, the mechanisms involved remain unclear, and nothing is known about their influence on cancer-specific survivals. Therefore, the purpose of this study was to examine the impact of bacterial infections on cancer survival in a cohort of colorectal cancer patients. In addition, the number of infections and neutrophil counts were assessed with respect to cancer-specific survival.
Two cohorts of colorectal cancer patients treated at the A.C. Camargo Cancer Center between January 2006 and April 2010 were retrospectively examined. All cases of infection occurring within this period, as registered in the data bank of the Hospital Infection Control Service, were included. Controls were identified from a chart survey of cancer patients that were hospitalized for other non-infectious reasons, such as surgery, chemotherapy and deep venous thrombosis, among others. This study was approved by the local ethics committee.
All patients with colorectal cancer identified using the International Disease Code designations C18 (colon) and C20 (rectum) that had been hospitalized for cancer treatment (chemotherapy, radiotherapy or surgery) or for treatment of concomitant complications up to one year after the end of cancer-specific treatment were included in the study. Patients with bacterial infections suspected from elevated white cell count and fever, confirmed by isolation of a bacterial agent, or determined by the physician or health care team based on symptoms, signs and lab results (elevated C-reactive protein or pro-calcitonin) were assigned to the infected group. Results from bacteriological analysis were searched for any biological material collected (blood, urine, sputum, liquor, etc.). The control group consisted of patients hospitalized for cancer-specific treatment, without any history or evidence of infection and negative bacteriological test results.
The following were criteria for exclusion: chronically immunosuppressed patients, such as transplant patients (due to the use of corticosteroids or cyclosporine); human immunodeficiency virus carriers; use of any immunosuppressive drug on a chronic basis; diagnosed autoimmune disease or primary immune deficiency; known viral or parasitic infection. Patients with an infection but treated in the ambulatory setting were also excluded due to the difficulties with collection and tracking of corresponding details. Patients hospitalized for pulmonary thromboembolism, stroke, acute myocardial infarction, uncontrolled diabetes, malignant hypercalcemia or other serious non-infectious complications not directly related to infection were also excluded.
Microsoft Excel 2007 and R software (R Development Core Team 2011; http://www.r-project.org) were used for all analyses. Cancer-specific survival was defined as the time between cancer diagnosis and death by cancer. Patients dying from other causes or lost to follow-up were censored for analyses. Survival curves were plotted using the Kaplan-Meier method. Log-rank tests were used to compare survival according to the variables of interest. All variables that achieved a P≤ 0.2 in univariate analyses were included for a multivariate analysis using a Cox test assuming a proportional hazards model. The variables were removed from the analysis in descending order according to the descriptive level, until all remaining variables were significant (indicated by a P < 0.05). The supposition of proportional hazard was verified using graphs of ln[s (t)], where s indicates estimated survival, and t represents time. The association between infection and neutrophilia was determined by a χ2 test.
One-hundred and six patients were included in the analyses and divided into infected (n = 58) and control (n = 48) groups (Table 1). Patient ages ranged from 23 to 91 years, with a median of 55 years. The majority of patients were male (57/106, 53.77%) with stage III disease (45/106, 44.11%). A total of 86 bacteriologic events were recorded (Table 2).
| Infection | Total | P value | ||
| No | Yes | |||
| Gender | ||||
| F | 26 (54) | 23 (40) | 49 (46.23) | 0.1358 |
| M | 22 (46) | 35 (60) | 57 (53.77) | |
| Age | ||||
| < 65 | 36 (80) | 37 (65) | 73 (71.57) | 0.0935 |
| ≥ 65 | 9 (20) | 20 (35) | 29 (28.43) | |
| Stage | ||||
| I | 2 (4) | 7 (12) | 9 (8.65) | 0.2875 |
| II | 12 (25) | 12 (21) | 24 (23.00) | |
| III | 24 (50) | 21 (38) | 45 (44.11) | |
| IV | 10 (21) | 16 (29) | 26 (24.24) | |
| Treatment | ||||
| Adjuvant | 27 (69) | 24 (48) | 51 (48.11) | 0.0528 |
| Adjuvant + neoadjuvant | 9 (23) | 13 (26) | 22 (20.75) | |
| Surgery | 3 (8) | 13 (26) | 16 (15.10) | |
| Others | - | - | 17 (16.04) | |
| Metastasis | ||||
| Não | 32 (71) | 44 (76) | 76 (73.78) | 0.5866 |
| Sim | 13 (29) | 14 (24) | 27 (26.22) | |
| T stage | ||||
| 1 + 2 | 6 (14) | 11 (20) | 17(17.70) | 0.4385 |
| 3 + 4 | 36 (86) | 43 (80) | 79 (82.30) | |
| Lymph node involvement | ||||
| 0 | 17 (41) | 21 (38) | 38 (39.17) | 0.6928 |
| 1 + 2 | 24 (59) | 35 (62) | 59 (60.83) | |
| Bacteria (etiological agents) | Classification | Diagnosis of Colorectal Cancer |
| Staphylococcus aureus (S. aureus) | Gram-positive | 15 |
| Escherichia coli | Gram-negative | 11 |
| Pseudomonas aeruginosa (P. aeruginosa) | Gram-negative | 10 |
| Staphylococcus epidermidis | Gram-positive | 6 |
| Enterobacter cloacae | Gram-negative | 7 |
| Klebsiella pneumoniae | Gram-negative | 6 |
| Serratia marcescens | Gram-negative | 1 |
| Enterobacter aerogenes | Gram-negative | 4 |
| Proteus mirabilis | Gram-negative | 1 |
| Acinetobacter baumannii | Gram-negative | 3 |
| Coagulase negative Staphylococcus | Gram-negative | 3 |
| Enterococcus faecalis | Gram-positive | 1 |
| E. faecium | Gram-positive | 2 |
| Citrobacter freundii | Gram-negative | 2 |
| Enterobacter cloacae + P. aeruginosa | Gram-negative | 1 |
| Staphylococcus lugdunensis | Gram-positive | 2 |
| Klebsiella pneumoniae + | Gram-negative | 1 |
| Escherichia coli | ||
| S. aureus + Klebsiela | Gram-positive and | 1 |
| Gram-negative | ||
| Pseudomonas aeruginosa + S. aureus | Gram-positive and | 1 |
| Gram-negative | ||
| Citrobacter amalonaticus | Gram-negative | 1 |
| Enterococcus avium | Gram-positive | 1 |
| Serratia odorifera | Gram-negative | 1 |
| Klebsiella pneumoniae + Pseudomonas aeruginosa | Gram-negative | 1 |
| P. aeruginosa + Klebsiella pneumoniae | Gram-negative | 1 |
| Staphylococcus saprophyticus | Gram-positive | 1 |
| Staphylococcus sp | Gram-positive | 1 |
| Streptococcus viridans | Gram-positive | 1 |
| Total | 86 |
The median follow-up for colorectal cancer patients was 31.5 mo. The risk of death significantly increased with the presence and number of infections (P = 0.02) (Figure 1A and B). The risk of death was also higher in patients who developed neutrophilia (P = 0.02) (Figure 1C). Moreover, neutrophilia was associated with the number of infections (P = 0.01), developing in 26.51% (22/83) of patients with only one infection and in 55.56% (15/27) of those with two or more infections. As these patients in general had infections in more than one place, the sites were evaluated separately. The most common infection sites were surgical wounds, the urinary tract, and abdominal cavity. There was no difference in survival with respect to the location of the infection. Survival time was also analyzed according to disease stage. As expected, patients with more advanced stages (III, IV) had a worse survival compared to those with an earlier stage (I, II) cancer. Moreover, advanced tumor stages (T3, T4) were associated with a shorter colorectal cancer survival (P = 0.05).
Results of a multivariate analysis indicated that variable treatment did not influence survival time of colorectal cancer patients. However, presence of infection and cancer staging were identified as independent prognostic factors for colorectal cancer (P < 0.05) (Table 3). The risk of death in patients was almost three-fold higher in patients with bacterial infections or advanced cancer stages (III, IV).
| Variabel | Category | HR | 95%CI | P value |
| Infecion | no | 1 | - | |
| yes | 2.62 | 1.26-5.45 | 0.01 | |
| Stage | I + II | 1 | - | |
| III + IV | 2.63 | 1.08-6.39 | 0.03 |
Finally, overall survival was analyzed according to patient age to account for a possible bias in the results due to immunosenescence. Immunosenescence is an age-related dysfunction of the immune system in which there is a decline in the efficiency of vaccines and reduced immune protection[7]. Colorectal cancer patients older than 65 years had a poorer cancer-specific survival compared to patients younger than 65 years (P = 0.04) (Figure 1D).
The present study demonstrates that the occurrence of infection during or after the end of treatment is associated with a poorer colorectal cancer survival. These results corroborate findings from previous animal studies, such as the tumor tolerance-directed, rather than antitumor-directed, adaptive immune response induced by H. pylori[8]. However, Bohman et al[9] presented a retrospective review of 18 glioblastoma patients from a single institution with postoperative infections over a 10-year period. These patients did not differ from an age- and treatment-matched group of 51 glioblastoma patients, though other prognostic factors, such as Karnofsky Performance Scale status and type of chemotherapy, were not considered. Thus, although targeted immunotherapy may provide antitumoral effects, simple infection does not appear to do so.
Another finding of the current study is that individuals with two or more infections had poorer cancer-specific survival. Although there are no reports from experimental animal models testing this hypothesis, we can speculate that individuals that develop more than one infection have an already disturbed immune system. On the other hand, a greater number of infections could also result in the release of a larger amount of inflammatory mediators that may favor tumor growth and progression.
We initially anticipated a higher frequency of neutropenia in our patients, but conversely, observed neutrophilia, which was associated with a poorer cancer-specific survival. Mueller and Fusenig showed that inflammatory cells, mainly neutrophils, contribute to angiogenesis and promote tumor growth; thus, patients with neutrophilia had a poorer survival[10]. Although the literature suggests a protective role of neutrophils in cancer, tumor-associated neutrophils have been found to foster malignancy in certain situations, through the liberation of growth stimulating signals, proteases that degrade the matrix, and mediators of angiogenesis[11,12].
The results of the present study also demonstrate that increasing age impacts colorectal cancer survival. Indeed, Akbar and Henson reported that senescence in human T-cells may limit long-term specific immune responses[13]. In this way, persistent viruses may induce senescence in specific T-cell populations stimulated to repeatedly proliferate over the patient’s lifetime, evidenced by immunosenescence acceleration via restriction of the T-cell repertoire induced by cytomegalovirus infection[7].
In conclusion, this study demonstrates that rather that producing a protective effect, infection is an independent predictor of poor colorectal cancer survival. Therefore, the follow-up of colorectal cancer patients should include infection-directed therapies.
We would like to thank Dr. Marcello Ferretti Fanelli for allowing access to the Oncology Department registry, and to Dr. Ivan Leonardo Avelino França e Silva for allowing us to search the Hospital Infection Control Service data bank. We would also like to thank Aline Santos Damascena for review of all the statistical analyses.
An important question remains among physicians regarding the influence of infection in cancer-specific survival. There is some evidence that infections exert antitumoral effects, whereas other studies have indicated a poorer prognosis for cancer mortality in relation to infection.
There is evidence that bacterial infections can contribute to resolution of neoplasms, but the mechanisms involved are still unclear, and nothing is known about their influence on cancer-specific survival. The purpose of this study was to examine the impact of bacterial infections on colorectal cancer survival.
Although many physicians believe that infection can improve cancer-specific survival through immune system activation, the results of this study on colorectal cancer patients indicate that infection has a negative impact.
This study documents the influence of bacterial infection during the treatment course for colorectal cancer. As the results indicate a poorer prognosis, special attention is necessary for those patients who develop infection during chemotherapy treatment.
Immunosenescence is an age-related gradual deterioration of the immune system. Cancer-specific survival is defined as the time between cancer diagnosis and death by cancer.
The authors of this study analyzed cancer-specific survival with regard to bacterial infections among patients receiving treatment for colorectal cancer. The results indicate that bacterial infections reduce survival, and should therefore be promptly and effectively managed in these patients.
P- Reviewer: de la Cadena MP, Kucherlapati MH, Zhong WX S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5765] [Article Influence: 240.2] [Reference Citation Analysis (0)] |
| 2. | de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1719] [Article Influence: 132.2] [Reference Citation Analysis (1)] |
| 3. | Hobohm U. Fever therapy revisited. Br J Cancer. 2005;92:421-425. [PubMed] |
| 4. | Rahir G, Moser M. Tumor microenvironment and lymphocyte infiltration. Cancer Immunol Immunother. 2012;61:751-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Zhao M, Geller J, Ma H, Yang M, Penman S, Hoffman RM. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Natl Acad Sci USA. 2007;104:10170-10174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 199] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 6. | Paterson Y, Guirnalda PD, Wood LM. Listeria and Salmonella bacterial vectors of tumor-associated antigens for cancer immunotherapy. Semin Immunol. 2010;22:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Mekker A, Tchang VS, Haeberli L, Oxenius A, Trkola A, Karrer U. Immune senescence: relative contributions of age and cytomegalovirus infection. PLoS Pathog. 2012;8:e1002850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Arnold IC, Hitzler I, Müller A. The immunomodulatory properties of Helicobacter pylori confer protection against allergic and chronic inflammatory disorders. Front Cell Infect Microbiol. 2012;2:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Bohman LE, Gallardo J, Hankinson TC, Waziri AE, Mandigo CE, McKhann GM, Sisti MB, Canoll P, Bruce JN. The survival impact of postoperative infection in patients with glioblastoma multiforme. Neurosurgery. 2009;64:828-34; discussion 834-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1323] [Cited by in RCA: 1315] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 11. | Brú A, Souto JC, Alcolea S, Antón R, Remacha A, Camacho M, Soler M, Brú I, Porres A, Vila L. Tumour cell lines HT-29 and FaDu produce proinflammatory cytokines and activate neutrophils in vitro: possible applications for neutrophil-based antitumour treatment. Mediators Inflamm. 2009;2009:817498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol. 2012;82:296-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 271] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 13. | Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol. 2011;11:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 340] [Article Influence: 24.3] [Reference Citation Analysis (0)] |