Published online Oct 14, 2014. doi: 10.3748/wjg.v20.i38.13904
Revised: May 21, 2014
Accepted: June 14, 2014
Published online: October 14, 2014
Processing time: 234 Days and 12.3 Hours
Gastric sleeve gastrectomy has become a frequent bariatric procedure. Its apparent simplicity hides a number of serious, sometimes fatal, complications. This is more important in the absence of an internationally adopted algorithm for the management of the leaks complicating this operation. The debates exist even regarding the definition of a leak, with several classification systems that can be used to predict the cause of the leak, and also to determine the treatment plan. Causes of leak are classified as mechanical, technical and ischemic causes. After defining the possible causes, authors went into suggesting a number of preventive measures to decrease the leak rate, including gentle handling of tissues, staple line reinforcement, larger bougie size and routine use of methylene blue test per operatively. In our review, we noticed that the most important clinical sign or symptom in patients with gastric leaks are fever and tachycardia, which mandate the use of an abdominal computed tomography, associated with an upper gastrointrstinal series and/or gastroscopy if no leak was detected. After diagnosis, the management of leak depends mainly on the clinical condition of the patient and the onset time of leak. It varies between prompt surgical intervention in unstable patients and conservative management in stable ones in whom leaks present lately. The management options include also endoscopic interventions with closure techniques or more commonly exclusion techniques with an endoprosthesis. The aim of this review was to highlight the causes and thus the prevention modalities and find a standardized algorithm to deal with gastric leaks post sleeve gastrectomy.
Core tip: Gastric leak is one of the most feared complications after a sleeve gastrectomy. Routine oversewing of the staple line decreases the hemorrhagic complications but may not decrease the leak rate. Fever and tachycardia are the two most important clinical factors in the detection of gastric leaks and should never be neglected. The treatment modality should be based on the clinical status of the patient and the timing of the leak. Complete endoscopic approach via natural orifices transluminal endoscopic surgery, diversion using a stent and closure with glue or clips is a reasonable option in selected patients and specialized centers.
- Citation: Abou Rached A, Basile M, El Masri H. Gastric leaks post sleeve gastrectomy: Review of its prevention and management. World J Gastroenterol 2014; 20(38): 13904-13910
- URL: https://www.wjgnet.com/1007-9327/full/v20/i38/13904.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i38.13904
Laparoscopic sleeve gastrectomy (LSG) is a surgical approach to treat morbid obesity. It restricts the stomach’s size to induce satiety and resects fundal ghrelin-producing cells to decrease appetite[1,2].
LSG has become a very frequent procedure in bariatric surgery, due to its simplicity and efficacy compared to the gastric bypass procedure[3,4].
The fact that this technique has erroneously been considered simple and easy has led to its adoption by a large number of surgeons. Compared to gastric bypass and biliopancreatic diversion, its complications can be even more severe[5]. Staple line leaks, bleeding, and strictures are the commonly reported complications following LSG. Based on the data of 12799 LSGS, the International Sleeve Gastrectomy Expert Panel Consensus Statement 2011, the leak rate was 1.06%[4], but the leak rate can vary between 1% and 3% for primary procedure[6] and more than 10% in revision procedures[7-9].
According to the United Kingdom Surgical Infection Study Group, a gastric leak was defined as “the leak of luminal contents from a surgical join between two hollow viscera”. It can also be an effluent of gastrointestinal content through a suture line, which may collect near the anastomosis, or exit through the wall or the drain[10].
Leaks can be classified based either on the time of onset, clinical presentation, site of leak, radiological appearance, or mixed factors (Table 1).
| Number of patients | Proximal third | Mid-third | Distal third | Posterior wall | Not located | |
| Mui et al[64], 2008 | 70 patients, 1 leak (1.42) | 1 (100) | ||||
| Burgos et al[18], 2009 | 214 patients, 7 leaks (3.3) | 6 (85.6) | 1 (14.3) | |||
| Csendes et al[16], 2010 | 334 patients, 16 leaks (4.66) | 14 (87.5) | 2 (12.5) | |||
| Sakran et al[14], 2013 | 2834 patients, 44 leaks (1.5) | 33 (75) | 3 (6.8) | 3 (6.8) | 2 (4.5) | 3 (6.8) |
Csendes et al[11] defined early, intermediate and late leaks as those appearing 1 to 4, 5 to 9 and 10 or more days following surgery respectively. By clinical relevance and extent of dissemination, they defined type I or subclinical leaks as those that are well localized without dissemination into the pleural or abdominal cavity, nor inducement of systemic clinical manifestations, usually they are easy to treat medically. Type II are leaks with dissemination into abdominal or pleural cavity, or the drains with consequent severe and systemic clinical manifestations.
Based on both clinical and radiological findings, type A are microperforations without clinical or radiographic evidence of leak, while type B are leaks detected by radiological studies but without any clinical finding, and finally, type C are leaks presenting with both radiological and clinical evidence[12].
Gastric leaks can be due to mechanical or ischemic causes. According to Baker et al[13] stapler misfiring, or direct tissular injury are categorized as “mechanical-tissular” causes and usually appear within 2 d of surgery (early), compared to the “ischemic causes” that usually appear on day 5-6 post operatively (post op) (intermediate).
In a multicenter experience with 2834 patients, leaks post LSG included improper vascularization due to an aggressive dissection especially of the posterior attachments of the upper sleeve, thermal injuries to the gastric tube by ultrasonic devices (harmonic, Ligasure), stapler devices misfiring, stapling of the orogastric tube[14].
Patients with distal stenosis are more likely to have proximal leaks, because of gastric emptying impairment leading to increased intraluminal pressure and decreased compliance of the gastric tube[15,16].
Other mechanisms concerning gastric leak post sleeve are still obscure, with a case report that presented 16 mo after surgery[17].
Some authors advocate gentle handling of tissues when using ultrasonic devices and staplers, avoiding distal stenosis[16,18], in addition to sustaining steady compression on the staple device before firing to washout the fluids from the tissues[13].
Does staple line reinforcement or buttressing prevent or decrease the leak rate is a question that was addressed in several studies, some of them concluded that reinforcement with oversewing decreases the leakage rate[19], some of these authors were based on retrospective, non-controlled studies[20], others recommend the use of PSD (Peri Strips Dry, a bovine pericardium with collagen matrix) to decrease the leakage rate[21-23]. Ser et al[24] recommend oversewing despite their admission that their high leakage rate in their first cases (40 cases out of 118) is probably attributed to the learning curve.
Fibrin sealants (Tissucol) were also addressed in some studies with good impact in term of decreasing leakage rate[25,26].
Large randomized prospective trials and a recent meta-analysis showed no significant difference between reinforcement (by oversewing or Seamguard) and without reinforcement in term of leakage rate[27,28], other large series reported no leakage without any reinforcement[29], most of the others agree that reinforcement decreases the complication rate in term of bleeding but not in term of leak[30,31].
Based on what we mentioned previously concerning the impact of increased intraluminal pressure on the gastric leak formation[14,16], Márquez et al[5] leave the nasogastric tube in place for 24 h post op to decrease intraluminal pressure.
The size of the bougie to be used for calibration is also a subject of controversies, with bougie size ranging between 32 and 60 fr[32], with the rational that using smaller sizes leads to better outcome in term of sustained excessive weight loss[33], but on the other hand a large systematic review taking 4888 patients and another large meta-analysis of 9991 patients suggest that larger bougie size may decrease the leak rate, but further randomized studies are needed to assure the exact effect of the bougie size on the leak rate[14,16,25].
Based on the fact that operative detection of a technically induced staple line defect can be treated with prompt closure, other modalities are adopted by a wide number of authors and surgeons including the routine use of methylene blue test during surgery for detection of leaks, with high sensitivity and specificity[34-36], intraoperative endoscopy or air leak test[37,38]. But we should keep in consideration that a negative intraoperative methylene blue test does not eliminate the presence of a leak[14,39].
The use of closed suction drain routinely near the staple line, despite that it is performed by the majority of surgeons, may not detect leak, and may not be helpful also in the drainage of the collection[31,32].
The cornerstones in the revisional bariatric surgery include clear identification of the existing anatomy necessitating extensive dissection and adhesiolysis, taking in consideration the stomach wall thickness due to fibrosis or edema, pushing the surgeon to appropriately choose the size of stapler’s height (usually 4.5 mm), oversewing of the gastric tube at the level of the staple line, leak test, and gastrostomy tube[40].
Based on the increased incidence of leakage in the revision surgeries, some authors advocate two steps procedure, gastric band removal followed by the sleeve with a delay between 6 to 12 wk[41,42].
Debates still exist on which diagnostic modality is the most sensitive and specific concerning the diagnosis of a post sleeve gastrectomy leakage, but all of them agree that early detection is associated with better outcome, and that a high index of suspicion is the cornerstone in the detection and diagnosis of leaks[14,43,44].
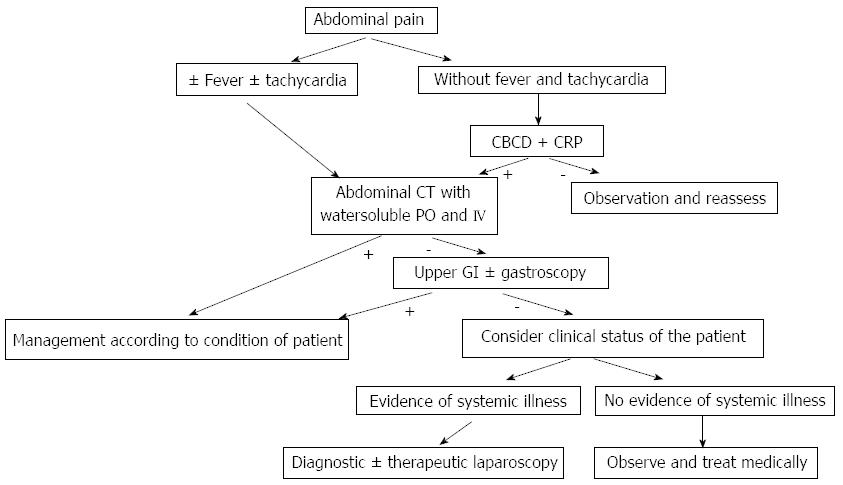
Clinical presentation can vary widely between totally asymptomatic patients diagnosed with routine imaging studies (upper gastrointrstinal series¡) post op[36], that are considered type A as mentioned previously[12], to the signs and symptoms of a septic shock including fever, abdominal pain, peritonitis, leucocytosis, tachycardia, hypotension[12] (Figure 1).
Unexplained fever and tachycardia post op should raise the index of suspicion of a possible complication and push the surgeon to perform further radiological investigations to R/O the presence of leak[16].
As for Csendes et al[16] and Dakwar et al[17], fever is the most important clinical factor in the diagnosis of gastric leak post sleeve gastrectomy.
Others agree that tachycardia is the earliest[18], and most important and constant clinical finding indicating the presence of a gastric leak[45], and a tachycardia above 120 beat/min is a powerful indicator of leak and systemic compromise[46].
Early leaks usually present with sudden abdominal pain, accompanied with fever and tachycardia in most cases, while late leaks tend to present with insidious abdominal pain commonly associated with fever[31].
Laboratory studies including CBCD, CRP are neither sensitive nor specific, and they rarely contribute to make a diagnosis[14].
Computed tomography (CT) of the abdomen with IV and PO water soluble contrast is considered as a part of the diagnostic workup of patients with suspected leak, with the presence of abdominal collection or free fluid, extravasation of contrast into the abdominal cavity or the drain tube, or persistent pneumoperitoneum as diagnostic findings of leak or fistula[47].
CT is considered to be the best non-invasive modality for detection and confirmation of a gastric leak[16,31,48]. These results are also supported in another multicenter experience showing that CT had the highest detection rate of gastric leaks in up to 86% of patients[14].
This superiority of CT scan over other invasive and non-invasive modalities is questioned by some investigators, lying on the fact that obesity and large body dimensions [body mass index (BMI) over 50] produce artefacts that reduce the image quality, added to the technical difficulties imposed by the large body weight and dimensions that may overcome the ability of the framework to support and thus they recommend upper gastrointestinal (UGI) radiography and endoscopy instead[49].
Now concerning the routine performance of the gastrografin swallow test 24-72 h post op, it is still an area of large debate. While a large recent retrospective review of 712 patient conducted by Wahby et al[36] have shown its inability to detect post op leakage, they still recommend it to be done routinely, especially that it can detect other complications like strictures and anatomical consequences of the sleeve[18,47,50], but at the same time, they recommend to do routine methylene blue test per operatively as mentioned previously.
Be aware, knowing that a normal test cannot rule out a fistula, it contrarily can have deleterious effects on the delay of diagnosis when a “normal test” covers a leakage[41,51].
Even in the setting of positive diagnosis with CT scan of a leak, an upper gastrointestinal gastrografin swallow is of great importance to identify the magnitude and the level of the leak[31].
One study mentioned that measuring the amylase level in the drain, when in doubt, has a high level of sensitivity and specificity for detecting fistula post gastric bypass surgery, but it was abandoned because it mandates leaving an unnecessary drain in place for more than 7-10 d at least so that an early or intermediate fistula can be diagnosed[52].
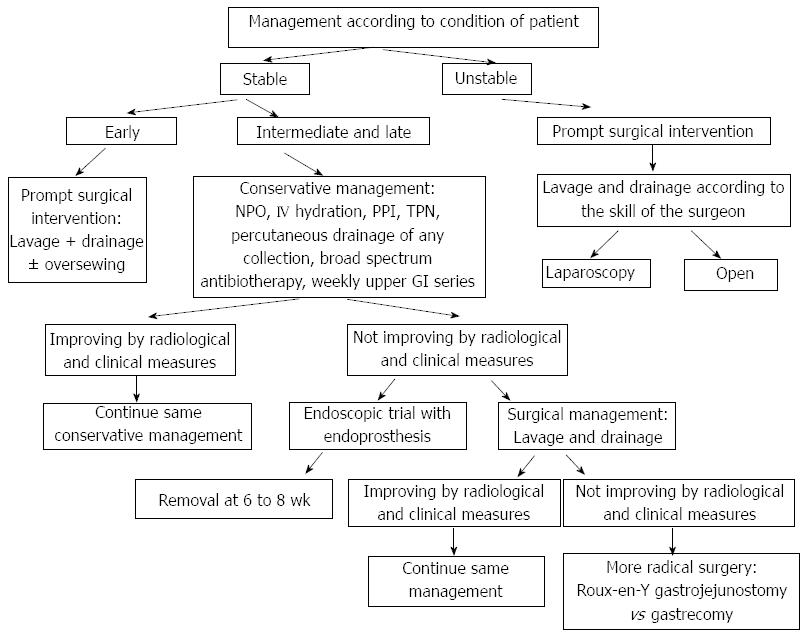
The management of leak post sleeve gastrectomy imposes a lot of controversies and difficulties in the adoption of a standard algorithm, due to the paucity of prospective randomized trials that are considered unethical to perform in this situation (Figure 2).
Based on the First International Summit for Sleeve Gastrectomy, the treatment may include early oversewing, drainage (open or laparoscopic), endoscopic clipping, stenting or using fibrin glue, sometimes the use of a Roux limb or total gastrectomy as the last resort[34].
The adoption of Csendes et al[16] model of classification for gastric fistula post sleeve may constitute the first step in the establishment of such an algorithm or protocol, based on 3 characteristics: Time of appearance (early, intermediate and late); Location (proximal, mid or distal gastric); Severity or magnitude (type I and II).
Unstable patient upon presentation justify prompt surgical intervention by laparoscopic or open means for washout and drainage at least, that may be coupled with debridement and suturing of the orifice if the condition of the patient and the tissues, and the skills and experience of the surgeon permit[18,36].
Immediate surgical intervention with washout drainage and suture if the tissue was in an early stage of inflammation in patients with early leaks showed better outcome than the more conservative approach. While the adoption of a more conservative approach for intermediate and late leaks in clinically stable patients is more reasonable with adequate hydration, proton pump inhibitors, nil per os, nutritional support, percutaneous drainage of any collection and broad spectrum antibiotherapy[16,18,27,36], with a follow up weekly by upper gastrointrstinal series to ensure healing, when any concern about healing, more invasive approaches may be considered.
Most patients who underwent suturing for their fistula failed to close directly due either to persistence of the leak (orifice cannot be identified clearly) or failure of the suture (inflamed and friable tissue)[13], especially after the third day post op[16]. For that, a simultaneous endoscopic intervention and insertion of a guide wire (rendez-vous) from the stomach lumen so that the surgeon can identify clearly the gastric defect to suture it can be used[36].
If the leak does not heal after several weeks, usually 2 wk, endoscopic management can be considered with wide range of success rate according to the studies[53,54].
Closure techniques: (1) Endoclips were used initially for hemostasis, later on trials to treat esophageal, colonic and duodenal mucosal defects and perforations were extrapolated to be used in post sleeve gastrectomy leakage[55], now the new over the scope clips (OTSC) have more promising results, but they are limited for very small mucosal defects and microperforations[56], and are inefficacious in inflammatory or edematous mucosa, demanding technical skills; and (2) Sealant materials including fibrin glue and cyanoacrylates. Fibrin glue acts by dual effect, as a plug directly occluding the defect and as a fibroblast promoter to enhance wound healing, thus it is absorbed after 4 wk and replaced by connective scar tissue[57,58].
Exclusion techniques - endoprosthesis (stents): Initially stents were used to treat stenosis, it was shown that they decrease the intraluminal pressure, which may be part of the pathophysiology of the gastric leak post sleeve as mentioned above[14], so its use gained a widespread in the management of proximal and middle gastric leak[51] due to the advantage of the ability to resume per os feeding and discharge the patient home, but the migration index is high, reaching 30%, with the same rate when comparing self-expanding metallic stents (SEMS) and self-expanding polyester stents (SEPS)[59].
Nguyen et al[54] used self-expanding stents with a success rate of 100%. The gastric sleeve leaks usually take more than 6 wk to heal (average of 45 d) compared to healing time in laparoscopic Roux-En-Y gastric bypass leaks (30 d)[16], at the same time keeping the stents for long periods risks to damage the underlying mucosa, especially with uncovered stents, ideally most authors agree that 6-8 wk is the optimal removal time, but these prosthesis should be observed closely with the possibility to remove them after 4 wk[14,54,60].
A complete endoscopic approach was also suggested by Bège et al[61], without the need for any surgical intervention that consists of 3 stages of endoscopic treatment: Washout and drainage using natural orifices transluminal endoscopic surgery (NOTES); Diversion using a stent; Closure with glue or clips.
Patients who fail all these measures, need a definitive surgical intervention with more aggressive and radical treatment, including either conversion to gastric bypass, or a Roux-En-Y with a jejunal limb oversewn over the fistula, or finally in some cases a total gastrectomy with esojejunal anastomosis[37,62,63].
LSG has now gained a wide spread among surgeons; its apparent simplicity to perform hides a number of tricks and pitfalls to avoid, and a number of general principles to stick to.
The most dreadful complication of this apparently simple procedure is gastric leak, which implies a long hospital stay, morbidities and sometime mortalities. Its management is variable, with no standard algorithm to follow, but most of the data demonstrates that the management should be planned based on the clinical evaluation, time of diagnosis and finally the location of the leak.
On the other hand and in the absence of a clear approach and guidelines for the management of gastric leaks post sleeve gastrectomy, appears the importance of prevention, by the simple adherence to general surgical principles, and the particular considerations of sleeve gastrectomies.
P- Reviewer: Koch TR, Lirici MM, Mickevicius A S- Editor: Ding Y L- Editor: A E- Editor: Du P
| 1. | Serra C, Pérez N, Bou R, Bengochea M, Martínez R, Baltasar A. Laparoscopic sleeve gastrectomy. A bariatric procedure with multiple indications. Cir Esp. 2006;79:289-292. [PubMed] |
| 2. | Marceau P, Cabanac M, Frankham PC, Hould FS, Lebel S, Marceau S, Lescelleur O, Biron S. Accelerated satiation after duodenal switch. Surg Obes Relat Dis. 2005;1:408-412. [PubMed] |
| 3. | Msika S, Castel B. Present indications for surgical treatment of morbid obesity: how to choose the best operation? J Visc Surg. 2010;147:e47-e51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Rosenthal RJ, Diaz AA, Arvidsson D, Baker RS, Basso N, Bellanger D, Boza C, El Mourad H, France M, Gagner M. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of > 12,000 cases. Surg Obes Relat Dis. 2012;8:8-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 712] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 5. | Márquez MF, Ayza MF, Lozano RB, Morales Mdel M, Díez JM, Poujoulet RB. Gastric leak after laparoscopic sleeve gastrectomy. Obes Surg. 2010;20:1306-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Fuks D, Verhaeghe P, Brehant O, Sabbagh C, Dumont F, Riboulot M, Delcenserie R, Regimbeau JM. Results of laparoscopic sleeve gastrectomy: a prospective study in 135 patients with morbid obesity. Surgery. 2009;145:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Lacy A, Ibarzabal A, Pando E, Adelsdorfer C, Delitala A, Corcelles R, Delgado S, Vidal J. Revisional surgery after sleeve gastrectomy. Surg Laparosc Endosc Percutan Tech. 2010;20:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Foletto M, Prevedello L, Bernante P, Luca B, Vettor R, Francini-Pesenti F, Scarda A, Brocadello F, Motter M, Famengo S. Sleeve gastrectomy as revisional procedure for failed gastric banding or gastroplasty. Surg Obes Relat Dis. 2010;6:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Iannelli A, Schneck AS, Ragot E, Liagre A, Anduze Y, Msika S, Gugenheim J. Laparoscopic sleeve gastrectomy as revisional procedure for failed gastric banding and vertical banded gastroplasty. Obes Surg. 2009;19:1216-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Peel AL, Taylor EW. Proposed definitions for the audit of postoperative infection: a discussion paper. Surgical Infection Study Group. Ann R Coll Surg Engl. 1991;73:385-388. [PubMed] |
| 11. | Csendes A, Burdiles P, Burgos AM, Maluenda F, Diaz JC. Conservative management of anastomotic leaks after 557 open gastric bypasses. Obes Surg. 2005;15:1252-1256. [PubMed] |
| 12. | Welsch T, von Frankenberg M, Schmidt J, Büchler MW. Diagnosis and definition of anastomotic leakage from the surgeon’s perspective. Chirurg. 2011;82:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Baker RS, Foote J, Kemmeter P, Brady R, Vroegop T, Serveld M. The science of stapling and leaks. Obes Surg. 2004;14:1290-1298. [PubMed] |
| 14. | Sakran N, Goitein D, Raziel A, Keidar A, Beglaibter N, Grinbaum R, Matter I, Alfici R, Mahajna A, Waksman I. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc. 2013;27:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 285] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 15. | Yehoshua RT, Eidelman LA, Stein M, Fichman S, Mazor A, Chen J, Bernstine H, Singer P, Dickman R, Beglaibter N. Laparoscopic sleeve gastrectomy--volume and pressure assessment. Obes Surg. 2008;18:1083-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 277] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 16. | Csendes A, Braghetto I, León P, Burgos AM. Management of leaks after laparoscopic sleeve gastrectomy in patients with obesity. J Gastrointest Surg. 2010;14:1343-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 17. | Dakwar A, Assalia A, Khamaysi I, Kluger Y, Mahajna A. Late complication of laparoscopic sleeve gastrectomy. Case Rep Gastrointest Med. 2013;2013:136153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Burgos AM, Braghetto I, Csendes A, Maluenda F, Korn O, Yarmuch J, Gutierrez L. Gastric leak after laparoscopic-sleeve gastrectomy for obesity. Obes Surg. 2009;19:1672-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 19. | Baltasar A, Serra C, Pérez N, Bou R, Bengochea M, Ferri L. Laparoscopic sleeve gastrectomy: a multi-purpose bariatric operation. Obes Surg. 2005;15:1124-1128. [PubMed] |
| 20. | Angrisani L, Cutolo PP, Buchwald JN, McGlennon TW, Nosso G, Persico F, Capaldo B, Savastano S. Laparoscopic reinforced sleeve gastrectomy: early results and complications. Obes Surg. 2011;21:783-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Angrisani L, Lorenzo M, Borrelli V, Ciannella M, Bassi UA, Scarano P. The use of bovine pericardial strips on linear stapler to reduce extraluminal bleeding during laparoscopic gastric bypass: prospective randomized clinical trial. Obes Surg. 2004;14:1198-1202. [PubMed] |
| 22. | Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 569] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 23. | Daskalakis M, Berdan Y, Theodoridou S, Weigand G, Weiner RA. Impact of surgeon experience and buttress material on postoperative complications after laparoscopic sleeve gastrectomy. Surg Endosc. 2011;25:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Ser KH, Lee WJ, Lee YC, Chen JC, Su YH, Chen SC. Experience in laparoscopic sleeve gastrectomy for morbidly obese Taiwanese: staple-line reinforcement is important for preventing leakage. Surg Endosc. 2010;24:2253-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Sapala JA, Wood MH, Schuhknecht MP. Anastomotic leak prophylaxis using a vapor-heated fibrin sealant: report on 738 gastric bypass patients. Obes Surg. 2004;14:35-42. [PubMed] |
| 26. | Liu CD, Glantz GJ, Livingston EH. Fibrin glue as a sealant for high-risk anastomosis in surgery for morbid obesity. Obes Surg. 2003;13:45-48. [PubMed] |
| 27. | Dapri G, Cadière GB, Himpens J. Reinforcing the staple line during laparoscopic sleeve gastrectomy: prospective randomized clinical study comparing three different techniques. Obes Surg. 2010;20:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 28. | Parikh M, Issa R, McCrillis A, Saunders JK, Ude-Welcome A, Gagner M. Surgical strategies that may decrease leak after laparoscopic sleeve gastrectomy: a systematic review and meta-analysis of 9991 cases. Ann Surg. 2013;257:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 295] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 29. | Kasalicky M, Michalsky D, Housova J, Haluzik M, Housa D, Haluzikova D, Fried M. Laparoscopic sleeve gastrectomy without an over-sewing of the staple line. Obes Surg. 2008;18:1257-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Tan JT, Kariyawasam S, Wijeratne T, Chandraratna HS. Diagnosis and management of gastric leaks after laparoscopic sleeve gastrectomy for morbid obesity. Obes Surg. 2010;20:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 31. | Stamou KM, Menenakos E, Dardamanis D, Arabatzi C, Alevizos L, Albanopoulos K, Leandros E, Zografos G. Prospective comparative study of the efficacy of staple-line reinforcement in laparoscopic sleeve gastrectomy. Surg Endosc. 2011;25:3526-3530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Milone L, Strong V, Gagner M. Laparoscopic sleeve gastrectomy is superior to endoscopic intragastric balloon as a first stage procedure for super-obese patients (BMI & gt; or =50). Obes Surg. 2005;15:612-617. [PubMed] |
| 33. | Atkins ER, Preen DB, Jarman C, Cohen LD. Improved obesity reduction and co-morbidity resolution in patients treated with 40-French bougie versus 50-French bougie four years after laparoscopic sleeve gastrectomy. Analysis of 294 patients. Obes Surg. 2012;22:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | de Aretxabala X, Leon J, Wiedmaier G, Turu I, Ovalle C, Maluenda F, Gonzalez C, Humphrey J, Hurtado M, Benavides C. Gastric leak after sleeve gastrectomy: analysis of its management. Obes Surg. 2011;21:1232-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 35. | Albanopoulos K, Alevizos L, Linardoutsos D, Menenakos E, Stamou K, Vlachos K, Zografos G, Leandros E. Routine abdominal drains after laparoscopic sleeve gastrectomy: a retrospective review of 353 patients. Obes Surg. 2011;21:687-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Wahby M, Salama AF, Elezaby AF, Belgrami F, Abd Ellatif ME, El-Kaffas HF, Al-Katary M. Is routine postoperative gastrografin study needed after laparoscopic sleeve gastrectomy? Experience of 712 cases. Obes Surg. 2013;23:1711-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Deitel M, Crosby RD, Gagner M. The First International Consensus Summit for Sleeve Gastrectomy (SG), New York City, October 25-27, 2007. Obes Surg. 2008;18:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 266] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 38. | Gagner M, Deitel M, Kalberer TL, Erickson AL, Crosby RD. The Second International Consensus Summit for Sleeve Gastrectomy, March 19-21, 2009. Surg Obes Relat Dis. 2009;5:476-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 256] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 39. | Casella G, Soricelli E, Rizzello M, Trentino P, Fiocca F, Fantini A, Salvatori FM, Basso N. Nonsurgical treatment of staple line leaks after laparoscopic sleeve gastrectomy. Obes Surg. 2009;19:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 40. | Shimizu H, Annaberdyev S, Motamarry I, Kroh M, Schauer PR, Brethauer SA. Revisional bariatric surgery for unsuccessful weight loss and complications. Obes Surg. 2013;23:1766-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 41. | Acholonu E, McBean E, Court I, Bellorin O, Szomstein S, Rosenthal RJ. Safety and short-term outcomes of laparoscopic sleeve gastrectomy as a revisional approach for failed laparoscopic adjustable gastric banding in the treatment of morbid obesity. Obes Surg. 2009;19:1612-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Ramalingam G, Anton CK. Our 1-year experience in laparoscopic sleeve gastrectomy. Obes Surg. 2011;21:1828-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Dallal RM, Bailey L, Nahmias N. Back to basics--clinical diagnosis in bariatric surgery. Routine drains and upper GI series are unnecessary. Surg Endosc. 2007;21:2268-2271. [PubMed] |
| 44. | Bertucci W, White S, Yadegar J, Patel K, Han SH, Blocker O, Frickel D, Kadell B, Mehran A, Gracia C. Routine postoperative upper gastroesophageal imaging is unnecessary after laparoscopic Roux-en-Y gastric bypass. Am Surg. 2006;72:862-864. [PubMed] |
| 45. | Gonzalez R, Sarr MG, Smith CD, Baghai M, Kendrick M, Szomstein S, Rosenthal R, Murr MM. Diagnosis and contemporary management of anastomotic leaks after gastric bypass for obesity. J Am Coll Surg. 2007;204:47-55. [PubMed] |
| 46. | Hamilton EC, Sims TL, Hamilton TT, Mullican MA, Jones DB, Provost DA. Clinical predictors of leak after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Surg Endosc. 2003;17:679-684. [PubMed] |
| 47. | Ballesta C, Berindoague R, Cabrera M, Palau M, Gonzales M. Management of anastomotic leaks after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2008;18:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 48. | Aurora AR, Khaitan L, Saber AA. Sleeve gastrectomy and the risk of leak: a systematic analysis of 4,888 patients. Surg Endosc. 2012;26:1509-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 437] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 49. | Jurowich C, Thalheimer A, Seyfried F, Fein M, Bender G, Germer CT, Wichelmann C. Gastric leakage after sleeve gastrectomy-clinical presentation and therapeutic options. Langenbecks Arch Surg. 2011;396:981-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 50. | Gonzalez R, Nelson LG, Gallagher SF, Murr MM. Anastomotic leaks after laparoscopic gastric bypass. Obes Surg. 2004;14:1299-1307. [PubMed] |
| 51. | Goitein D, Goitein O, Feigin A, Zippel D, Papa M. Sleeve gastrectomy: radiologic patterns after surgery. Surg Endosc. 2009;23:1559-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | Maher JW, Bakhos W, Nahmias N, Wolfe LG, Meador JG, Baugh N, Kellum JM. Drain amylase levels are an adjunct in detection of gastrojejunostomy leaks after Roux-en-Y gastric bypass. J Am Coll Surg. 2009;208:881-84; discussion 881-84;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Conio M, Blanchi S, Repici A, Bastardini R, Marinari GM. Use of an over-the-scope clip for endoscopic sealing of a gastric fistula after sleeve gastrectomy. Endoscopy. 2010;42 Suppl 2:E71-E72. [PubMed] |
| 54. | Nguyen NT, Nguyen XM, Dholakia C. The use of endoscopic stent in management of leaks after sleeve gastrectomy. Obes Surg. 2010;20:1289-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 55. | Chuttani R, Barkun A, Carpenter S, Chotiprasidhi P, Ginsberg GG, Hussain N, Liu J, Silverman W, Taitelbaum G, Petersen B. Endoscopic clip application devices. Gastrointest Endosc. 2006;63:746-750. [PubMed] |
| 56. | Mennigen R, Colombo-Benkmann M, Senninger N, Laukoetter M. Endoscopic closure of postoperative gastrointestinal leakages and fistulas with the Over-the-Scope Clip (OTSC). J Gastrointest Surg. 2013;17:1058-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 57. | Petersen B, Barkun A, Carpenter S, Chotiprasidhi P, Chuttani R, Silverman W, Hussain N, Liu J, Taitelbaum G, Ginsberg GG. Tissue adhesives and fibrin glues. Gastrointest Endosc. 2004;60:327-333. [PubMed] |
| 58. | Hwang TL, Chen MF. Randomized trial of fibrin tissue glue for low output enterocutaneous fistula. Br J Surg. 1996;83:112. [PubMed] |
| 59. | Eubanks S, Edwards CA, Fearing NM, Ramaswamy A, de la Torre RA, Thaler KJ, Miedema BW, Scott JS. Use of endoscopic stents to treat anastomotic complications after bariatric surgery. J Am Coll Surg. 2008;206:935-98; discussion 935-98;. [PubMed] |
| 60. | Lalor PF, Tucker ON, Szomstein S, Rosenthal RJ. Complications after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2008;4:33-38. [PubMed] |
| 61. | Bège T, Emungania O, Vitton V, Ah-Soune P, Nocca D, Noël P, Bradjanian S, Berdah SV, Brunet C, Grimaud JC. An endoscopic strategy for management of anastomotic complications from bariatric surgery: a prospective study. Gastrointest Endosc. 2011;73:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 62. | Baltasar A, Bou R, Bengochea M, Serra C, Cipagauta L. Use of a Roux limb to correct esophagogastric junction fistulas after sleeve gastrectomy. Obes Surg. 2007;17:1408-1410. [PubMed] |
| 63. | Baltasar A, Serra C, Bengochea M, Bou R, Andreo L. Use of Roux limb as remedial surgery for sleeve gastrectomy fistulas. Surg Obes Relat Dis. 2008;4:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 64. | Mui WL, Ng EK, Tsung BY, Lam CC, Yung MY. Laparoscopic sleeve gastrectomy in ethnic obese Chinese. Obes Surg. 2008;18:1571-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |