INTRODUCTION
Colorectal cancer (CRC) is a largely preventable cancer, but still causes approximately 50000 deaths each year in the United States[1]. The majority of patients suffering from CRC are over 50 years of age, with generally equal gender incidence[2]. Recent declines in CRC incidence and mortality are attributed to reduced exposure to the risk factors, improved treatment, early detection and polypectomy[3]. Despite this, CRC remains the third commonest adult cancer with approximately 1 in 19 adults diagnosed with CRC during their lifetime[2].
In the United States, CRC has relative high rates of cancer-related morbidity and mortality (10%)[3]. A study carried on in 2008 detected that among the 142950 diagnosed CRC people, 52857 died from the disease. Although both the incidence and mortality rates of CRC have decreased significantly in 2007 compared to 1975, those would be 59.5 vs 44.7 per 100000 and 28.6 vs 16.7[4]. The result above is coming to be the basis of many worldwide screening and surveillance programs. Considering that the majority of early cancers are clinically insidious, people are interested in screening the average risk population for genuine CRC and precancerous conditions. While there are many potential screening modalities such as double contrast barium enema, fecal occult blood testing (FOBT), flexible sigmoidoscopy, and conventional colonoscopy (CC), particularly interesting approaches are positron emission tomography (PET)/computed tomography colonography (CTC), magnetic resonance colonography (MRC) and CTC. They are so-called virtual colonoscopy (VC). The rational combination use, advantages and limitations of these modalities are discussed in this review.
RISK FACTORS FOR CRC
Prevalence of CRC increases with age[5,6] and reaches the highest rate when people come to their sixties. Males have more chances of suffering this diseases compared to females[6]. Apart from age, sex, and ethnicity, lifestyle factors such as obesity, smoking, high fat diet, and physical inactivity increase the risk of CRC[7]. Any of the following factors such as over ten years of history of Crohn’s disease and ulcerative colitis, a previous history of CRC or adenomas, a cholecystectomy which was performed for more than ten years, and a family history of CRC, etc.[8-10] can increase a person’s chances of developing CRC, too.
Adenomatous polyps have long been considered as the sole preneoplastic lesions leading to CRC. On the other side, hyperplastic polyps which are often detected in the distal colon are now regarded as harmless lesions, although disagreements exist[11,12]. This generally accepted belief has been challenged lately, as it turned out that these polyps and other similar lesions commonly named “serrated polyps” can be precursors to CRC[13,14].
COLONOSCOPY FOR CRC SCREENING
For detecting and preventing CRC, colonoscopy is imperfect despite being an outstanding test. After investigating more than 12000 colonoscopies, a study revealed that the level of endoscopists’ continuing medical education, the quality of the endoscopic devices, the patients’ age and gender, and the quality of bowel preparation were all factors associated with the adenoma detection rate[15]. Based on the early reports, we believed that colonoscopic screening could reduce risk for CRC by 90%. Therefore, CC was preferred to be the modality for both screening and preventing CRC in the United States for over a decade. In a French study, the polyp detection rate was 35.5% (1159/3266)[16]. In the United States, CC was still to be the main CRC screening method in 2012[17], because the data strongly support that CC can significantly reduce the incidence and mortality rates of left-sided CRC. However, researchers and clinicians have great interest in its limited benefit for right-sided CRC and find that it is likely multifactorial.
Subsequent studies found that while CC is still an outstanding screening and detection tool for CRC, it has several important limitations. The limitations include (1) the mechanics of the procedure such as the inability to detect all colonic polyps, the risk of colonic perforation, adverse consequences of sedation and bleeding; and (2) issues with patient perception regarding CC which might take part in driving patient non-adherence to recommended testing[16,17].
Because early detection and removal of potential neoplastic polyps are the key points of CRC screening and prevention, CC is still irreplaceable in current screening and prevention programs[18]. CC must perform in high risk population. Despite it is proved that CC and polypectomy can reduce CRC incidence and mortality[19] as a tool for screening, CC is still imperfect. The epidemiologic data collected from North American suggest that CC may have little effect in protecting against right-sided CRC[20-22]. Several factors are involved in the conclusion. Some are dependent on the operator, such as removing identified polyps completely, achieving an adequate bowel preparation before examination, and the most important thing, the mucosal inspection quality performed by CC. Some are out of the control of the operator, such as the biology of the tumor and polyp. The former includes employing appropriate maneuvers, spending sufficient time and ensuring high cecal intubation rates to examine the entire mucosal surface on scope withdrawal. Additionally, to identify and subsequently remove those easily missed flat or diminutive lesions, we should recognize them first[23].
Because of the so many limitations of CC and variability in quality indicators measured by operators, methods that could help to enhance polyp and adenoma detection have been investigated to improve the performance of colonoscopy. The use of water during the insertion phase of CC instead of air, or water-exchange, and cap-assisted colonoscopy has received recent attention. Usage of these two simple methods has theoretical and practical advantages and each individual technique could address the limitations of the other in some degree[15,24].
In a 2000 CC study conducted in France, Coriat et al[24] found an adenoma detection rate of 31% in complete colonoscopies, while the same thing accomplished by Denis et al[25] found an adenoma detection rate of 30%[26]. However, detection rates in other studies with a larger number of patients were about 20%[27,28]. The difference might be caused by the following factors: a cecal intubation rate < 90% caused by poor bowel preparation; variety of indications, with about 25% patients either having a CC for abdominal pain or changes in bowel habits or younger than 50.
In 2011, 427865 (95%CI: 389108-466622) polypectomies had been performed in France and the overall complication rate was 1.1%. The incidences of intestinal perforation, hemorrhage and fever were 0.1% (1/1159), 0.6% (7/1159) and 0.2% (2/1159), respectively. Patients’ gender and age, the intake of anticoagulant or antiplatelet drugs, a pedunculated/flat/sessile polyp type, and the polyps’ localization did not significantly affect the rate of complications. However, the complication rate increased as the size of resected polyps enlarged, from 0.2% (1/556) for polyps ≤ 5 mm to 8.2% (6/73) for polyps > 20 mm (likelihood ratio of 8.8, P = 0.003). The only intestinal perforation in this study occurred after polypectomy[16].
Capsule endoscopy is the latest developed of the emerging CRC screening tests, but it is much more expensive than CC. New endoscopic techniques have some advantages for resolution of focus, such as high definition endoscopy. Other developing techniques around colonoscopy including retro-viewing colonoscopy, balloon colonoscopy or 330-degree viewing colonoscopy try to enhance the efficacy by reducing the adenoma miss rate in right-sided, non-polypoid lesions[29,30].
The future of colorectal screening will depend on many factors such as insurance coverage of the various options, acceptability, cost and efficacy. Some combinations of tests, such as colonoscopy with interval fecal DNA testing, are hoped to not only lower the costs into an acceptable range, but also promote its risk/benefit ratio. However, whatever the future holds, CC will be the keystone of CRC screening for not a short time. High quality CC and recognized performance improvement measures will maximize its effectiveness.
CTC FOR CRC
Vining et al[31] had presented the first virtual colon images for 20 years since the 1994 meeting of the Society of Gastrointestinal Radiologists. That was the birth of “virtual colonoscopy” or “CTC”, the name widely used now.
CTC is a minimally invasive sedation-free imaging modality that has been shown to be comparable to optical colonoscopy in the detection of clinically significant colon polyps and CRC[32-35] and has been endorsed by some national guidelines as an acceptable option for CRC screening[36-38]. In addition to the excellent performance measures, the risks and complications related to this examination are very low[39]. Potentially, the addition of CTC to CRC screening options could markedly impact current low adherence rates, likely in a cost-effective manner[40]. A recent cost-effectiveness analysis indicated that modest increases in patient adherence to CRC screening guidelines with greater availability of CTC would place CTC among the cost-effective CRC screening options[41].
CTC in CRC screening has a present and a potential role. The present role is used in the case of incomplete CC to replace barium enema and integrate this method into established screening programs. The potential role of CTC screening is the combination of it with sigmoidoscopy, fecal occult blood test and CC, taken as a first-line method. In fact, various scientific societies have approved the authentic use of CTC screening for average-risk individuals. These include the American College of Radiology, the American Cancer Society, and the United States Multisociety Task Force on Colorectal Cancer. Nevertheless, other entities have thought that the evidence is inadequate to warrant the use of CTC as a mass screening method, such as the United States Preventive Services Task Force[42]. Lately, Medicare has also refused to refund for CTC screening[8]. However, there are many advantages in the use of CTC as a CRC screening method. These include its non-invasiveness, patient comfort, safety, high accuracy, detection of extracolonic lesions, full assessment of the colon in almost all patients and cost-effectiveness. The principal possible disadvantage of a CTC screening is the patients’ exposure to ionizing radiation. But this is not an important question, because low-dose equipments are now usually applied. These protocols convey a dose similar to or a little higher than the yearly radiation exposure of any person. There is indirect evidence showing that such a dose of radiation exposure does not increase the incidence of cancers[41,42].
CTC TECHNIQUE
Patients usually consume a standard low-volume bowel preparation on the day before the procedure, consisting of either magnesium citrate with bisacodyl or a 2-L polyethylene glycol bowel preparation alone. Single dose of 2% barium sulfate and diatrizoate is used for tagging colonic stool and fluid, respectively. Before imaging, a small flexible rectal catheter is placed and the colon is insufflated using an automated carbon dioxide delivery system that is both pressure and volume regulated. Patients undergo multi-row detector CT in the supine and prone positions with a low-dose CTC protocol. Additional decubitus views are obtained in cases with areas of persistent luminal collapse. No sedation or IV medications are administered as part of the CTC examination[16,31,42].
ANATOMICAL IMAGING BY CT SCREENING
Although elevated serum carcinoembryonic antigen levels are often present in CRC, they are neither sensitive nor specific enough to be used as a screening tool for asymptomatic patients[43]. CTC allows a minimally invasive imaging examination of the entire large bowel. Compared to optical colonoscopy, the risk for colonic perforation during screening is extremely low, being 0.005%[44] for asymptomatic patients and up to 0.06% for symptomatic patients[45]. Use of carbon dioxide with an insufflator that regulates pressure rather than room air for gas insufflation of the colon may further reduce the incidence of perforation[39,44]. In CTC, high resolution image acquisition of the entire large intestine in a single breath hold is permitted by the use of multi-row detector CT. Combined 3D and 2D analysis with specific post-processing software allows for ease of polyp detection, depiction of lesions and location[36].
For optimal assessment, it is important to prepare the bowel adequately and to inflate the colon with gas. Newer techniques such as fecal tagging reduce the need for vigorous bowel preparation[46] and decrease false positives from the presence of adherent fecal matter. In contrast with optical colonoscopy, extra-colonic structures are also evaluated in the same examination by CTC. Hellström et al[47] showed that potentially important extra-colonic findings, such as lymphadenopathy, aortic aneurysms and solid hepatic and renal masses, were present in 23% of patients. An Imaging Network National CT Colonography Trial has been conducted by the American College of Radiology. This trial included 2500 patients across 15 institutions in the United States, and has shown comparable accuracy between CTC and standard colonoscopy. Pickhardt et al[34] and Ramos et al[48] reported a sensitivity of 89% for adenomas greater than 5 mm. For invasive CRC, the pooled CTC sensitivity was higher at 96%. As with other screening techniques, CTC accuracy improves with lesion size. All patients with one or more polyps larger than 10 mm or 3 or more polyps larger than 6 mm should be recommended for CC[49]. However, at present it is still controversial about the direction of patients with fewer than three polyps of which the largest one is 6 to 9 mm or smaller[50,51]. For patients with suspected CRC, the diagnostic accuracies of contrast-enhanced CTC were even better. Using the tumor, node, and metastasis system, rates of 95%, 85%, and 100% were achieved. The sensitivity of both CTC and CC for cancer detection were both 100%, while the overall sensitivity of CTC was even higher than initial CC for polyp detection (90% vs 78%)[52]. The main drawback of CTC is radiation exposure. A single CTC study results in that the dose to the colon as an organ was estimated at 7 to 13 millisievert (mSv), and this amount is 0.044% more than the lifelong danger of colon cancer[53]. More efficient low dose protocols (estimated organ dose ranges of 5 to 8 mSv) have been shown to be feasible with encouraging results[54].
CT plays an important role in the management of CRC. The use of CTC as a screening tool for CRC has been validated and is expected to rise over time. The results of prior studies suggest that CT is suboptimal for assessment of local T stage and moderate for N stage disease. Recent advances in CT technology are expected to lead to some improvement in staging accuracy. At present, the main role of CT in pretreatment imaging assessment lies in its use for the detection of distant metastases, especially in the liver. In a select group of patients, routine post-treatment surveillance by CT confers survival benefits. Imaging plays an important role in screening for CRC. According to the current American Cancer Society guidelines for CRC screening, 5-yearly CTC is recommended for asymptomatic patients with average risk[4]. In patients with known CRC, CT plays an important role in both pretreatment staging of disease and assessing for response to treatment. Traditionally, this has been done by anatomical imaging assessment on CT. Advances in technology have further elevated the role of CT, by promoting functional imaging by PET and perfusion studies[31].
MRC
MRC is a noninvasive method for evaluating the entire colon. Potential uses include staging of colorectal pathology and detection of cancer and precancerous lesions. It also allows for the evaluation of extra-colonic pathologies including cancer metastases. In the context of CRC screening, the absence of ionizing radiation in MRC provides an advantage over CTC. Bowel preparation is performed in a way similar to that required for CTC or CC and different substances for bowel preparation are commercially available[42]. Patients must be screened for general contraindications to MRI including the presence of metallic implants or severe claustrophobia. As with CTC, distension of the colon is a prerequisite for procedure and can be achieved using water, air, or carbon dioxide. Also similar to the procedure in CTC, patients can be imaged in the prone and supine positions[31,55].
Dark lumen and bright lumen techniques of MRC
Methods of MRC are classified into bright lumen and dark lumen manners. In the bright lumen technique, colorectal lesions are shown as dark filling defects of low signal on a bright background of distended colon; while in the dark lumen technique, colorectal lesions are shown as white defects on a dark background of distended colon. In the dark lumen technique, the intravenous application of paramagnetic contrast agents allows visualization of the colorectal wall, discriminating it from the dark colonic lumen. This reduces the incidence of false positive findings. Residual stools or air bubbles that might look like polyps in the bright lumen technique remain dark[55]. The bright lumen technique is, however, less affected by movement of the patient and may be preferable in patients unable to hold their breath[56].
Sensitivity of MRC for cancer detection
The prevalence of cancer in related studies ranged from 0% to 72.7%[57]. A total of 58 cancers were found in 1305 patients. There was no report about whether all the cancers were detected by MRC; however, from the 54 remaining cancers, 53 were detected by MRC. Overall, the MRC detection rate for cancer was 98.2%[58-60]. Studies reported lesions using the size categories of larger than 10 mm in diameter, 5 to 10 mm, and less than 5 mm, as well as using a combination of these categories (Figure 1). The cut-off threshold did vary slightly between studies, thus we considered three size categories, large, medium, and small for identified polyps. Where sufficient data was available, additional size categories were calculated (e.g., for medium to large or all size polyps) by grouping other size categories if such was not reported. Generally, investigators agree that polyps less than or equal to 5 mm in diameter have a very low likelihood to become malignant. This causes some of them to ignore documenting polyps so small[60]. The significance of medium-sized polyps has aroused debate on sensitivity and specificity, as well as the appropriate interval at which the examination should be repeated. We included all types of polyps. Thirteen studies reported polyps regardless of their pathology while one[61] reported adenomatous polyps only.
Figure 1 Combination use of computed tomography, computed tomography colonography and conventional colonoscopy in large bowel examination and management.
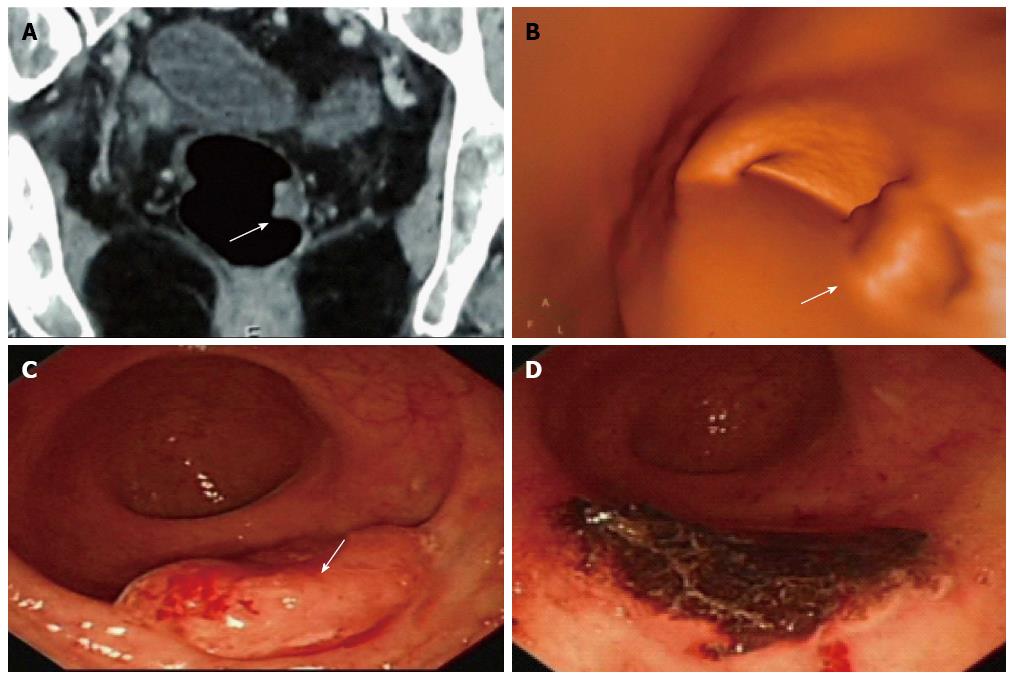
A 62-year-old woman complained of blood stool for several days and was detected with multi-polyps in colorectal cancer screening. Virtual and conventional colonoscopy all had positive results (single white arrow) and pathology after removal under conventional colonoscopy (CC) proved that the most suspected polyp had turned to be moderately differentiated adenocarcinoma. A: Lesion revealed by cross-sectional computed tomography (CT); B: Same lesion by CT colonography (CTC); C: By CC; D: CC after removal of the cancer. The case illustrated the different roles of CT, CTC and CC, as well as the combination use of these modalities. The invasiveness of CC might explain injury and swelling of the lesion, and the role of CC in polypectomy is demonstrated.
Sensitivity and specificity of MRC for detection of patients with polyps
Investigators use two different ways to analyze accuracy of MRC. One focuses on the accuracy in identification of patient with polyps, the other on that in identification of individual polyps themselves. From a screening point of view, analysis aiming at for per-patient data is more meaningful than per-polyp data as it stresses the use of MRC as a screening method. An evidence-based analysis from Health Quality Ontario summarized the number of true and false positives and true and false negatives for MRC for detection of patients with colorectal polyps of different sizes and drew the conclusion that the overall performance of MRC for identification of patients with large polyps (> 10 mm) was excellent (95%). However, sensitivity decreased with decreasing polyp size. When the polyp size dropped down under 5 mm the detection rate of MRC became pretty low (10%)[58-60,62].
Dark lumen vs bright lumen
The dark lumen technique provided a further improvement in the diagnostic accuracy of MRC as demonstrated by Lauenstein et al[63]. This study found both the sensitivity and specificity of dark lumen to be higher than bright lumen. The former identified all the 11 polyps larger than 5 mm in diameter (sensitivity 100%), while the latter missed two polyps measuring 7 and 8 mm in diameter (sensitivity 81.8%). The dark lumen technique can differentiate residual stool from colorectal lesions. In this study, the dark lumen technique presented no false positive results (specificity 100%), while the bright lumen technique produced five false positive results because of an inability to draw this distinction.
When it comes to MRI gradient strength, most clinical studies assessing the performance characteristics of MRC have been carried out at 1.5 T. Although experience with MRC at 3 T is limited, the clinical results are promising. With the consideration of the advancement in the technique and learning curve, the sensitivity of MRC for polyps would become higher than studies before. The shortage in detection of polyps of small size may be changed in future[58-60,62].
PET/CT COLONOGRAPHY
The protocol of CTC without bowel preparation is safer and better-tolerated than full laxation protocols, but its comparative sensitivity and specificity may be reduced. Uptake of 18F-fluorodeoxyglucose (18F-FDG) by colonic neoplasia is characteristic, and the combination of non-laxative CTC with PET could enhance accuracy. CRC is the most common malignancy of the gastrointestinal tract and is the third most frequent malignancy in humans. The development of CT and MRI provides an early diagnosis of CRC and an adjustment of the therapeutic decision[61]. The major drawback of radiological imaging such as CT and MRI is the lack of characteristic functional data. A number of studies appear to confirm the role of PET/CT and virtual colonography in the judgment of primary lesions. PET/CT is useful for assessing lymph node metastases in pre-operative staging of primary, recurrent and metastatic cancer[64,65]. PET/CT colonoscopy provides more accurate diagnosis and staging of CRC than ever. The use of these diagnostic tools has certain disadvantages. The cost is expensive for their installation. In some procedures, more ionizing radiation exposure is needed from both radiological and nuclear aspects. There are difficulties in combination use of these modalities and acquisition of fusion imaging. Non-rigid image registration is a natural extension to rigid registration in order to achieve a good match. Non-rigid registration more or less overcomes some problems in image integration by registering the surfaces of organs and structures within two data sets[64].
PET/CT colonography has been reported to detect all polyps 10 mm or larger, and all of these polyps were 18F-FDG-avid. However, only half of the 6-9 mm polyps and none of the 5 mm or smaller polyps were 18F-FDG-avid. In a highly enriched cohort of 17 patients undergoing PET/CT colonography after full bowel preparation, Gollub et al[66] reported 18F-FDG avidity in 13%, 35%, and 59% of polyps sized 1-5 mm, 6-9 mm, and at least 10 mm, respectively. However, in a small feasibility study, Mainenti et al[67] reported 18F-FDG avidity in none of 12 polyps 9 mm or smaller but in all of the 5 polyps 10 mm or larger. These data suggest that polyps 10 mm or larger are often 18F-FDG-avid, whereas those below the 10-mm threshold are frequently not. The radiologist is more familiar to CT interpretation. Some strategies can be applied to overcome interpretation difficulties and increase sensitivity for smaller lesions. For example, high diagnostic accuracy was reported in a selected population with fecal occult blood test positive results when bowl preparation was limited, and computer-aided detection improved significantly the sensitivity of less-experienced radiologist readers[68,69]. We were able to demonstrate the technical feasibility and patient preference for PET/CT colonography over CC (which were our main aims) and benefits to reader confidence.
CTC VS MRC
A meta-regression analysis compared CTC with MRC for their diagnostic accuracy in patients with CRC, using CC results as comparison. In CTC, the total sensitivity was 95% and the total specificity was 95%. In MRC, the total sensitivity was 95% and the total specificity was 95%, too. The meta-regression analysis demonstrated that MRC and CTC with 16-slice or 64-slice scanners have equal sensitivity for the diagnosis of CRC, as well as for the diagnosis of large and medium sized polyps; however, MRC does not carry the associated risks of ionizing radiation[70]. Most CRCs and large colorectal polyps can be detected by MRC or CTC with 16-slice or 64-slice scanners. Nevertheless, about 20% of medium-sized colorectal polyps can not be detected by both techniques. Neither of them can reliably detect small polyps. For the detection of small polyps, the sensitivity of MRC is much lower than that of CTC.
This meta-regression analysis demonstrated that the diagnostic precision of both MRC and CTC is similar in detecting CRC. Both techniques have high discrimination for cases presenting with CRC and are suitable for elderly persons with suspected CRC. There are such drawbacks of CT/MR colonoscopy as missing small polyps or lesions. Diagnostic accuracy is influenced by intra-luminal and or intravenous contrast agents, as well as quality and size of the study. To make a precise comparison, it is necessary to conduct studies in the same patient group to evaluate CC, CTC and MRC[62,70-73].
COVENTIONAL COLONOSCOPY VS VIRTUAL COLONOSCOPY
Since it has been confirmed that “CTC is highly specific for the detection of colorectal polyps and tumors”[74,75], researchers designed three important studies for further investigation, including two large, multicenter trials comparing CTC to CC in asymptomatic subjects. The first is the American College of Radiology Imaging Network (ACRIN) trial. This trial was conducted in the United States with a typical screening group of people at average risk[33]. Another is the Italian Multicenter Polyps Accuracy CTC study (IMPACT) trial conducted in patients referred for a result of positive FOBT and in a mixed patient cohort of symptomless individuals at higher-than- average risk[35]. The third is the Special Interest Group in Gastrointestinal and Abdominal Radiology trial. It is a multicenter trial conducted on patients with symptoms in the United Kingdom for the detection of CRC[76].
Both the IMPACT and ACRIN trials reported high per-patient sensitivity and per-patient specificity. The ACRIN trial was 78%-84% for polyps larger than 6 mm and 90% for polyps larger than 10 mm. The IMPACT trial reported greatly high specificity, with a rate more than 85% not influenced by lesion size[74,75]. The ACRIN trial had a positive predictive value (PPV) of 23% for polyps no larger than 10 mm, so low as its main downside. This might produce negative impression on a screening program, with factors of patient discomfort, useless CC, development of complications, awkwardness of radiologists and greater expenses. The IMPACT trial had much better PPV (62%) for lesions > 6 mm. Other studies conducted in high-experience centers also obtained much better PPV, such as in several Korean hospitals and in the University of Wisconsin. The former had a PPV of 69% for lesions larger than 6 mm and 92% for lesions larger than 10 mm. The latter achieved a PPV of 91.5%[77,78].
However, both the ACRIN and the IMPACT trials reported rather high negative predictive values that reached nearly 100%. This is of great significance in giving the patients with negative results confidence in the examination. The Munich Colorectal Cancer Prevention Trial also obtained exceptionally good results[32,79]. It is a single-center study in which around 300 asymptomatic individuals were subject to low dose CTC, which was compared with other screening methods such as FOBT, sigmoidoscopy and CC.
A screening program presented by the University of Wisconsin is worthy of mentioning[80]. More than 3000 individuals were enlisted in 2 years and divided into two nonrandomized groups receiving CTC and CC. There was no significant statistical difference in detection rate for advanced adenomas between the two groups, with 3.2% for CTC and 3.4% for CC, respectively. The CTC group had the superiority of much fewer polypectomies and no complications. On the contrary, the CC group had seven perforations.
Both the radiological and gastroenterological communities had several unsettled arguments, such as the significance of polyps smaller than 6 mm, the detection rate for nonpolypoid, flat lesions, how to manage those 6-9 mm lesions, and the effect of findings outside the colon. A systematic review[81] of published papers reported the distribution of advanced adenomas in screening patient groups without symptoms. It concluded that small polyps have a minimal clinical impact. Advanced lesions were found in 0.9%, 4.9%, 1.7%, and 73.5% of patients whose largest polyp size was no larger than 5 mm, 6-9 mm, smaller than 10 mm, and larger than 10 mm, respectively. If the smallest size for polypectomy indication is set at 6 mm, over 95% of patients with advanced adenomas would be identified. But when the minimal size was indicated as 10 mm, only 88% of such patients would be identified. From a cost-effectiveness point of view, it is very inefficient to detect and remove all polyps including those smaller than 5 mm. The cost per year of life rises to more than $460000, absolutely unacceptable in terms of cost-effectiveness[82]. However, substantial education of patients and primary care providers is needed to convince them not to eliminate the small polyps identified by CTC, but to follow by CC. In fact, according to a recently published survey[83], most patients, primary care providers and gastroenterologists would not take this choice because they fear the missing of precancerous lesions. There is also a disagreement over the management of intermediate (6 to 9 mm) lesions. According to American Cancer Society CRC screening guidelines, any polyp with the size of 6 mm or larger should be rather recommended for CC and polypectomy. However, there is no evidence from cost-effectiveness[84,85] as well as follow-up[86] studies supporting this indication. In future, referral for CC and polypectomy might be substituted by polyp follow-up. Studies on the rate of advanced adenoma occurring in polyps with the size of 6-9 mm with CC and subsequent polypectomy supported these issues about size of lesions[87]. A possible downside of CTC would be its reduced ability to detect non-polypoid, flat lesions[84].
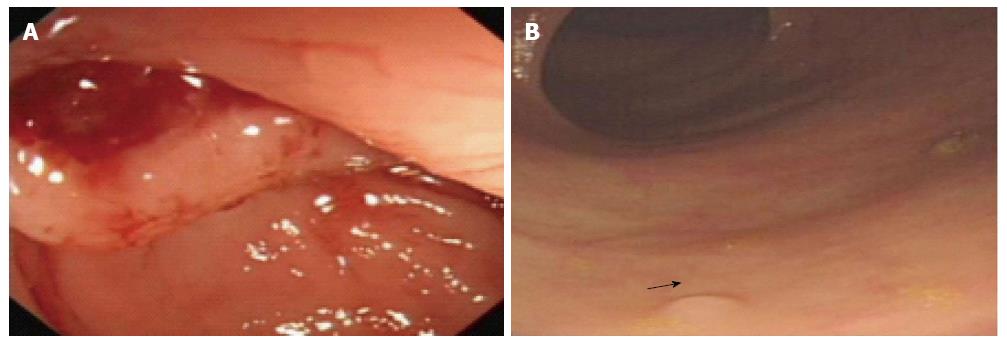
MRC has high accuracy as a modality full of promise for finding out colorectal polyps with a diameter > 5 mm. MRC can be used to estimate disease activity in inflammatory bowl disease, including spreading[88,89]. The diagnostic accuracy of MRC in detecting colon lesions was reported similar to that of CC. But MRC has the advantage of minimal invasion, usually with neither sedation nor analgesia during examination. This advantage also reduces risk of serious complications including bleeding, perforation and death (Figure 2A). All large bowel segments can be assessed with multi-sectional imaging availability and 3 dimensional fly-through program of MRC. A recent study showing the insufficiency of preoperative colonic evaluation by CC reported that up to 54% of all preoperative colon evaluations in patients with CRC and up to 17%-23% of regular colonoscopies are incomplete[90]. In this situation, MRC could be used as a practicable and effective approach to evaluating the entire colon in patients with incomplete CC. MRC could be used as a safe, patient friendly alternative examination with high sensitivity, because of the minimum invasiveness, and the excellent ability to evaluate extra-intestinal, intramural components of lesions of the colon, cancer metastasis and any other lesions[90].
Figure 2 Examples of risk and merit of conventional colonoscopy.
A: A 51-year-old woman was found with perforation of the colon during conventional colonoscopy; B: A 68-year-old man was detected with multi-polyps with the smallest diameter of 2 mm (arrow) in one routine colorectal cancer screening.
Most of the patients considered that MRC is less unpleasant than CC. The majority of them have a preference for MRC compared to CC as an examination of the colon in future. MRC also seems to take less time than CC in post-procedural monitoring for patients and medical personnel[91,92]. When some segments cannot be accessed by CC, MRC is often applied successfully to the evaluation of most of the colonic segments. The identification of additional disease by MRC emphasizes that secondary diagnostic step is needed on the background of incomplete CC. Use of MRC for CRC screening is worthy investigating. However, at the present time, wide implementation of MRC for CRC screening is not likely, largely because of the lack of skillful readers[93].
COST-EFFECTIVENESS ISSUES
As a screening test CTC is effective for detection of colorectal neoplasia[41,94,95]. Nevertheless, CTC is usually less effective and sometimes more costly than CC. If the sensitivity of CTC for 1-cm adenomas is 83% or lower, CC is a dominant strategy (Figure 2B). CTC can be reasonably cost-effective for CRC screening among Medicare enrollees when its diagnostic accuracy is high, and if the refund rate for each scan is considerably less than that for CC[41,94]. According to the majority of international thresholds, CTC seemed to have good cost-effectiveness compared to flexible sigmoidoscopy, and CTC may also come to be a reasonable substitute to CC[95].
A helical CT or MR scan is involved in VC of the abdomen and pelvis to determine the presence of polyps and cancer in the colon and rectum. Compared to CC, these two modalities are promising in sensitivity in revealing larger polyps. Caution should be taken before it is clinically implemented because of the existence of significant variation among readers and learning curves. After reviewing the evidence on the cost-effectiveness of CRC screening from several published studies, Lansdorp-Vogelaar et al[96] concluded the following 4 consensuses[36,96-99]: (1) All CRC screening strategies are cost-effective compared to no screening; (2) There is no consensus on what is the preferred CRC strategy for a given willingness to pay; (3) Generalization of cost-effectiveness analyses from one country to another cannot be done, because screening costs, resource capacity and population preferences differ; and (4) CTC, stool DNA and capsule endoscopy are not yet cost-effective compared to FOBT and endoscopy screening.
DERIVATIVE TECHNIQUES FOR CRC SCREENING
Stool DNA testing
An acceptable substitute for CRC screening is stool-based DNA testing. It is noninvasive and in every-day clinical practice can establish the diagnosis of adenomatous polyps and CRC in early stage. For patients with inflammatory bowl disease, stool DNA testing with its advanced technology has proved highly distinctive for revealing sporadic CRC and advanced precancerous lesions[100]. Yehya et al[101] demonstrated that stool DNA testing could reveal CRC-related high molecular weight p53 DNA in stool samples with an overall sensitivity of 56.3% and specificity of 100%.
The fecal immunochemical test (FIT) is a rather novel method for stool test which also identifies occult blood in the stool, also called the immunochemical fecal blood test. CRC screening was evaluated economically by Heitman et al[102] in North American subjects with average risk. All the related screening modalities were included as well as the current CRC treatment expenses. It was found that annual FIT was less expensive and more effective in comparison with all the other modalities. Its sensitivity for cancer is high (81%) and sensitivity for advanced adenomas is moderate (54%). Among the lifetimes of every 100000 patients with average-risk, the number of CRC and related deaths could be reduced from 4857 to 1782, and from 1393 to 457, respectively, while saving CAN$68 per person. Finally they concluded that in comparison with all the other methods (including no screening), annual FIT was less expensive and more effective than not only FOBT but also CC, assuming the mid-range testing characteristics. So they suggested that FIT should be regarded as the method of choice for CRC screening for patients aged between 50 and 75 years in North America with average risk.
On the other hand, Sharaf et al[103] recently explored the comparative effectiveness and cost-effectiveness of screening strategies including those recommended by the United States Preventive Services Task Force. These involved colonoscopy every 10 years, flexible sigmoidoscopy (FS) every 5 years, annual FOBT, annual FIT, and the combination FS/FIT. They concluded that CC may be cost-effective compared with FIT and FS, on the basis of the relative rates of uptake and compliance and the protective benefit of CC in the proximal colon.
PET/MRI colonography
The integration of MRI and PET could compensate for their drawbacks and provide a few benefits compared to sole MRI or PET. When the two outstanding diagnosis imaging modalities are integrated into one device, the precision of diagnosis is further improved. PET/MRI promotes the registration accuracy of molecular features and changes in metabolism of the diseases and presents accurate link to anatomical features and details in morphology[104].
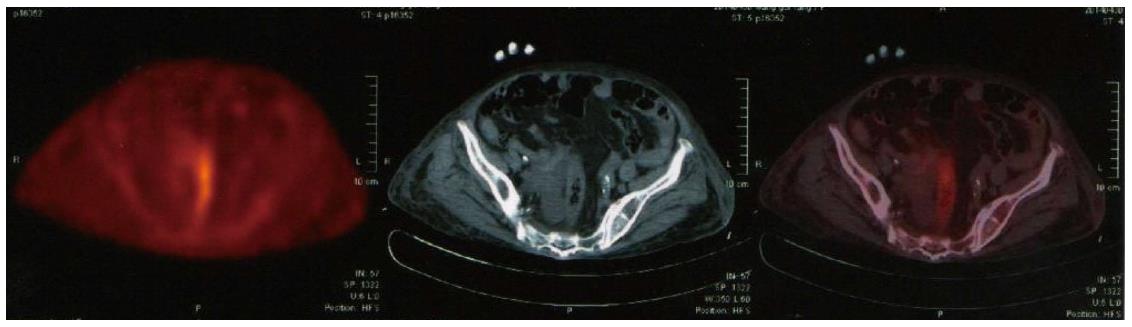
However, PET/MRI still does not match entirely the diagnostic accuracy of standard low-dose PET/CT according to the research result of Appenzeller et al[105]. Thus, it might only be used as a back-up solution in limited number of patients. Therefore, more time needs to be invested on the MRI part including higher matrix, more breath-holds, and additional surface coil acquired sequences, to make PET/MRI an equivalent to the standard low-dose PET/CT (Figure 3). In the near future PET/MRI may serve as a modern effective multimodality technique in clinical oncology, presenting great capability for imaging applications apart from connection of functional and anatomic images[106].
Figure 3 Standard low-dose positron emission tomography/computed tomography showing the colon and rectum.
Single photon emission computed tomography
Single photon emission computed tomography (SPECT) has impressive superiorities over the conventional two-dimensional imaging technique in producing more information about radionuclide distribution within the body. SPECT employs radioactive tracers and a scanner to record data that a computer constructs into two- or three-dimensional (3-D) images. The imaging procedure is executed by the application of a gamma camera to collect multiple 2-D projections from multiple angles. A computer is then used to do a tomographic reconstruction algorithm to the multiple projections, and then a set of 3-D data records is generated. Tumor tissue can be detected inside the body where the radioactive material is taken up by the cells. When SPECT is combined with X-ray CT (SPECT/CT), 3-D radionuclide imaging from SPECT is combined with anatomical information from CT, with both functional and anatomical imaging integrated[107]. Currently, this immunoscintigraphy plays a role in detection of recurrence and metastases of CRC[108].
The integration of PET and SPECT (PET/SPECT) is believed to provide detailed explanation of physiological and pathological information, medical descriptions and clinical diagnoses. Molecular imaging makes it possible to noninvasively estimate various important features of cancer in patients, including such as metabolic disorders, cell proliferation, tumor hypoxia, and receptor aspects[109]. PET/SPECT scanners are now available to perform imaging for oncologic studies including staging patients with CRC, and it is important that knowledge regarding the usefulness of these images, and their limitations, be spread to health care providers[110].
CONCLUSION
CRC is a largely preventable cancer but remains the third commonest adult cancer with approximately 1 in 19 adults diagnosed with CRC during their lifetime. All CRC screening strategies are cost-effective compared to no screening. With the rapid progress in computer processing strength, several superiorities are presented by CT, MR and PET/CT colonography over the diagnostic methods of traditional radiology, and VC also presents important data not only for primary diagnosis, but also for treatment of disease, revealing possible complications and follow-up. Currently CC still cannot be replaced by CT, MR and PET/CT colonography. However, combined with several derivative techniques including stool DNA testing, PET/MRI colonography and computer-aided detection, these modalities may further increase the sensitivity and specificity of imaging methods in screening of CRC and the resource of CC may be saved for those patients who are in need of treatment. Theses modalities also could be used as practicable and effective approaches to observing the whole colon in patients who had insufficient CC. At the present time, wide implementation of them for CRC screening is still not likely, largely because of the lack of skillful readers. It is also important that knowledge regarding the usefulness of these images, and their limitations, be spread to health care providers.