Published online Oct 14, 2014. doi: 10.3748/wjg.v20.i38.13756
Revised: April 21, 2014
Accepted: June 12, 2014
Published online: October 14, 2014
Processing time: 135 Days and 2.7 Hours
Gastric cancer is one of the most frequent and lethal malignancies worldwide because of high frequency of metastasis. Tumor cell motility and invasion play fundamental roles in cancer metastasis. Recent studies have revealed that the Rho/Rho-associated protein kinases (ROCK) pathway plays a critical role in the regulation of cancer cell motility and invasion. In addition, the Rho/ROCK pathway plays important roles in invasion and metastasis on the basis of its predominant function of cell cytoskeletal regulation in gastric cancer. According to the current understanding of tumor motility, there are two modes of tumor cell movement: mesenchymal and amoeboid. In addition, cancer cell movement can be interchangeable between the mesenchymal and amoeboid movements under certain conditions. Control of cell motility through the actin cytoskeleton creates the potential for regulating tumor cell metastasis. In this review we discuss Rho GTPases and ROCK signaling and describe the mechanisms of Rho/ROCK activity with regard to motility and metastasis in gastric cancer. In addition, we provide an insight of the therapeutic potential of targeting the Rho/ROCK pathway.
Core tip: Gastric cancer is one of the main causes of cancer-associated death in the worldwide. The poor prognosis associated with gastric cancer is mainly related to metastasis and cell motility is vital for several steps involved in the metastasis. Rho GTPases could affect cancer progression including cytoskeletal dynamics. This study aimed at gaining further insight into the mechanism of Rho/Rho-associated protein kinases pathway mediated gastric cancer metastasis, particularly with regard to cell movement.
- Citation: Matsuoka T, Yashiro M. Rho/ROCK signaling in motility and metastasis of gastric cancer. World J Gastroenterol 2014; 20(38): 13756-13766
- URL: https://www.wjgnet.com/1007-9327/full/v20/i38/13756.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i38.13756
Gastric cancer is one of the most common and lethal malignancies worldwide[1,2]. The poor prognosis associated with gastric cancer is mainly related to lymph node and peritoneal metastasis, the major causes of tumor recurrence in gastric cancer. The invasion of cancer cells into the lymphatic vessels or peritoneal cavity facilitates secondary growth in distant organs. Cell motility is vital for the several steps involved in cancer metastasis such as invasion, intravasation, and extravasation.
The ability of cancer cells to invade into surrounding tissue is one of the hallmarks of cancer, which requires increased cell motility driven by remodeling of the cytoskeletal system and cell contact with the extracellular matrix (ECM)[3]. The Rho family of small GTPases (Rho, Rac, and Cdc42) play well-characterized roles in the regulation of actin cytoskeleton organization and dynamics[4]; furthermore, Rho GTPases act as important regulators of cellular homeostasis[5-7]. Rho-associated protein kinases (ROCK, also known as Rho kinase) belong to a family of serine/threonine kinases modulated by interactions with Rho GTPases to promote actin-myosin-mediated contractile force generation by phosphorylating a variety of downstream target proteins; thus, controlling cell motility and metastasis[8-11]. ROCK signaling plays crucial roles in a range of human diseases and is now considered as a potential target for the treatment of several diseases, including diabetic nephropathy[12], as well as diseases of the central nervous and the cardiovascular system[13-15].
Accumulating evidence suggests that Rho/ROCK activity is associated with dissemination of various tumors to distant organs, including mammary[16], ovarian[17] and hepatocellular[18] cancers. However, the involvement of Rho GTPases in gastric cancer is not well understood. Herein, we review the evidences supporting a positive association between Rho/ROCK and gastric cancer motility. In addition, we discuss the potential for the application of Rho/ROCK in therapy targeting gastric cancer.
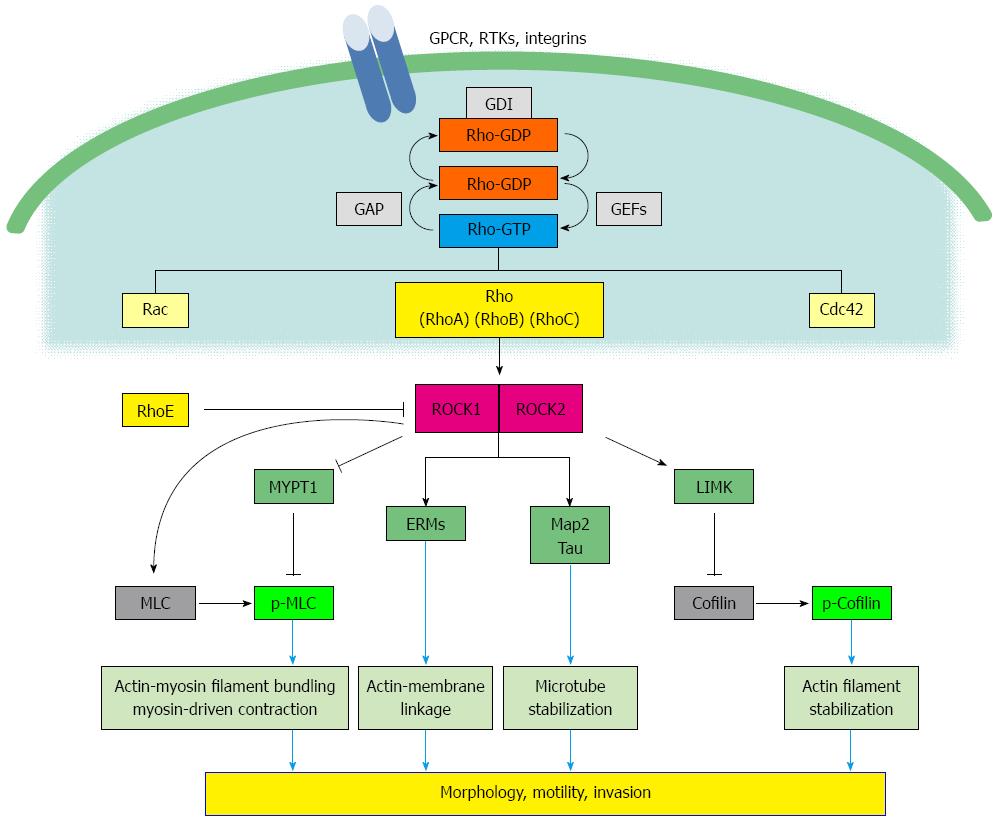
The general roles of Rho/ROCK signaling has been previously reviewed[19,20]. Figure 1 presents an overview of the Rho/ROCK signaling pathway. Rho GTPases, members of the Ras superfamily of small GTP-binding proteins, are divided into three major classes: Rho, Rac, and Cdc42. The three Rho GTPases RhoA, RhoB and RhoC have the potential to interact with the same downstream effectors[21]. In contrast, RhoE has been said to show the inhibitory potential[22]. Specific functions of the isoforms of the Rho family are summarized in Table 1[23]. Upstream signals stimulate dissociation and the binding of GTP, which leads to conformational change in the effector-binding region of GTPase resulting in interaction with downstream targets[24]. Rho GTPases act as molecular switches to translate extracellular signals into intracellular changes in the actin cytoskeleton[25]. The activity of Rho GTPases is usually controlled by three types of interaction molecules, guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs), and guanine nucleotide dissociation inhibitors (GDIs). Their activity is regulated by nucleotide binding or subcellular localization[4,19] (Figure 1). Rho GDIs protect Rho GTPases from ubiquitination[26] or degradation[27].
| Protein | Localization | Function | Effector proteins | Ref. |
| Rho | ||||
| RhoA | Cytoplasm | Regulation of the actin polymerization, basement membrane disassembly and cortical contractility | ROCK and mDia1 | [23,60] |
| Plasma membrane | Regulation of membrane bleb production | |||
| Regulation of the turnover of cell–extracellular matrix adhesions at the cell rear | ||||
| RhoB | Endosomes | Regulation of the adhesive properties and wound healing in fibroblast | p76RBE and DIAPH1 (diaphanous homolog 1) | [23] |
| Lysosomes | Retardation of growth factor receptor trafficking | |||
| Negative regulation of cell survival | ||||
| Inhibition of metastasis | ||||
| RhoC | Cytoplasm | Promotion of metastasis (invasion) | ROCK and mDia1 | [23,24] |
| Plasma membrane | Regulation of actin polymerization within invadopodia protrusions | |||
| ROCK | ||||
| ROCK1 | Plasma membrane | Destabilization of the actin cytoskeleton through regulating MLC phosphorylation | MLC and RhoE | [38,39] |
| Regulation of cell migration by leading and trailing edges of motile cells | ||||
| ROCK2 | Cytoplasm | Stabilization of the actin cytoskeleton through regulating cofilin activity | LINK and Cofilin | [37,39] |
| Plasma membrane | Regulation of myosin II–dependent phagocytosis | |||
| Negative regulation of cell migration |
GTPases bind to effector molecules that activate downstream targets. ROCKs were originally isolated as downstream targets of RhoA[28-30]. ROCK is a serine/threonine kinase that phosphorylates downstream targets involved in cytoskeletal rearrangement. The ROCK family comprises ROCK1 (also known as ROCKI, p160ROCK or ROKβ) and ROCK2 (also known as ROCKII, ROKα or Rho kinase)[31]. These proteins were originally isolated as RhoA-GTP interacting proteins, which share 65% overall identity and 92% identity particularly in their kinase domains[8]. Active RhoA-GTP interacts with the C-terminal domain of ROCK that promote the formation of stress fibers and focal adhesions, cell junctions and cell cycles[10,31].
The ROCK family elevates myosin and mediates muscle contraction, in addition to neurite retraction driven by actomyosin contraction through phosphorylation of two major substrates: myosin light chain (MLC) and myosin phosphatase 1 (MYPT1)[32]. Intermediate filaments, ezrin/radixin/moesin (ERM) family proteins, collapsing response mediator protein 2 (CRMP2), calponin and adducin have been identified as downstream targets of ROCK proteins[33]. Tau and MAP2 modulate the microtubule structure and dynamics[34]. Activated myosin connects the actin filaments to form stress fibers that generate actomyosin forces to facilitate cell movement. LIM kinase (LIMK) is another important downstream effector of Rho[35]. LIMK phosphorylates cofilin and blocks cofilin-mediated actin filament disassembly[36] Therefore, cofilin is often referred to as the terminal effector of the cell signaling cascades that regulate the cytoskeletal rearrangement (Figure 1). Recently, differences in activity between the two isoforms (ROCK1 and 2) have been obseved (Table 1)[37,38]. A recent study has shown that ROCK1 mediated destabilization of actin cytoskeleton through regulating MLC2 phosphorylation In contrast, ROCK2 played a role for stabilizing actin cytoskeleton via cofilin[39]. Firstly, ROCK1 protein is mainly found in organs such as liver, kidney, and lung, whereas ROCK2 protein is mainly expressed in muscle and brain tissue. Several paper showed the inhibitory effect of RhoE on ROCK1, but not ROCK2 activity[22,40]. Activity of MLC and MYPT was affected after silencing ROCK I, but not ROCK II[41]. LIM kinase is downstream of p21-activated kinase[42] and is not phosphorylated by full-length ROCK1[36].
Rho/ROCK activity is regulated by both protein regulator signaling and cell surface receptors. The Rho subfamily (RhoA, RhoB and RhoC) share 85% amino acid identity. Despite of this similarity, the three isoforms have different cellular functions[43] (Table 1). Rho was found to be activated in various cancers, such as breast, colon, and lung cancer, as well as metastatic melanoma[43-46]. Overexpression of RhoA signaling elements has been detected in several human tumors, including those of the urinary tract, and cervicx[47-49]. Rho overexpression also contributes to malignant phenotype in gastric cancer[49]. Enhanced expression of RhoC was revealed to be correlated with a motile and invasive phenotype of gastric cancer cells[50-52]. In contrast, RhoB significantly inhibited the proliferation, migration, and invasion of gastric cancer cells[53]. Interestingly, gastric cancer cells with a high expression of RhoA are resistant to chemotherapeutic drugs, such as taxol or vincristine, implying that treatment strategies aimed at inactivation of RhoA may have potential in improving the efficacy of these chemotherapeutic drugs[54]. Additionally, RhoGDI is involved in gastric tumor growth and metastasis, suggesting it to be a useful marker for tumor progression in gastric cancer[55].
Scirrhous gastric cancer, is a diffusely infiltrating Borrmann type 4 carcinoma (also known as linitis plastica-type carcinoma) has a worse prognosis than other types of gastric cancer[56], reflecting their rapid and progressive invasion and frequent metastasis to the peritoneum[57,58]. Our previous study described that the expression level of active RhoA was higher in scirrhous-type gastric cancer cell line, OCUM-2MD3 and MKN-45 than in an intestinal-type gastric cancer cell line, MKN-74[59]. Shinto et al[60] revealed that TGF-β significantly upregulated the activity of RhoA and myosin phosphorylation in diffuse-type gastric cancer cells.
Somatic mutations in RHO genes (RHOA, RHOB, and RHOC) have been found in certain solid cancers, including those in the breast, lung, ovary, and intestine[5,61]. In addition, somatic mutations in ROCK genes have been identified in certain cancers. ROCK was overexpressed in testicular and bladder cancers[62,63]. Furthermore, mutations have been identified in the ROCK2 gene in gastric cancer[64]. On the other hand, application of MicroRNA-148a resulted in suppression of tumor cell invasion and metastasis by downregulating ROCK1 in gastric cancer, suggesting that ROCK1 may be closely related with metastatic process in this type of malignancy[65].
The Rho/ROCK pathway plays multiple roles in the distant metastasis of cancer cells[24,34,66-68]. Zhang et al[69] found that selective suppression of RhoA by small interfering RNA (RNAi) or a pharmacologic inhibitor reduced the proliferation of gastric cancer cells. RhoC stimulates the proliferation of gastric cancer cells through recruitment of IQ-domain GTPase-activating protein 1 (IQGAP1)[70]. Lin et al[71] reported that IL-6 induces AGS gastric cancer cell invasion through activation of the c-Src/RhoA/ROCK signaling pathway. High expression of RhoA is correlated with lymph node metastasis, tumor stage, histologically diffuse type, and poor survival of patients with gastric cancer. RhoA RNAi caused a decrease in ROCK1 expression but an increase in caspase-3/cleaved-caspase-8[72]. miR-10b is a Twist-induced microRNA which stimulate camcer cell invasion by the upregulation of RhoC and AKT phosphorylation through HOXD10[73]. In contrast, one of the Rho GTPase family member RhoE inhibits RhoA signaling in part by binding to the ROCK1[22]. RhoE also increased hypoxia-induced epithelial-mesenchymal transition (EMT) of cancer cells through hypoxia-inducible factor (HIF)-1a signaling[74].
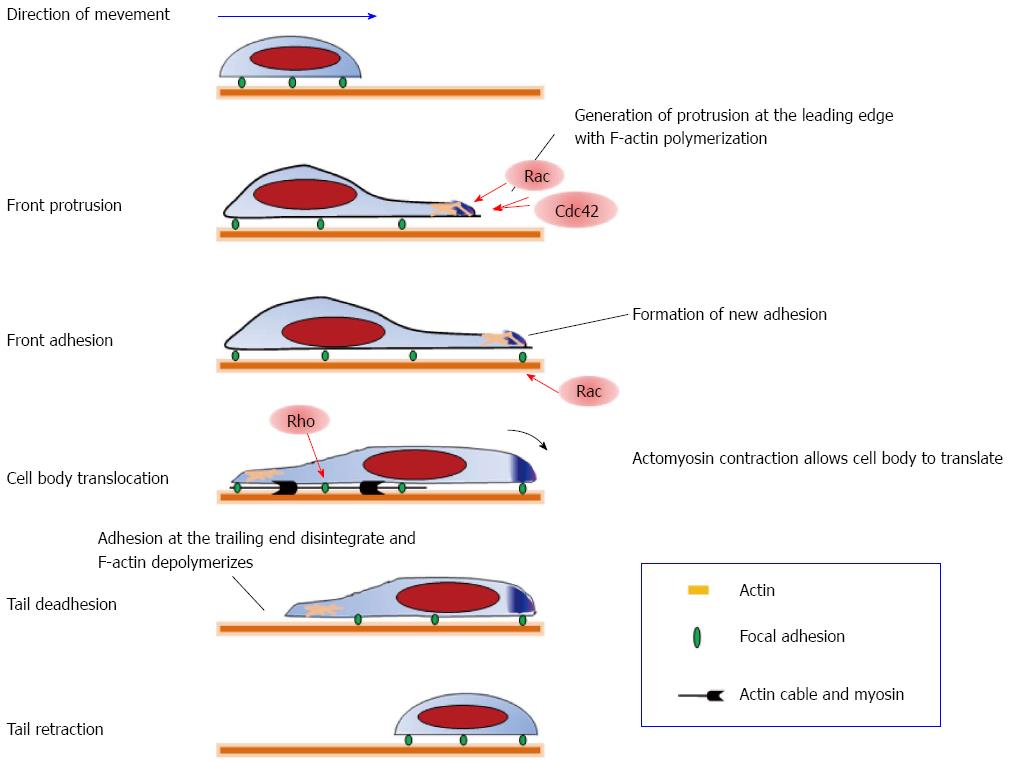
Several studies have established the role of Rho/ROCK signaling in tumor cell motility[75]. Directed cell movement consists of cycles of five processes: membrane protrusion at the leading edge, adhesion of protrusions to the substrate, translocation of the cell body, tail deadhesion from the substrate, and retraction of the trailing edge[76] (Figure 2). Reorganization of the actin cytoskeleton is involved in these processes which is regulated by Rho GTPases[77]. Among a various responses, reorganization of actin cytoskeleton is the most studied. Cdc42, Rac, and Rho are key regulators of actin assembly and control the formation of filopodia, lamellipodia, and stress fibers[24]. Activated Rac and Cdc42 induce reorganization of the actin cytoskeleton at the leading edge[78]: localized actin polymerization at the leading edge pushes the membrane forward in finger-like structures known as filopodia and in sheet-like structures known as lamellipodia. These structures generate the locomotive force in migrating cells. On the other hand, Rho regulates the assembly of contractile actomyosin filaments[79]. Rho has always been assumed to act at the rear of migrating cells for inducing tail detachment, but active Rho has recently been found to localize in membrane ruffles and lamellipodia under certain conditions. Thus, during cell movement, Rac and Cdc42 stimulate formation of protrusions at the leading edge, whereas Rho induces retraction at the trailing edge (Figure 2). This coordinated reorganization permits cell movement toward a target.
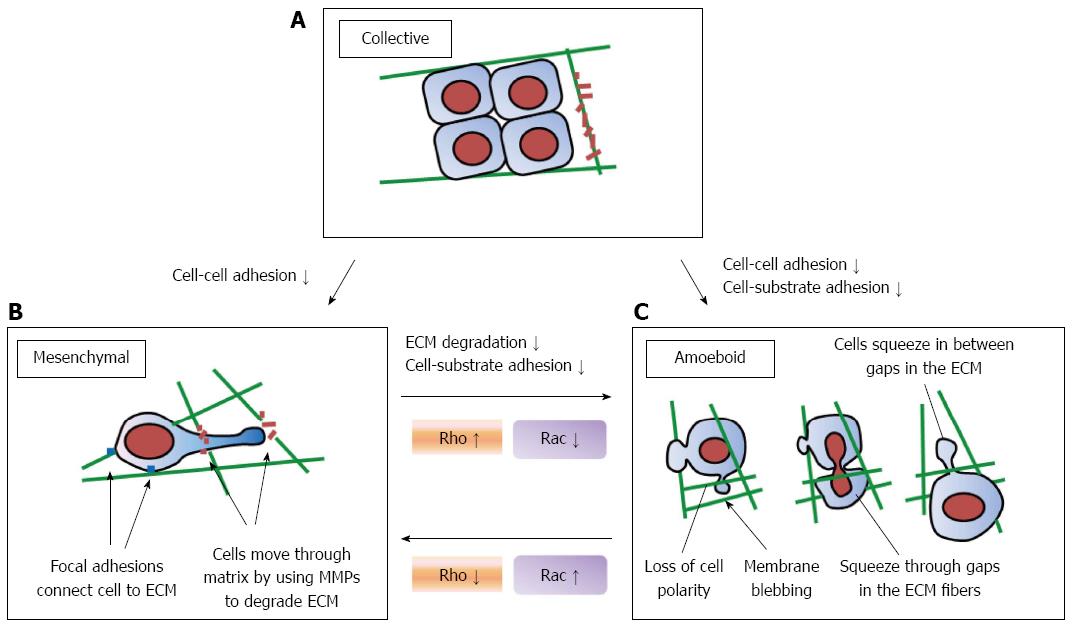
It was recently demonstrated that cancer cells display two different modes of cell motility: EMT and amoeboid transition[80,81] (Figure 3). In the case of epithelial tumors, cells move in groups through the extracellular matrix and maintain cell–cell adhesions in sheet-like structures (Figure 3A). As epithelial cancer cells advances, the function of cadherin is suppressed and they move as single cells[82]. This transition from collective to individual motility is termed EMT[83]. When cultured in three-dimensional matrices, cancer cells that undergo individual cell movement similar to that of dedifferentiated epithelial tumor cells exhibit two typical morphologies: an elongated and a rounded morphology. These two forms use different motility mechanisms, which are termed as mesenchymal and amoeboid movements. This review primarily discusses the single-cell motility strategies of tumor cells, referred to as amoeboid and mesenchymal. With respect to the mesenchymal movement, the elongated morphology of cells is dependent on integrin-mediated adhesion[84]. Mesenchymal movement requires degradation of the ECM, similar to collective movement (Figure 3B). Elongated cells are polarized, creating an obvious frontal extension comprising of one or more leading pseudopodes, and form integrin-dependent adhesions with the substrate. In addition, matrix-degrading proteases such as the matrix metalloproteinase (MMP) and serine protease families (μPA/μPAR) accumulate at the leading edge of moving cells, which causes localized proteolysis[85]. Elongated cells form a pathway and overcome tissue boundaries by degradation of the ECM. In contrast, cells with amoeboid movement migrate in an integrin-independent manner (Figure 3C). When rounded cells migrate through the ECM, they change their shape and squeeze themselves into the gaps. This amoeboid movement is arguably primitive and in some ways the most effective form of cell movement that does not require degradation of the ECM, which is observed in hematopoietic stem cells, leukocytes, and certain tumor cells[86-88].
The amoeboid movement of the rounded cells is driven by actomyosin-based cortical contraction (stiffening and contracting of the cell cortex) along the plasma membrane. Actin–myosin contractility for bleb retraction is provided by signaling through Rho/ROCK[88-90]. The enhanced contractility of cells that use amoeboid-like invasive strategies enables them to squeeze through gaps in ECM fibers and adapt their shape to the preexisting spaces, or to exert sufficient force to deform the surrounding ECM. RhoA is important in both amoeboid and mesenchymal movement. RhoA-ROCK signaling is proposed to induce actomyosin-based cortical contractility leading to amoeboid movement through blebbing. Epithelial cells expressing constitutively active RhoA can detach from epithelial sheets and acquire a rounded, bleb-associated mode of motility[91]. Although it is known that Rho/ROCK plays a critical role in promoting adaptation to the cellular environment, the regulation of RhoA in determining morbility is beginning to be unraveled. Phosphoinositide-dependent protein kinase 1 has been identified as a regulator of amoeboid cell motility through maintenance of ROCK1 activity, and it prevents inhibition of ROCK1 through RhoE[40]. In addition, Smurf1[92], EphA2[93], and loss of p53[94] have been identified as positive regulators of mesenchymal-amoeboid transition (MAT).
The amoeboid and mesenchymal types of movement are mutually interchangeable: MAT or amoeboid–mesenchymal transition (AMT)[95]. Whereas remodeling of the ECM is required for mesenchymal movement, in the presence of protease inhibitors, cancer cells change to amoeboid and pass through the ECM[85,88]. This ability leads to difficulty in repression of cancer cell invasion by protease inhibitors. To regulate cancer cell motility, both mesenchymal and amoeboid movement must be repressed simultaneously. If cell–ECM interactions are weakened, mesenchymal movement can change to amoeboid movement (Figure 3B and C). Known mechanisms leading to MAT are the abrogation of pericellular proteolysis by protease inhibitors, strengthening of the Rho/ROCK signal pathways, and weakening of integrin–ECM interactions by antagonists. These transitions are associated with P-MLC2 levels, actin localization, RhoA activity, or membrane blebbing[95].
Both mesenchymal and amoeboid movements are based on reorganization of the actin cytoskeleton, but their requirements for Rho and Rac signaling differ. A siRNA screen for Rho-GEFs and Rho-GAPs has revealed specificities in the activation of RhoA and Rac that directly affect mesenchymal and amoeboid movement[96]. With respect to mesenchymal movement, membrane protrusions at the leading edge are formed in a Rac-dependent manner. Inactivation of Rac induces a rounded phenotype. This effect has also been observed with the inactivation of either NEDD9 or DOCK3, both of which mediate the activation of Rac[97]. In contrast, Rho signaling is not essential for mesenchymal movement[88]. In regard to amoeboid migration, the actin cytoskeleton is reorganized along the plasma membrane, causing dynamic membrane blebbing along the cell surface[88]. This cortical actin reorganization is dependent on Rho/ROCK signaling[98]. Inhibitors of Rho/ROCK signaling suppress the formation of membrane blebs and inhibit amoeboid migration.
The common features of scirrhous gastric cancer include rapidly progressive motility and invasion, and a high frequency of metastasis to the peritoneum[58]. Scirrhous gastric cancer cells proliferate with fibrosis when the cancer cells invade the submucosa containing abundant stromal cells. The proliferative and invasive ability of scirrhous gastric cancer cells is closely associated with the growth factors produced by organ-specific fibroblasts. Threrfore, fibroblasts are a key determinant in the malignant progression of gastric cancer[99,100]. Scirrhous gastric cancer cells with high peritoneal metastatic ability exihited ameboid types of cell movement. Inhibition of Rho/ROCK signaling by ROCK inhibitor, Y-27632, induced an elongated morphology and increased the invasive ability of scirrhous gastric cancer cells. The fact that Rac1 inhibitor reduced the invasive ability of ROCK inhibitor suggests that the invasive ability of scirrhous gastric cancer cells is related to Rac activity. Y-27632 converted scirrhous gastric cancer cells from rounded to elongated shape, suggesting that inhibition of the RhoA/ROCK pathway is associated with AMT[59]. Thus, mesenchymal and amoeboid movements are dependent on Rac and Rho signaling, respectively. Furthermore, the balance between these signaling systems may determine the type of cell migration. The inhibition of proteases, particularly MMPs, can change the mode of migration from mesenchymal to amoeboid, whereas inhibition of ROCK may alter it from amoeboid to mesenchymal. Although it is known that the amoeboid movement is clearly Rho/ROCK-dependent, there is an indication that Rho signaling is implicated in the mesenchymal process. Interestingly, a recent study showed that vincristine-treated gastric cancer cells activated GEF-H1/RhoA/ROCK/MLC signaling and promoted MAT[101].
Despite the poor mortality of patients with gastric cancer, only trastumab is available for molecular targeting therapy against gastric cancer[102]. Several preclinical and clinical studies have utilized inhibitors of Rho/ROCK signaling for anticancer therapeutics in prostate, lung, melanoma, glioblastoma, and several other tumor types with remarkable success[103-106]. The fact that Rho/ROCK is highly expressed in advanced gastric cancer suggests that its inhibition could be a potential therapeutic target for metastatic gastric cancer. Aberrant activation of ROCK may contribute to disastrous physiological consequences such as blood vessel constriction and extensive retraction of neuritis[107,108].
The most frequently used inhibitors of the Rho-ROCK pathway can be categorized into three classes: those inhibiting ROCK (RIs), geranylgeranyl transferase-1 (GGTIs), and 3-hydroxy-3-methylglutaryl-Coenzyme A (HMG-CoA) reductase[11]. Several ROCK inhibitors are currently the subject of clinical trials. For instance, fasudil (HA1077), a potent adenosine triphosphate (ATP) competitor for ROCK, has become prominent in the treatment of human diseases by ROCK inhibitors[109,110]. Other ROCK inhibitors such as Wf-536, H1152, and RKI-1447 have all been revealed to reduce tumor progression in hepatocellular cancer[111,112] and lung cancers[113], melanoma[114], and breast cancers[115], but no clinical report has utilized these inhibitors in gastric cancer.
Despite the interest in ROCK as a cancer therapeutic target, ROCK inhibitors have not yet progressed to clinical use. Because a common problem with kinase inhibitors is their tendency toward non-selectivity, there is an urgent need for alternative approaches to specifically target Rho/ROCK signaling pathways. Selective phosphorylation-specific antibodies for ROCK2 or ROCK1 may represent a new type of optimized inhibitor. The ROCK inhibitor, Y27632 and fasudil are not yet optimized for in vivo use[11]. However, these problems associated with inhibitors may be overcome by research using inactive RhoA expressing cells[116], siRNA targeting RhoA[117] and ROCK knockout mice[118,119]. Several groups have identified miRNAs targeting the RhoGTPase pathways in various cancer cell models, yielding a new source of knowledge regarding the underlying mechanisms regulating these pathways[120].
MAT, the likelihood of escape by cells that can switch between motility modes, makes it difficult to regulate the motility of all cancer cells by a single strategy. Rho/ROCK signaling contributes to amoeboid motility by promoting integrin, a protease-independent mode of tumor cell invasion. Our study implies that combined inhibition of ROCK and Rac restored the enhanced invasion of scirrhous gastric cancer cell lines[59]. These data suggest that drug combinations may produce greater anticancer effects by completely blocking the independent targets[121]. The combined inhibition of ROCK and MRCK kinases was found to be more effective in blocking actomyosin-mediated cell activities than either in isolation[122]. Further mechanistic studies as well as unbiased screening efforts are needed to identify additional potential agents among ROCK inhibitors.
In this review, we described the role of Rho GTPases and its effector protein as well as ROCK in gastric cancer progression and metastasis. The Rho/ROCK pathway is important in the regulation of pathways leading to enhanced malignancy of gastric cancer. Reorganization of the actin cytoskeleton plays a central role in the motility of cancer cells. A single tumor cell’s invasive strategy follows either a mesenchymal or amoeboid pattern, and certain cell types can use both modes of invasiveness and undergo transition between them. Inhibition of actin polymerization by Rho/ROCK inhibitors is a good candidate for cancer therapy. To completely elucidate the details of cancer cell motility, it is important to investigate more precisely the mechanisms underlying regulation of the actin cytoskeleton.
Although Rho GTPases- or ROCK-based therapy appear to have a potential role in gastric cancer therapy, the results of further clinical trials are still pending. Thus, it is necessary to identify new mechanisms that may offer great potential for defining new drug target sites, and to attempt use of a novel strategy for more selective therapeutic intervention. Several questions remain to be completely answered with regard to the optimization of Rho/ROCK target therapy, as outlined below: (1) what are the precise underlying mechanisms involved in MAT and AMT? Although MAT in response to protease inhibitors or integrin antagonists has been proposed to be a key event in the dissemination of invasive cells, the identification of factors regulating this conversion is still in its infancy; (2) can we identify response biomarkers to Rho/ROCK inhibition? The optimal patient subgroup that would benefit from single-agent ROCK inhibitory treatment remains to be determined. Further efforts to better define the molecular determinants of Rho/ROCK therapy response would be needed to unleash the complete impact of the targeted therapeutics; (3) what tumor types are most probable to benefit from Rho/ROCK inhibitory treatment? Accordingly, Rho/ROCK activation during amoeboid movement is believed to be relatively high, suggesting that Rho/ROCK is closely correlated with the progression and dedifferentiation of gastric cancer. Thus, it is vital to investigate more precisely the effect of Rho/ROCK inhibition in relation to the types of gastric cancer; and (4) if ROCK inhibitors are to be used for sustained periods of therapy, what are the potential chemoresistance liabilities?
It is believed that the clarification of these questions will provide an insight into how gastric cancer cells are integrated with various signaling pathways, which includes Rho/ROCK pathway. Till date, investigations of the signaling that govern these events in gastric cancer have only just begun, and new findings may contribute to the identification of new antimetastatic therapeutic targets for gastric cancer treatment.
P- Reviewer: Lu XM, Villanueva A S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Smyth EC, Cunningham D. Targeted therapy for gastric cancer. Curr Treat Options Oncol. 2012;13:377-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25538] [Article Influence: 1824.1] [Reference Citation Analysis (7)] |
| 3. | Fidler IJ. Critical determinants of cancer metastasis: rationale for therapy. Cancer Chemother Pharmacol. 1999;43 Suppl:S3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Wennerberg K, Der CJ. Rho-family GTPases: it’s not only Rac and Rho (and I like it). J Cell Sci. 2004;117:1301-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 448] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 5. | Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582:2093-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 612] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 6. | Schmitz AA, Govek EE, Böttner B, Van Aelst L. Rho GTPases: signaling, migration, and invasion. Exp Cell Res. 2000;261:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 418] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 7. | Knaus UG. Rho GTPase signaling in inflammation and transformation. Immunol Res. 2000;21:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Shi J, Wei L. Rho kinase in the regulation of cell death and survival. Arch Immunol Ther Exp (Warsz). 2007;55:61-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 9. | Liu S, Goldstein RH, Scepansky EM, Rosenblatt M. Inhibition of rho-associated kinase signaling prevents breast cancer metastasis to human bone. Cancer Res. 2009;69:8742-8751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 421] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 11. | Amin E, Dubey BN, Zhang SC, Gremer L, Dvorsky R, Moll JM, Taha MS, Nagel-Steger L, Piekorz RP, Somlyo AV. Rho-kinase: regulation, (dys)function, and inhibition. Biol Chem. 2013;394:1399-1410. [PubMed] |
| 12. | Komers R. Rho kinase inhibition in diabetic nephropathy. Curr Opin Nephrol Hypertens. 2011;20:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Kubo T, Yamaguchi A, Iwata N, Yamashita T. The therapeutic effects of Rho-ROCK inhibitors on CNS disorders. Ther Clin Risk Manag. 2008;4:605-615. [PubMed] |
| 14. | Saito M, Ohmasa F, Shomori K, Dimitriadis F, Ohiwa H, Shimizu S, Tsounapi P, Kinoshita Y, Satoh K. Rhos and Rho kinases in the rat prostate: their possible functional roles and distributions. Mol Cell Biochem. 2011;358:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Zhou Q, Gensch C, Liao JK. Rho-associated coiled-coil-forming kinases (ROCKs): potential targets for the treatment of atherosclerosis and vascular disease. Trends Pharmacol Sci. 2011;32:167-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 16. | Tang Y, Olufemi L, Wang MT, Nie D. Role of Rho GTPases in breast cancer. Front Biosci. 2008;13:759-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Cheng KW, Agarwal R, Mills GB. Ras-superfamily GTP-ases in ovarian cancer. Cancer Treat Res. 2009;149:229-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Grise F, Bidaud A, Moreau V. Rho GTPases in hepatocellular carcinoma. Biochim Biophys Acta. 2009;1795:137-151. [PubMed] |
| 19. | Ridley AJ. Historical overview of Rho GTPases. Methods Mol Biol. 2012;827:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp Cell Res. 2000;261:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 403] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 21. | Madaule P, Axel R. A novel ras-related gene family. Cell. 1985;41:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 348] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | Riento K, Villalonga P, Garg R, Ridley A. Function and regulation of RhoE. Biochem Soc Trans. 2005;33:649-651. [PubMed] |
| 23. | Wheeler AP, Ridley AJ. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res. 2004;301:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 374] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 24. | Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3598] [Cited by in RCA: 3694] [Article Influence: 160.6] [Reference Citation Analysis (0)] |
| 25. | Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4808] [Cited by in RCA: 4803] [Article Influence: 177.9] [Reference Citation Analysis (0)] |
| 26. | Doye A, Mettouchi A, Lemichez E. Assessing ubiquitylation of Rho GTPases in mammalian cells. Methods Mol Biol. 2012;827:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Boulter E, Garcia-Mata R, Guilluy C, Dubash A, Rossi G, Brennwald PJ, Burridge K. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol. 2010;12:477-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 272] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 28. | Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313-5327. [PubMed] |
| 29. | Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem. 1995;270:29051-29054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 559] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 30. | Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996;15:2208-2216. [PubMed] |
| 31. | Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1440] [Cited by in RCA: 1514] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 32. | Somlyo AV, Phelps C, Dipierro C, Eto M, Read P, Barrett M, Gibson JJ, Burnitz MC, Myers C, Somlyo AP. Rho kinase and matrix metalloproteinase inhibitors cooperate to inhibit angiogenesis and growth of human prostate cancer xenotransplants. FASEB J. 2003;17:223-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S, Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140:647-657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 691] [Cited by in RCA: 714] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 34. | Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken). 2010;67:545-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 635] [Cited by in RCA: 760] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 35. | Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1395] [Cited by in RCA: 1426] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 36. | Sumi T, Matsumoto K, Nakamura T. Specific activation of LIM kinase 2 via phosphorylation of threonine 505 by ROCK, a Rho-dependent protein kinase. J Biol Chem. 2001;276:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 237] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 37. | Mertsch S, Thanos S. Opposing signaling of ROCK1 and ROCK2 determines the switching of substrate specificity and the mode of migration of glioblastoma cells. Mol Neurobiol. 2014;49:900-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Morgan-Fisher M, Wewer UM, Yoneda A. Regulation of ROCK activity in cancer. J Histochem Cytochem. 2013;61:185-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 39. | Shi J, Wu X, Surma M, Vemula S, Zhang L, Yang Y, Kapur R, Wei L. Distinct roles for ROCK1 and ROCK2 in the regulation of cell detachment. Cell Death Dis. 2013;4:e483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 40. | Pinner S, Sahai E. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nat Cell Biol. 2008;10:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 214] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 41. | Yoneda A, Morgan-Fisher M, Wait R, Couchman JR, Wewer UM. A collapsin response mediator protein 2 isoform controls myosin II-mediated cell migration and matrix assembly by trapping ROCK II. Mol Cell Biol. 2012;32:1788-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 810] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 43. | Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1094] [Cited by in RCA: 1083] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 44. | Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int J Cancer. 1999;81:682-687. [PubMed] |
| 45. | Burbelo P, Wellstein A, Pestell RG. Altered Rho GTPase signaling pathways in breast cancer cells. Breast Cancer Res Treat. 2004;84:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 46. | Carr KM, Bittner M, Trent JM. Gene-expression profiling in human cutaneous melanoma. Oncogene. 2003;22:3076-3080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | Kamai T, Kawakami S, Koga F, Arai G, Takagi K, Arai K, Tsujii T, Yoshida KI. RhoA is associated with invasion and lymph node metastasis in upper urinary tract cancer. BJU Int. 2003;91:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | He M, Cheng Y, Li W, Liu Q, Liu J, Huang J, Fu X. Vascular endothelial growth factor C promotes cervical cancer metastasis via up-regulation and activation of RhoA/ROCK-2/moesin cascade. BMC Cancer. 2010;10:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Pan Y, Bi F, Liu N, Xue Y, Yao X, Zheng Y, Fan D. Expression of seven main Rho family members in gastric carcinoma. Biochem Biophys Res Commun. 2004;315:686-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 50. | Liu N, Zhang G, Bi F, Pan Y, Xue Y, Shi Y, Yao L, Zhao L, Zheng Y, Fan D. RhoC is essential for the metastasis of gastric cancer. J Mol Med (Berl). 2007;85:1149-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Wang ZN, Xu HM, Jiang L, Zhou X, Lu C, Zhang X. Positive association of RhoC gene overexpression with tumour invasion and lymphatic metastasis in gastric carcinoma. Chin Med J (Engl). 2005;118:502-504. [PubMed] |
| 52. | Kondo T, Sentani K, Oue N, Yoshida K, Nakayama H, Yasui W. Expression of RHOC is associated with metastasis of gastric carcinomas. Pathobiology. 2004;71:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Zhou J, Zhu Y, Zhang G, Liu N, Sun L, Liu M, Qiu M, Luo D, Tang Q, Liao Z. A distinct role of RhoB in gastric cancer suppression. Int J Cancer. 2011;128:1057-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Kang WK, Lee I, Park C. Characterization of RhoA-mediated chemoresistance in gastric cancer cells. Cancer Res Treat. 2005;37:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 55. | Harding MA, Theodorescu D. RhoGDI signaling provides targets for cancer therapy. Eur J Cancer. 2010;46:1252-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 56. | Ikeguchi M, Miyake T, Matsunaga T, Yamamoto M, Fukumoto Y, Yamada Y, Fukuda K, Saito H, Tatebe S, Tsujitani S. Recent results of therapy for scirrhous gastric cancer. Surg Today. 2009;39:290-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | Tahara E. Genetic pathways of two types of gastric cancer. IARC Sci Publ. 2004;327-349. [PubMed] |
| 58. | Yashiro M, Chung YS, Kubo T, Hato F, Sowa M. Differential responses of scirrhous and well-differentiated gastric cancer cells to orthotopic fibroblasts. Br J Cancer. 1996;74:1096-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Matsuoka T, Yashiro M, Kato Y, Shinto O, Kashiwagi S, Hirakawa K. RhoA/ROCK signaling mediates plasticity of scirrhous gastric carcinoma motility. Clin Exp Metastasis. 2011;28:627-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Shinto O, Yashiro M, Kawajiri H, Shimizu K, Shimizu T, Miwa A, Hirakawa K. Inhibitory effect of a TGFbeta receptor type-I inhibitor, Ki26894, on invasiveness of scirrhous gastric cancer cells. Br J Cancer. 2010;102:844-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 61. | Gómez del Pulgar T, Benitah SA, Valerón PF, Espina C, Lacal JC. Rho GTPase expression in tumourigenesis: evidence for a significant link. Bioessays. 2005;27:602-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 62. | Kamai T, Tsujii T, Arai K, Takagi K, Asami H, Ito Y, Oshima H. Significant association of Rho/ROCK pathway with invasion and metastasis of bladder cancer. Clin Cancer Res. 2003;9:2632-2641. [PubMed] |
| 63. | Kamai T, Yamanishi T, Shirataki H, Takagi K, Asami H, Ito Y, Yoshida K. Overexpression of RhoA, Rac1, and Cdc42 GTPases is associated with progression in testicular cancer. Clin Cancer Res. 2004;10:4799-4805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 190] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 64. | Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, Davies H, Teague J, Butler A, Stevens C. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2512] [Cited by in RCA: 2295] [Article Influence: 127.5] [Reference Citation Analysis (0)] |
| 65. | Zheng B, Liang L, Wang C, Huang S, Cao X, Zha R, Liu L, Jia D, Tian Q, Wu J. MicroRNA-148a suppresses tumor cell invasion and metastasis by downregulating ROCK1 in gastric cancer. Clin Cancer Res. 2011;17:7574-7583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 66. | Bryan BA, Dennstedt E, Mitchell DC, Walshe TE, Noma K, Loureiro R, Saint-Geniez M, Campaigniac JP, Liao JK, D’Amore PA. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J. 2010;24:3186-3195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 215] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 67. | del Peso L, Hernández-Alcoceba R, Embade N, Carnero A, Esteve P, Paje C, Lacal JC. Rho proteins induce metastatic properties in vivo. Oncogene. 1997;15:3047-3057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 116] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 68. | Wong CC, Wong CM, Au SL, Ng IO. RhoGTPases and Rho-effectors in hepatocellular carcinoma metastasis: ROCK N’Rho move it. Liver Int. 2010;30:642-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | Zhang S, Tang Q, Xu F, Xue Y, Zhen Z, Deng Y, Liu M, Chen J, Liu S, Qiu M. RhoA regulates G1-S progression of gastric cancer cells by modulation of multiple INK4 family tumor suppressors. Mol Cancer Res. 2009;7:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 70. | Wu Y, Tao Y, Chen Y, Xu W. RhoC regulates the proliferation of gastric cancer cells through interaction with IQGAP1. PLoS One. 2012;7:e48917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Lin MT, Lin BR, Chang CC, Chu CY, Su HJ, Chen ST, Jeng YM, Kuo ML. IL-6 induces AGS gastric cancer cell invasion via activation of the c-Src/RhoA/ROCK signaling pathway. Int J Cancer. 2007;120:2600-2608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 72. | Xu XT, Song QB, Yao Y, Ruan P, Tao ZZ. Inhibition of RhoA/ROCK signaling pathway promotes the apoptosis of gastric cancer cells. Hepatogastroenterology. 2012;59:2523-2526. [PubMed] |
| 73. | Liu Z, Zhu J, Cao H, Ren H, Fang X. miR-10b promotes cell invasion through RhoC-AKT signaling pathway by targeting HOXD10 in gastric cancer. Int J Oncol. 2012;40:1553-1560. [PubMed] |
| 74. | Zhou J, Li K, Gu Y, Feng B, Ren G, Zhang L, Wang Y, Nie Y, Fan D. Transcriptional up-regulation of RhoE by hypoxia-inducible factor (HIF)-1 promotes epithelial to mesenchymal transition of gastric cancer cells during hypoxia. Biochem Biophys Res Commun. 2011;415:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 75. | Olson MF, Sahai E. The actin cytoskeleton in cancer cell motility. Clin Exp Metastasis. 2009;26:273-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 431] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 76. | Lauffenburger DA. Cell motility. Making connections count. Nature. 1996;383:390-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 77. | Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1061] [Cited by in RCA: 1106] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 78. | Small JV, Stradal T, Vignal E, Rottner K. The lamellipodium: where motility begins. Trends Cell Biol. 2002;12:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 682] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 79. | Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235-1244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1126] [Cited by in RCA: 1143] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 80. | Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2304] [Cited by in RCA: 2338] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 81. | Yamazaki D, Kurisu S, Takenawa T. Regulation of cancer cell motility through actin reorganization. Cancer Sci. 2005;96:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 470] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 82. | Lozano E, Betson M, Braga VM. Tumor progression: Small GTPases and loss of cell-cell adhesion. Bioessays. 2003;25:452-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 83. | Friedl P. Prespecification and plasticity: shifting mechanisms of cell migration. Curr Opin Cell Biol. 2004;16:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 462] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 84. | Tamariz E, Grinnell F. Modulation of fibroblast morphology and adhesion during collagen matrix remodeling. Mol Biol Cell. 2002;13:3915-3929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 186] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 85. | Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, Strongin AY, Bröcker EB, Friedl P. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160:267-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1107] [Cited by in RCA: 1078] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 86. | Friedl P, Borgmann S, Bröcker EB. Amoeboid leukocyte crawling through extracellular matrix: lessons from the Dictyostelium paradigm of cell movement. J Leukoc Biol. 2001;70:491-509. [PubMed] |
| 87. | Wang W, Wyckoff JB, Frohlich VC, Oleynikov Y, Hüttelmaier S, Zavadil J, Cermak L, Bottinger EP, Singer RH, White JG. Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res. 2002;62:6278-6288. [PubMed] |
| 88. | Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 909] [Cited by in RCA: 904] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 89. | Tournaviti S, Hannemann S, Terjung S, Kitzing TM, Stegmayer C, Ritzerfeld J, Walther P, Grosse R, Nickel W, Fackler OT. SH4-domain-induced plasma membrane dynamization promotes bleb-associated cell motility. J Cell Sci. 2007;120:3820-3829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 90. | Charras GT, Hu CK, Coughlin M, Mitchison TJ. Reassembly of contractile actin cortex in cell blebs. J Cell Biol. 2006;175:477-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 503] [Cited by in RCA: 465] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 91. | Vasiliev JM, Omelchenko T, Gelfand IM, Feder HH, Bonder EM. Rho overexpression leads to mitosis-associated detachment of cells from epithelial sheets: a link to the mechanism of cancer dissemination. Proc Natl Acad Sci USA. 2004;101:12526-12530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 92. | Sahai E, Garcia-Medina R, Pouysségur J, Vial E. Smurf1 regulates tumor cell plasticity and motility through degradation of RhoA leading to localized inhibition of contractility. J Cell Biol. 2007;176:35-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 93. | Parri M, Taddei ML, Bianchini F, Calorini L, Chiarugi P. EphA2 reexpression prompts invasion of melanoma cells shifting from mesenchymal to amoeboid-like motility style. Cancer Res. 2009;69:2072-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 94. | Gadea G, de Toledo M, Anguille C, Roux P. Loss of p53 promotes RhoA-ROCK-dependent cell migration and invasion in 3D matrices. J Cell Biol. 2007;178:23-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 95. | Panková K, Rösel D, Novotný M, Brábek J. The molecular mechanisms of transition between mesenchymal and amoeboid invasiveness in tumor cells. Cell Mol Life Sci. 2010;67:63-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 241] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 96. | Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 769] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 97. | Salhia B, Rutten F, Nakada M, Beaudry C, Berens M, Kwan A, Rutka JT. Inhibition of Rho-kinase affects astrocytoma morphology, motility, and invasion through activation of Rac1. Cancer Res. 2005;65:8792-8800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 136] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 98. | Worthylake RA, Lemoine S, Watson JM, Burridge K. RhoA is required for monocyte tail retraction during transendothelial migration. J Cell Biol. 2001;154:147-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 381] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 99. | Yashiro M, Chung YS, Sowa M. Role of orthotopic fibroblasts in the development of scirrhous gastric carcinoma. Jpn J Cancer Res. 1994;85:883-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 100. | Nakazawa K, Yashiro M, Hirakawa K. Keratinocyte growth factor produced by gastric fibroblasts specifically stimulates proliferation of cancer cells from scirrhous gastric carcinoma. Cancer Res. 2003;63:8848-8852. [PubMed] |
| 101. | Eitaki M, Yamamori T, Meike S, Yasui H, Inanami O. Vincristine enhances amoeboid-like motility via GEF-H1/RhoA/ROCK/Myosin light chain signaling in MKN45 cells. BMC Cancer. 2012;12:469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 102. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5309] [Article Influence: 353.9] [Reference Citation Analysis (3)] |
| 103. | Somlyo AV, Bradshaw D, Ramos S, Murphy C, Myers CE, Somlyo AP. Rho-kinase inhibitor retards migration and in vivo dissemination of human prostate cancer cells. Biochem Biophys Res Commun. 2000;269:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 182] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 104. | Rattan R, Giri S, Singh AK, Singh I. Rho/ROCK pathway as a target of tumor therapy. J Neurosci Res. 2006;83:243-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 105. | Routhier A, Astuccio M, Lahey D, Monfredo N, Johnson A, Callahan W, Partington A, Fellows K, Ouellette L, Zhidro S. Pharmacological inhibition of Rho-kinase signaling with Y-27632 blocks melanoma tumor growth. Oncol Rep. 2010;23:861-867. [PubMed] |
| 106. | Amine A, Rivera S, Opolon P, Dekkal M, Biard DS, Bouamar H, Louache F, McKay MJ, Bourhis J, Deutsch E. Novel anti-metastatic action of cidofovir mediated by inhibition of E6/E7, CXCR4 and Rho/ROCK signaling in HPV tumor cells. PLoS One. 2009;4:e5018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 107. | Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov. 2005;4:387-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 470] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 108. | Lee DL, Webb RC, Jin L. Hypertension and RhoA/Rho-kinase signaling in the vasculature: highlights from the recent literature. Hypertension. 2004;44:796-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 109. | Olson MF. Applications for ROCK kinase inhibition. Curr Opin Cell Biol. 2008;20:242-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 299] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 110. | Ying H, Biroc SL, Li WW, Alicke B, Xuan JA, Pagila R, Ohashi Y, Okada T, Kamata Y, Dinter H. The Rho kinase inhibitor fasudil inhibits tumor progression in human and rat tumor models. Mol Cancer Ther. 2006;5:2158-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 111. | Itoh K, Yoshioka K, Akedo H, Uehata M, Ishizaki T, Narumiya S. An essential part for Rho-associated kinase in the transcellular invasion of tumor cells. Nat Med. 1999;5:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 491] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 112. | Takamura M, Sakamoto M, Genda T, Ichida T, Asakura H, Hirohashi S. Inhibition of intrahepatic metastasis of human hepatocellular carcinoma by Rho-associated protein kinase inhibitor Y-27632. Hepatology. 2001;33:577-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 113. | Nakajima M, Hayashi K, Katayama K, Amano Y, Egi Y, Uehata M, Goto N, Kondo T. Wf-536 prevents tumor metastasis by inhibiting both tumor motility and angiogenic actions. Eur J Pharmacol. 2003;459:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 114. | Nakajima M, Hayashi K, Egi Y, Katayama K, Amano Y, Uehata M, Ohtsuki M, Fujii A, Oshita K, Kataoka H. Effect of Wf-536, a novel ROCK inhibitor, against metastasis of B16 melanoma. Cancer Chemother Pharmacol. 2003;52:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 115. | Patel RA, Liu Y, Wang B, Li R, Sebti SM. Identification of novel ROCK inhibitors with anti-migratory and anti-invasive activities. Oncogene. 2014;33:550-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 116. | Kolavennu V, Zeng L, Peng H, Wang Y, Danesh FR. Targeting of RhoA/ROCK signaling ameliorates progression of diabetic nephropathy independent of glucose control. Diabetes. 2008;57:714-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 117. | Peng F, Wu D, Gao B, Ingram AJ, Zhang B, Chorneyko K, McKenzie R, Krepinsky JC. RhoA/Rho-kinase contribute to the pathogenesis of diabetic renal disease. Diabetes. 2008;57:1683-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 118. | Zhou L, Liu F, Huang XR, Liu F, Chen H, Chung AC, Shi J, Wei L, Lan HY, Fu P. Amelioration of albuminuria in ROCK1 knockout mice with streptozotocin-induced diabetic kidney disease. Am J Nephrol. 2011;34:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 119. | Zhou Q, Mei Y, Shoji T, Han X, Kaminski K, Oh GT, Ongusaha PP, Zhang K, Schmitt H, Moser M. Rho-associated coiled-coil-containing kinase 2 deficiency in bone marrow-derived cells leads to increased cholesterol efflux and decreased atherosclerosis. Circulation. 2012;126:2236-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 120. | Valastyan S, Reinhardt F, Benaich N, Calogrias D, Szász AM, Wang ZC, Brock JE, Richardson AL, Weinberg RA. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell. 2009;137:1032-1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 663] [Cited by in RCA: 660] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 121. | Carragher NO, Walker SM, Scott Carragher LA, Harris F, Sawyer TK, Brunton VG, Ozanne BW, Frame MC. Calpain 2 and Src dependence distinguishes mesenchymal and amoeboid modes of tumour cell invasion: a link to integrin function. Oncogene. 2006;25:5726-5740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 122. | Heikkila T, Wheatley E, Crighton D, Schroder E, Boakes A, Kaye SJ, Mezna M, Pang L, Rushbrooke M, Turnbull A. Co-crystal structures of inhibitors with MRCKβ, a key regulator of tumor cell invasion. PLoS One. 2011;6:e24825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |