Published online Oct 7, 2014. doi: 10.3748/wjg.v20.i37.13625
Revised: April 1, 2014
Accepted: May 29, 2014
Published online: October 7, 2014
Processing time: 263 Days and 20.5 Hours
Inflammatory myofibroblastic tumors are usually treated by surgical resection. We herein report two cases of intra-abdominal inflammatory myofibroblastic tumors that were unresectable and underwent spontaneous regression without any treatment. Our case report and literature review show that regression is more common in the middle-aged and older male populations. Abdominal discomfort and fever were the most common symptoms, but the majority of patients had no obvious physical signs. There was no specific indicator for diagnosis. The majority of the lesions regressed within 3 mo and nearly all of the masses completely resolved within 1 year. We conclude that the clinical characteristics of inflammatory myofibroblastic tumors are variable and, accordingly, the disease needs to be subdivided and treated on an individual basis. Surgery is always the first-line treatment; however, for those masses assessed as unresectable, conservative therapy with intense follow-up should be considered.
Core tip: This article reports two rare cases involving the spontaneous regression of an unresectable intra-abdominal inflammatory myofibroblastic tumor (IMT) and summarizes the clinical characteristics of all published cases. The clinical characteristics of IMT are not clear and the treatment is controversial. By analyzing published papers, we discovered some common features of the disease and potential reasons for this unusual phenomenon. Our findings may be useful for the clinical diagnosis and treatment of IMT.
- Citation: Zhao JJ, Ling JQ, Fang Y, Gao XD, Shu P, Shen KT, Qin J, Sun YH, Qin XY. Intra-abdominal inflammatory myofibroblastic tumor: Spontaneous regression. World J Gastroenterol 2014; 20(37): 13625-13631
- URL: https://www.wjgnet.com/1007-9327/full/v20/i37/13625.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i37.13625
Inflammatory myofibroblastic tumor (IMT), also called inflammatory pseudotumor (IPT), is a rare disease commonly found in children and adolescents[1].
The etiology of IMT remains unclear, but is most likely associated with inflammation, trauma[2], viral infection[3], chromosome translocation[4], and gene fusion[5]. Although IMT has long been profiled as a benign lesion, it has presented aggressive behavior in some cases due to local spread, recurrence, and metastasis[6,7]. In 2003, IMT was classified as an intermediate neoplasm in the current World Health Organization (WHO) histologic typing[7] and characterized by a mixture of myofibroblasts, fibroblasts, lymphocytes, and plasma cells[8].
Surgery has long been regarded as the most effective and radical treatment. However, there were sporadic cases which reported the effectiveness of using antibiotics[9], corticosteroids[10], nonsteroidal anti-inflammatory drugs (NSAIDs)[2], and even non-intervention[11], which may provide new insights into the therapeutic approach for IMT, especially for unresectable lesions.
Herein, we present two cases of unresectable IMT arising from the abdominal cavity, both eventually resolving completely without any treatment. We also reviewed the relevant literature, with an emphasis on the spontaneous regression of intra-abdominal IMT, and summarized the clinical characteristics of these patients.
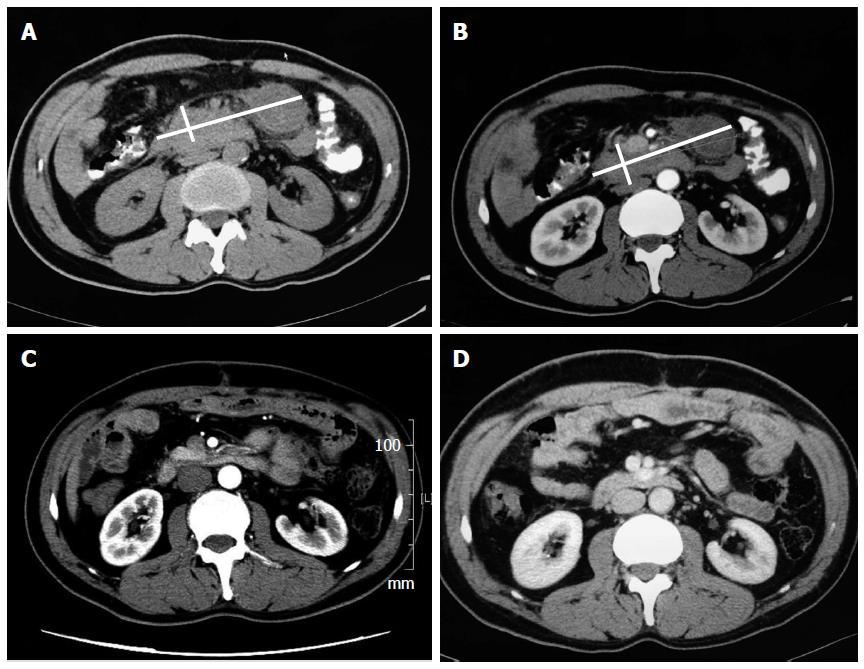
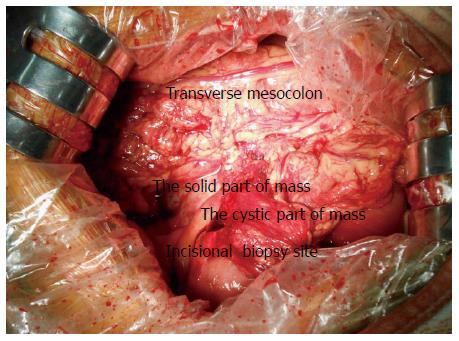
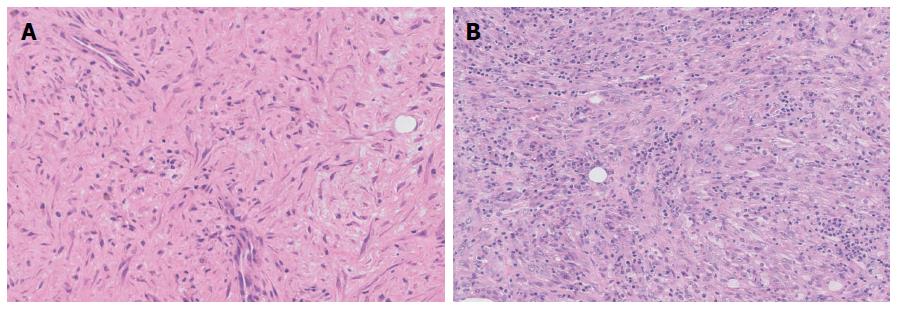
A 49-year-old male sought medical treatment with symptoms including paroxysmal abdominal pain, nausea, and vomiting, which recurred over 2 wk. A large mass was palpated in the epigastrium. Laboratory tests showed elevated neutrophil count (6.7 × 109/L), C-reactive protein (CRP) (27.9 mg/L), and lactic dehydrogenase (383 U/L). Tumor markers were normal except for CA125 (44.8 IU/mL). A computed tomography (CT) scan of the abdomen revealed the presence of a very large nodular mass in the upper quadrant of the peritoneum (Figure 1A and B). Although the diagnosis was not confirmed, the clinical evidence indicated that the lesions were likely to be a malignant tumor. Based on this diagnosis, the patient was admitted to surgery. During exploration, a 15 cm × 8 cm large mass was discovered in the retro-peritoneum that was attached to the posterior wall of the abdomen (Figure 2). We performed an incisional biopsy of the mass at different sites. The histological diagnosis was of a fibroinflammatory proliferation, with immunohistochemistry staining results showing negative expressions of CD34, desmin, and anaplastic lymphoma kinase (ALK), as well as a positive expression of SMA, which is consistent with IMT (Figure 3A). Because the mass was unresectable, we only performed gastrojejunostomy to relieve the obstruction. After surgery, the patient presented with delayed gastric empting and was completely symptom-free 3 wk later. Before discharging, the patient was re-examined by CT scan, which showed the apparent and spontaneous regression of the mass without a residual small tumor or inflammation remaining (Figure 1C). A CT scan three months later showed no indication of relapse (Figure 1D).
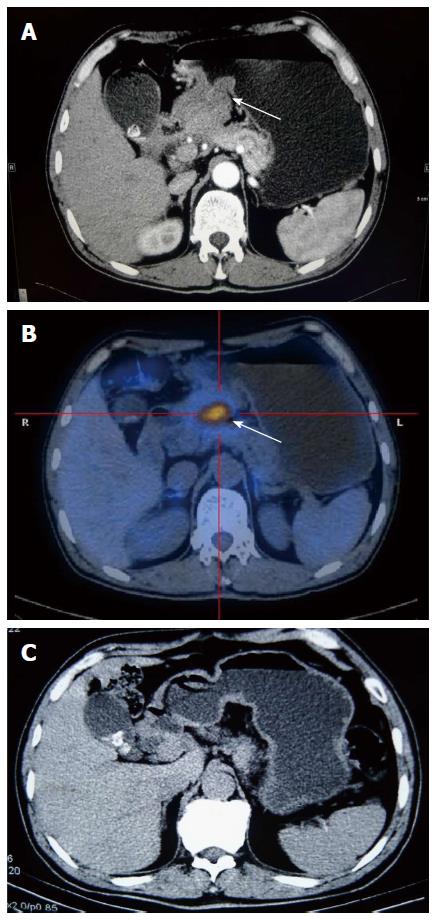
A 59-year-old man was hospitalized with a 1-mo history of abdominal distension, poor appetite, and weight loss of more than 5 kg. He was addicted to alcohol and tobacco and had uncontrolled diabetes mellitus. Clinical examination was unremarkable other than mild abdominal tenderness. Laboratory tests, including a routine hepatic function test, showed that C-reactive protein and tumor markers were all within the normal range. Gastroscopy detected an elevated lesion at the pylorus, which was likely from the extra-cavity. We twice performed an endoscopic biopsy, but both findings were of superficial gastritis. A CT scan and a PET-CT scan both demonstrated that a presumptive malignant mass was located between the gastric antrum and the neck of the pancreas (Figure 4A and B). Although we did not acquire a pathological diagnosis, we performed an exploratory laparotomy. During the surgery, we confirmed that the mass was located at the posterior wall of the gastric antrum and had invaded the pancreas, transverse mesocolon, and mesentery, which resulted in an inability to perform an en bloc resection. We only removed the subpyloric lymph nodes for a pathological assay. Histological examination showed conspicuous proliferation of myofibroblasts admixed with abundant inflammatory cells (Figure 3B), and the immunohistochemistry results demonstrated that CD34, desmin, and ALK were negative, while SMA was positive. Based on these findings, the diagnosis of IMT was made. The patient did not receive any treatment and became symptom-free one month later. The tumor mass regression was verified using a CT scan (Figure 4C). After discharging, the patient underwent a CT scan every three months at a local hospital and did not have any indication of recurrence during a one-year follow-up.
A systematic search of PubMed and Embase was performed. We listed 20 reports published from 1992 to date in Table 1, with a sum of 36 intra-abdominal IMT or IPT patients who had spontaneous regression without surgical excision[1,2,7-24]. In an effort to assess the biological behavior of IMT, we summarized the clinical characteristics of a total of 38 patients consisting of 36 reported cases as well as our two cases.
| Ref. | Year | Cases n | Location |
| Gollapudi et al[12] | 1992 | 1 | Liver |
| Jaïs et al[13] | 1995 | 1 | Liver |
| Su et al[2] | 2000 | 1 | Mesentery |
| Koea et al[10] | 2003 | 6 | Liver |
| Biecker et al[14] | 2003 | 1 | Liver |
| Thompson et al[15] | 2003 | 1 | Mesentery |
| Tanikawa et al[16] | 2003 | 1 | Periureteral |
| Przkora et al[17] | 2004 | 2 | Retro-peritoneum |
| Colakoglu et al[18] | 2005 | 1 | Liver |
| Koide et al[19] | 2006 | 1 | Liver |
| Tsou et al[9] | 2007 | 4 | Liver |
| Yamaguchi et al[20] | 2007 | 3 | Liver |
| Vassiliadis et al[21] | 2007 | 1 | Liver |
| Motojuku et al[22] | 2008 | 1 | Liver |
| Mattei et al[8] | 2008 | 1 | Retro-peritoneum |
| Goldsmith et al[23] | 2009 | 5 | Liver |
| Brage-Varela et al[24] | 2010 | 1 | Liver |
| Jerraya et al[11] | 2011 | 1 | Liver |
| Fragoso et al[7] | 2011 | 2 | Liver and pancreas |
| Shatzel et al[1] | 2012 | 1 | Mesentery |
Age and gender: Including our two cases, the median age of patients was 44 years (ranging from a few months to 79 years). The largest population was the middle-aged and elderly, with a percentage of 60.6%. There were 25 male patients, nearly twice as many as female patients.
Symptoms: Twenty-five of 31 patients were symptomatic at presentation (no data were available for 7 cases). The most common symptoms were abdominal pain (58.1%), fever (45.2%), weight loss (22.6%), appetite loss (12.9%), and nausea/vomiting (12.9%). Nearly 19.4% of patients were completely asymptomatic.
Physical examination: The majority of the 27 available cases did not show obvious signs of disease (70.4%). As the liver was the most frequent location, palpable enlargement of the liver was the most common sign (14.8%), followed by abdominal mass (7.4%) and tenderness (7.4%).
Laboratory examination: Nearly one in five patients had no aberrant findings (26 case files were available). Elevated white blood cell (WBC) count accounted for the highest percentage of findings (34.6%); liver function abnormity (30.8%), elevated CRP (19.2%), and erythrocyte sedimentation rate (ESR) (19.2%) were also frequent. Other abnormalities included hypoalbuminemia (15.4%), elevated tumor markers (15.4%), and anemia (15.4%).
Location: The liver was the most common location, making up 71.8% of all the reported cases. Retro-peritoneum, mesentery, gastrointestinal tract, and pancreas were also reported locations.
Size: The maximum diameter of the tumor was reported in 21 cases, and ranged from 1 cm to 15 cm. The average size was 6.1 cm.
Diagnosis: A total of 31 cases were diagnosed before treatment. Among them, 25 cases were diagnosed as IMT or IPT (80.6%) and led to conservative therapy. Nevertheless, 5 cases were diagnosed as malignant tumors, and so abdominal exploration was performed. All hepatic lesions were diagnosed before treatment, with the accuracy reaching 95.7%. However, only 77.8% of the masses located at the retro-peritoneum and mesentery could be diagnosed, and more than half of the masses were misdiagnosed as malignant tumors.
Management: Seventeen out of 38 cases did not receive any intervention vs 21 out of 38 cases that were managed medically. The most commonly used therapy was NSAIDs (15.8%), followed by steroids (13.2%) and antibiotics (10.5%). Of the remaining cases, 3 patients received a combination of medicines.
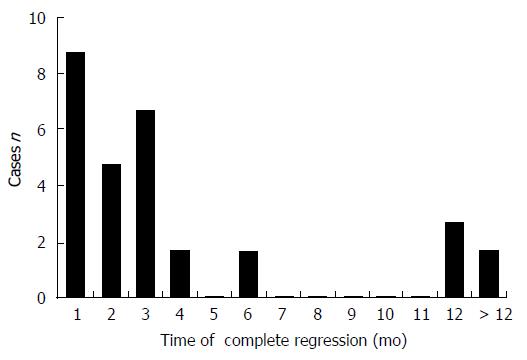
Outcome: All 38 patients were reported as thriving during the follow-up period. Thirty reports described the specific time that the tumor regressed, ranging from a few days to 3 years (Figure 5).
Although IMT and IPT were formerly regarded as the same disease, some authors argue they are distinct entities. As there is no uniform view at present, the use of these two terms synonymously in the literature has led to confusion in understanding the incidence and behavior of the disease. In this article, we still attribute IMT to the broad category of IPT for now. IMT was initially identified in the lungs in 1937. Since then, it has been described at various sites[12]. As a result of various origins, IMT may present with diverse clinical symptoms, physical signs, and laboratory findings, which makes it difficult to differentiate from other neoplasms[21]. Radiological appearance is nonspecific and insufficient to make a correct diagnosis. At present, percutaneous biopsy is considered the most useful tool. Meis et al[25] reported that most cases of IMT expressed actin, vimentin, and keratin. Chan et al[26] speculated that the expression of ALK may be a specific marker for IMT. However, a biopsy is unable to provide an absolutely certain diagnosis[21], especially for tumors located at the retro-peritoneum. Therefore, we recommend laparoscopic exploration first for these undiagnosed cases. Large samples taken during a laparoscopic biopsy may increase the reliability of the histopathology results.
The reasons for the spontaneous regression of IMT have not been clearly elucidated; however, by analyzing the published cases, we can generate some hypotheses for further investigation.
First, different tumor locations might influence the prognosis of IMT. Hepatic lesions have the most favorable outcome; in contrast, tumors occurring in the abdomen, pelvis, and retro-peritoneum tend to show more aggressive behavior and have a poor prognosis[6,27]. Location is also associated with age and gender. The mean age of patients with IMT is reported to be as low as 9.7 years, and females account for approximately 60% of patients. By contrast, liver lesions usually develop in men of middle or advanced ages[22].
Second, regression cases are more frequently observed in middle-aged and older patients. Therefore, age may have an impact on prognosis. Studies showed that ALK positivity was detected in 36% to 60% of cases and was associated with cytogenetic abnormalities[28] and local recurrence[27]. Interestingly, the ALK gene was expressed selectively in younger patients[26] and may contribute to the unfavorable prognosis of children and adolescents.
Third, recurrence is related to a number of factors, such as aneuploidy, atypia, and ganglion-like cells. Coffin et al[29] demonstrated that nearly 75% of aneuploid IMT had recurrence and malignant transformation. Hussong et al[30] reported that atypia and ganglion-like cells were detected much more in cases with recurrence and malignant transformation.
As discussed above, we conclude that the prognosis of IMT is closely related to location, age, and ALK positivity. This is also the area of dispute regarding the diagnosis of IMT and IPT. Vecchio suggested the IPT, rather than IMT, should be seriously considered when managing ALK-negative spindle cell lesions in adult patients[31]. Gleason also agreed that one should be wary of making the diagnosis of IMT in these populations and recommended taking many factors into consideration, including age, location, histological pattern, and ALK expression[32]. We deem that both IPT and IMT must be subdivided into different types according to location, age, histology, gene expression, and chromosome condition. Different types may have dramatically different outcomes and, as a result, the therapeutic approach should be determined accordingly.
Complete resection has been widely accepted as the mainstay treatment, although surgery could be destructive to adjacent structures and increase morbidity[2]. Adjuvant chemotherapy in conjunction with radiation therapy is also controversial[8,33].
With more spontaneous regression cases being described, there is an increasing realization of conservative management[10,13,34]. Although some articles have reported the recurrence[6] or malignant transformation[35,36] of IMT, Goldsmith et al[23] compared the mortality rate of 215 liver IMT patients between medical management and resection surgery and found no significant difference. In terms of conservative options, there is no obvious difference in overall outcome between patients receiving antibiotics, steroids, or no treatment[23].
Although conservative therapies have been effective for a number of patients, these treatments are all empirical, and the mechanisms are not well understood. Some recent studies[8,34] reported that the majority of IMT tissues were positive for COX-2 and VEGF staining, and proposed that NSAIDs could have an anti-angiogenic effect via the inhibition of COX-2. Others[22] found that many plasma cells infiltrating at IMT lesions were positive for IgG4, and speculated that IMT was associated with an immune process. Therefore, the administration of steroids might prevent an immune response and reduce systemic symptoms[10].
To date, the optimal treatment of IMT is far from conclusive and needs to be further studied. We agree that resection should be the initial treatment for those patients with mass effect symptoms, those where the tumor mass tends to increase or is easily excised, and those in which a certain diagnosis cannot be made by biopsy[21,22]. However, when the mass is assessed as unresectable by a CT scan or laparoscopic exploration, surgical procedures should be avoided and conservative therapy with antibiotics, steroids, NSAIDs, or observation along with intense follow-up, should be taken into consideration[2].
We thank the pathologists and the radiologists in our hospital for their technical assistance.
The symptoms of two middle-aged male patients were variable; one presented with abdominal pain and vomiting, and the other with abdominal distension, poor appetite, and weight loss.
The physical signs of the two cases were also variable. A large mass was palpated in the epigastrium for the first patient, while mild abdominal tenderness was observed during examination for the second.
Malignant tumors (angiosarcoma, cystadenocarcinoma, and metastatic tumors), benign neoplasms (focal nodular hyperplasia, hemangioma, and adenoma), and abscesses.
The first patient had an elevated neutrophil count (6.7 × 109/L), C-reactive protein (27.9 mg/L), lactic dehydrogenase (383 U/L), and CA125 (44.8 IU/mL), while the second had no remarkable abnormal laboratory results.
A computed tomography scan showed a large mass located in the abdominal cavity in both cases.
Histological examination showed a proliferation of myofibroblasts and an infiltration of inflammation cells. IHC staining showed CD34, desmin, and anaplastic lymphoma kinase (ALK) were negative, while SMA was positive in both cases.
Neither patient received curative resection or further treatment.
Spontaneous regression of an intra-abdominal inflammatory myofibroblastic tumor is seldom reported. The clinical and pathological characteristics of inflammatory myofibroblastic tumor are unclear and the treatment is controversial.
This case report represents the clinical characteristics of intra-abdominal inflammatory myofibroblastic tumors and also discusses the treatment of inflammatory myofibroblastic tumors. We recommend that conservative therapy should be considered when the tumor is unresectable, especially for middle-aged patients with negative ALK expression.
The authors have described two cases of intra-abdominal inflammatory myofibroblastic tumor which showed spontaneous resolution without intervention. The article highlights an important point which has important therapeutic implications.
P- Reviewer: Magro G, Park SB, Prabhu SM S- Editor: Ma YJ L- Editor: Rutherford A E- Editor: Wang CH
| 1. | Shatzel J, Wooten K, Ankola A, Cheney RT, Morrison CD, Skitzki JJ. Inflammatory myofibroblastic tumor of the mesentery: a clinical dilemma. Int J Clin Oncol. 2012;17:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Su W, Ko A, O’Connell T, Applebaum H. Treatment of pseudotumors with nonsteroidal antiinflammatory drugs. J Pediatr Surg. 2000;35:1635-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Arber DA, Kamel OW, van de Rijn M, Davis RE, Medeiros LJ, Jaffe ES, Weiss LM. Frequent presence of the Epstein-Barr virus in inflammatory pseudotumor. Hum Pathol. 1995;26:1093-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 217] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Sirvent N, Hawkins AL, Moeglin D, Coindre JM, Kurzenne JY, Michiels JF, Barcelo G, Turc-Carel C, Griffin CA, Pedeutour F. ALK probe rearrangement in a t(2; 11; 2)(p23; p15; q31) translocation found in a prenatal myofibroblastic fibrous lesion: toward a molecular definition of an inflammatory myofibroblastic tumor family? Genes Chromosomes Cancer. 2001;31:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Milne AN, Sweeney KJ, O’Riordain DS, Pauwels P, Debiec-Rychter M, Offerhaus GJ, Jeffers M. Inflammatory myofibroblastic tumor with ALK/TPM3 fusion presenting as ileocolic intussusception: an unusual presentation of an unusual neoplasm. Hum Pathol. 2006;37:112-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Lopez-Tomassetti Fernandez EM, Luis HD, Malagon AM, Gonzalez IA, Pallares AC. Recurrence of inflammatory pseudotumor in the distal bile duct: lessons learned from a single case and reported cases. World J Gastroenterol. 2006;12:3938-3943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Fragoso AC, Eloy C, Estevão-Costa J, Campos M, Farinha N, Lopes JM. Abdominal inflammatory myofibroblastic tumor a clinicopathologic study with reappraisal of biologic behavior. J Pediatr Surg. 2011;46:2076-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Mattei P, Barnaby K. Rapid regression of duodenal inflammatory myofibroblastic tumor after intravenous ketorolac: case report and review of the literature. J Pediatr Surg. 2008;43:1196-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Tsou YK, Lin CJ, Liu NJ, Lin CC, Lin CH, Lin SM. Inflammatory pseudotumor of the liver: report of eight cases, including three unusual cases, and a literature review. J Gastroenterol Hepatol. 2007;22:2143-2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Koea JB, Broadhurst GW, Rodgers MS, McCall JL. Inflammatory pseudotumor of the liver: demographics, diagnosis, and the case for nonoperative management. J Am Coll Surg. 2003;196:226-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Jerraya H, Jarboui S, Daghmoura H, Zaouche A. A new case of spontaneous regression of inflammatory hepatic pseudotumor. Case Rep Med. 2011;2011:139125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Gollapudi P, Chejfec G, Zarling EJ. Spontaneous regression of hepatic pseudotumor. Am J Gastroenterol. 1992;87:214-217. [PubMed] |
| 13. | Jaïs P, Berger JF, Vissuzaine C, Paramelle O, Clays-Schouman E, Potet F, Mignon M. Regression of inflammatory pseudotumor of the liver under conservative therapy. Dig Dis Sci. 1995;40:752-756. [PubMed] |
| 14. | Biecker E, Zimmermann A, Dufour JF. Spontaneous regression of an inflammatory pseudotumor of the liver. Z Gastroenterol. 2003;41:991-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Thompson RJ, Barrett AM, Dildey P. Congenital multifocal inflammatory pseudotumor: a case report. J Pediatr Surg. 2003;38:E17-E19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Tanikawa G, Takaha N, Nonomura N, Okuyama A. [Periureteral inflammatory pseudotumor: a case report]. Hinyokika Kiyo. 2003;49:595-597. [PubMed] |
| 17. | Przkora R, Bolder U, Schwarz S, Jauch KW, Spes J, Andreesen R, Mackensen A. Regression of nonresectable inflammatory myofibroblastic tumours after treatment with nonsteroidal anti-inflammatory drugs. Eur J Clin Invest. 2004;34:320-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Colakoglu O, Unsal B, Haciyanli M, Tunakan M, Buyrac Z, Yorukoglu G, Yazicioglu N, Genc H. A succesfully managed inflammatory pseudotumour of liver without surgery: report of a case. Acta Gastroenterol Belg. 2005;68:382-384. [PubMed] |
| 19. | Koide H, Sato K, Fukusato T, Kashiwabara K, Sunaga N, Tsuchiya T, Morino S, Sohara N, Kakizaki S, Takagi H. Spontaneous regression of hepatic inflammatory pseudotumor with primary biliary cirrhosis: case report and literature review. World J Gastroenterol. 2006;12:1645-1648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Yamaguchi J, Sakamoto Y, Sano T, Shimada K, Kosuge T. Spontaneous regression of inflammatory pseudotumor of the liver: report of three cases. Surg Today. 2007;37:525-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Vassiliadis T, Vougiouklis N, Patsiaoura K, Mpoumponaris A, Nikolaidis N, Giouleme O, Evgenidis N. Inflammatory pseudotumor of the liver successfully treated with nonsteroidal anti-inflammatory drugs: a challenge diagnosis for one not so rare entity. Eur J Gastroenterol Hepatol. 2007;19:1016-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Motojuku M, Oida Y, Morikawa G, Hoshikawa T, Nakamura T, Tajima T, Mukai M, Otsuka H, Akieda K, Hirabayashi K. Inflammatory pseudotumor of the liver: case report and review of literature. Tokai J Exp Clin Med. 2008;33:70-74. [PubMed] |
| 23. | Goldsmith PJ, Loganathan A, Jacob M, Ahmad N, Toogood GJ, Lodge JP, Prasad KR. Inflammatory pseudotumours of the liver: a spectrum of presentation and management options. Eur J Surg Oncol. 2009;35:1295-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Brage-Varela A, Estévez-Boullosa P, Pombo-Otero J, Blanco-Rodríguez M, Lago-Novoa M, Arnal-Monreal F. Multifocal hepatic inflammatory pseudotumor: spontaneous regression in a diabetic patient. Rev Esp Enferm Dig. 2010;102:507-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Meis JM, Enzinger FM. Inflammatory fibrosarcoma of the mesentery and retroperitoneum. A tumor closely simulating inflammatory pseudotumor. Am J Surg Pathol. 1991;15:1146-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 309] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 26. | Chan JK, Cheuk W, Shimizu M. Anaplastic lymphoma kinase expression in inflammatory pseudotumors. Am J Surg Pathol. 2001;25:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 163] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31:509-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 621] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 28. | Saleem MI, Ben-Hamida MA, Barrett AM, Bunn SK, Huntley L, Wood KM, Yelbuz TM. Lower abdominal inflammatory myofibroblastic tumor -an unusual presentation- a case report and brief literature review. Eur J Pediatr. 2007;166:679-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Coffin CM, Patel A, Perkins S, Elenitoba-Johnson KS, Perlman E, Griffin CA. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol. 2001;14:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 407] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 30. | Hussong JW, Brown M, Perkins SL, Dehner LP, Coffin CM. Comparison of DNA ploidy, histologic, and immunohistochemical findings with clinical outcome in inflammatory myofibroblastic tumors. Mod Pathol. 1999;12:279-286. [PubMed] |
| 31. | Vecchio GM, Amico P, Grasso G, Vasquez E, La Greca G, Magro G. Post-traumatic inflammatory pseudotumor of the breast with atypical morphological features: A potential diagnostic pitfall. Report of a case and a critical review of the literature. Pathol Res Pract. 2011;207:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol. 2008;61:428-437. [PubMed] |
| 33. | Kovach SJ, Fischer AC, Katzman PJ, Salloum RM, Ettinghausen SE, Madeb R, Koniaris LG. Inflammatory myofibroblastic tumors. J Surg Oncol. 2006;94:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 34. | Applebaum H, Kieran MW, Cripe TP, Coffin CM, Collins MH, Kaipainen A, Laforme A, Shamberger RC. The rationale for nonsteroidal anti-inflammatory drug therapy for inflammatory myofibroblastic tumors: a Children’s Oncology Group study. J Pediatr Surg. 2005;40:999-1003; discussion 1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Pecorella I, Ciardi A, Memeo L, Trombetta G, de Quarto A, de Simone P, di Tondo U. Inflammatory pseudotumour of the liver--evidence for malignant transformation. Pathol Res Pract. 1999;195:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Zavaglia C, Barberis M, Gelosa F, Cimino G, Minola E, Mondazzi L, Bottelli R, Ideo G. Inflammatory pseudotumour of the liver with malignant transformation. Report of two cases. Ital J Gastroenterol. 1996;28:152-159. [PubMed] |