Published online Sep 21, 2014. doi: 10.3748/wjg.v20.i35.12704
Revised: May 9, 2014
Accepted: June 12, 2014
Published online: September 21, 2014
Processing time: 169 Days and 20.3 Hours
Upper gastrointestinal bleeding refers to bleeding that arises from the gastrointestinal tract proximal to the ligament of Treitz. The primary reason for gastrointestinal bleeding associated with hepatocellular carcinoma is rupture of a varicose vein owing to pericardial hypotension. We report a rare case of gastrointestinal bleeding with hepatocellular carcinoma in a patient who presented with recurrent gastrointestinal bleeding. The initial diagnosis was gastric cancer with metastasis to the multiple lymph nodes of the lesser curvature. The patient underwent exploratory laparotomy, which identified two lesions in the gastric wall. Total gastrectomy and hepatic local excision was then performed. Pathological results indicated that the hepatocellular carcinoma had invaded the stomach directly, which was confirmed immunohistochemically. The patient is alive with a disease-free survival of 1 year since the surgery. Hepatocellular carcinoma with gastric invasion should be considered as a rare cause of upper gastrointestinal bleeding in hepatocellular carcinoma patients, especially with lesions located in the left lateral hepatic lobe. Surgery is the best solution.
Core tip: Hepatocellular carcinoma invading the stomach is a rare cause of upper gastrointestinal bleeding. It needed to be identified as gastric cancer with lymph nodes metastasis and hepatoid adenocarcinoma. The immunohistochemical result was important for the final diagnosis.
- Citation: Wu WD, Wu J, Yang HG, Chen Y, Zhang CW, Zhao DJ, Hu ZM. Rare cause of upper gastrointestinal bleeding owing to hepatic cancer invasion: A case report. World J Gastroenterol 2014; 20(35): 12704-12708
- URL: https://www.wjgnet.com/1007-9327/full/v20/i35/12704.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i35.12704
Upper gastrointestinal (GI) bleeding refers to bleeding that arises from the GI tract proximal to the ligament of Treitz and accounts for nearly 80% of significant GI hemorrhage. The causes of upper GI bleeding are categorized as either nonvariceal or bleeding related to portal hypertension. The nonvariceal causes account for approximately 80% of such bleeding, with peptic ulcer diseases being the most common[1]. China has a high incidence of hepatitis B (HBV), and patients may develop liver cirrhosis, portal hypertension, and hypersplenism; therefore, upper GI bleeding caused by rupture of a varicose vein is common. Other less common causes of upper GI bleeding include Mallory-Weiss tears, arteriovenous malformations, primary tumors, and erosive gastritis or duodenitis.
We report a case of a patient with hepatocellular carcinoma (HCC) who presented with massive upper GI bleeding owing to HCC invading the stomach and who was treated by local hepatic excision and total gastrectomy.
A 75-year-old Chinese male presented to our General Surgery ward in February 2013, with a history of recurrent upper GI hemorrhage. The GI bleeding recurred weekly, with a volume of 200-300 mL each time. The patient’s relatives declined surgery because of the high risk. Therefore, the patient received conservative treatment (hemostatics and blood transfusion) in the primary care unit, but the GI bleeding worsened, with volumes increasing to 500-600 mL with symptoms of shock. He was then transferred to our hospital.
The patient had been HBV-positive for 30 years and underwent right partial hepatectomy (local excision) for highly differentiated hepatocellular cancer, confirmed by histological examination, (preoperative AFP was negative) 10 years earlier. He also received preventive transarterial chemoembolization twice. Recurrence was detected 3 years later, located in the left lateral lobe. The result of preoperative indocyanine green testing was 24.3%, and at this level, hepatic insufficiency was a risk of repeat lobectomy. His family members rejected surgery. The HCC recurrence was finally managed by two transarterial chemoembolization procedures with no evidence of recurrence on follow-up since the procedures. He denied alcohol or smoking history, and he provided authorization to present the details of his case.
Results of the physical examination on admission to our hospital showed left upper quadrant tenderness and pale appearance. The results of laboratory workup revealed a hemoglobin level of 62 g/L, albumin level of 25 g/L, total bilirubin level of 31.3 μmol/L, and prothrombin time of 16.1 s. Tumor markers were normal, including AFP, CEA, CA199, CA153, CA125, and CA724. Serum was positive for hepatitis B surface antigen, but negative for hepatitis B e antigen. Serum anti-hepatitis C virus was also negative.
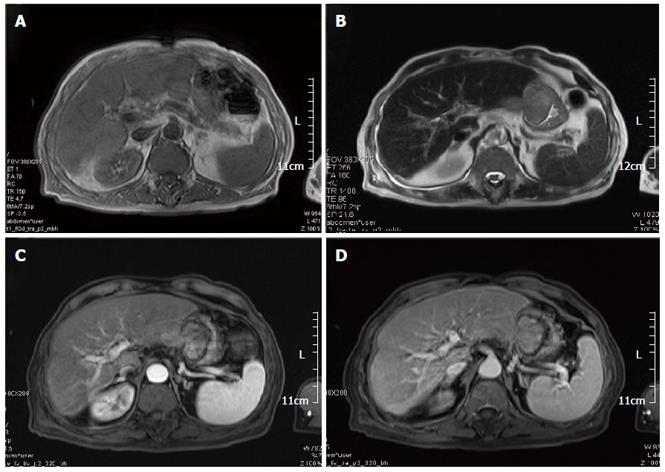
Abdominal contrast-enhanced MR (Figure 1) showed lesions localized in the left lateral hepatic lobe, and in the gastric lumen along the lesser curvature.
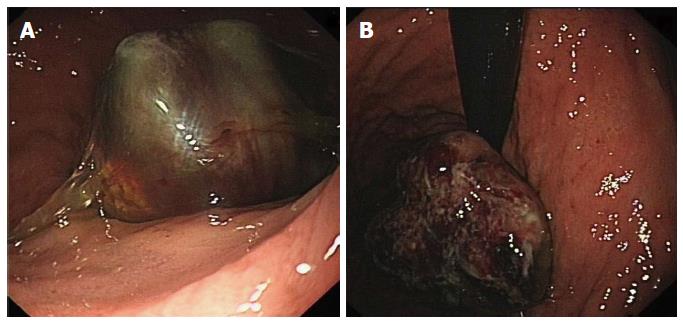
The mass was observed antegrade (Figure 2A) and retrograde (Figure 2B) by gastroscopy. The tumor was located 4 cm away from the cardia, along the lesser curvature.
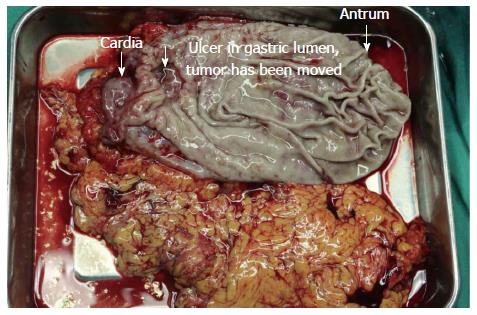
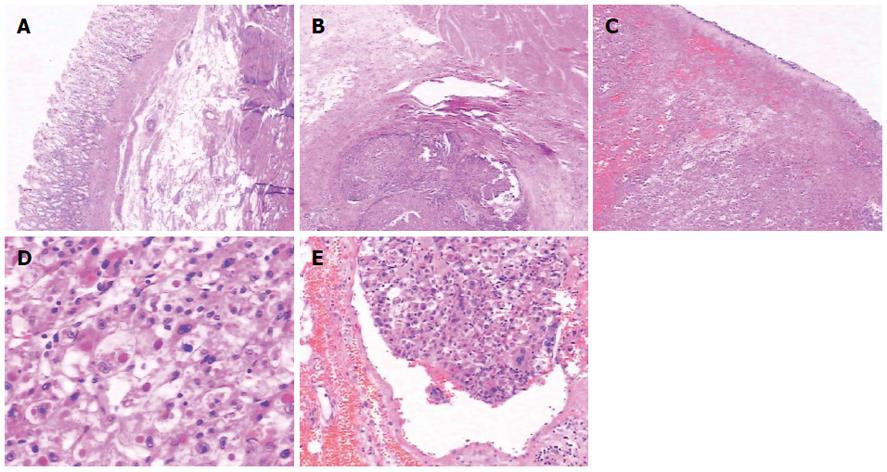
After physical and radiological examination, the diagnosis was gastric cancer with lesser curvature lymph node metastasis. Non-surgical treatment failed, and the patient underwent emergency surgery. We found two lesions in the gastric lumen, a larger one connected with the lesion in the left lateral hepatic lobe, and a smaller one located between the intact serosa and the mucosa (Figure 3). Pathological results (Figure 4) confirmed gastric cancer secondary to invasion by HCC in the left lateral hepatic lobe, and the margin was negative.
The patient underwent total gastrectomy and resection of the left lateral hepatic lobe followed by two episodes of preventive transhepatic arterial chemotherapy and embolization. Careful follow-up of this patient has revealed that he is alive with a 1-year disease-free survival since the surgery.
Extra-hepatic metastasis of HCC usually occurs in the lung, regional lymph nodes, and bone, and direct GI invasion is rare. Recently, Maruyama et al[2] reported a case of upper GI bleeding due to HCC direct invasion, and managed by transarterial embolization, but the patient died from hepatic failure 2 mo later.
In our case, according to the symptoms, blood test results, and emergent gastroscopy, the diagnosis was gastric cancer or gastric stromal tumor, initially. However, the final diagnosis was HCC invasion of the stomach.
Gastric invasion can occur from direct invasion or from metastatic lymph nodes along the lesser curvature or celiac trunk. Liver tumors treated by transarterial chemoembolization can invade the gastric lumen as a result of the inflammatory process, which results in promotion of subcapsular tumor adhesion to GI serosa, especially hepatic lesions located in the left lateral lobe[2]. In our case, there were two lesions in the gastric lumen, both along the lesser curvature, one near the cardia, and the other near the antrum. The larger was a result of direct gastric invasion by the HCC. The serosa and mucosa of the latter was intact, and separated from the liver. Therefore, we considered that when the HCC invaded the gastric lumen, it spread via blood and lymph vessels submucosally, which was supported by pathology (Figure 4E).
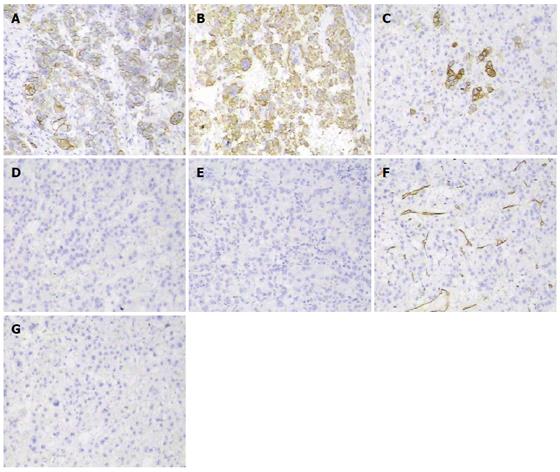
This case required differentiation of primary gastric cancer from liver metastasis (easily confirmed by gastroscopy and biopsy) and from hepatoid adenocarcinoma, which is a rare type of extrahepatic tumor that is morphologically similar to HCC and able to produce alpha-fetoprotein[3]. Hepatoid adenocarcinoma of the stomach frequently occurs in older people, with an average patient age of 63.5 years, and a male-to-female ratio of 2.32:1. The most common location is the antrum, followed by the cardia and fundus[4]. A diagnosis of hepatoid adenocarcinoma of the stomach depends on the characteristic histological features[5]. Histopathologically, the tumor is composed of two closely related areas, including hepatoid-like foci and adenocarcinomatous lesions. Tumor cells in the hepatoid foci resemble the morphology of HCC, and immunohistochemically, can be positive for AFP, alpha 1 antitrypsin (AAT) and alpha 1 antichymotrypsin (ACT). Also, polyclonal CEA staining shows a “canalicular” pattern, which is characteristic of hepatoid features. The adenocarcinomatous component may be well- or poorly-differentiated, often with clear cells and a papillary pattern[6]. Generally, in HCC, neighboring cirrhotic lesions are found, and tumor cells are positive for Hepar-1 antibody, a sensitive and specific immunohistochemical marker for hepatocyte differentiation, whereas in metastatic hepatoid adenocarcinoma of the stomach, Hepar-1 is often negative, and neighboring cirrhotic lesions are rare[7] (Figure 5). The prognosis is dismal when the GI tract is involved, and in cases of gastric invasion it is even worse, with less than one-year survival[8,9].
In conclusion, HCC with gastric invasion should be considered as a rare cause of upper GI bleeding in HCC patients, especially with lesions located in the left lateral hepatic lobe. Surgery could control bleeding and relieve symptoms, improving patients’ quality of life.
A 75-year-old Chinese male presented to our General Surgery ward in February 2013, with a history of recurrent upper gastrointestinal (GI) hemorrhage, with volumes increasing to 500-600 mL with symptoms of shock, and then he was transferred to our hospital.
According to the symptoms, blood test results, and emergent gastroscopy, the diagnosis was gastric cancer or gastric stromal tumor, initially.
This case required differentiation of primary gastric cancer from liver metastasis (easily confirmed by gastroscopy and biopsy) and from hepatoid adenocarcinoma, which is a rare type of extrahepatic tumor that is morphologically similar to hepatocellular carcinoma (HCC) and able to produce alpha-fetoprotein. The results of laboratory workup revealed moderate anemia, Child-Pugh B class hepatic function, normal tumor marker levels and positivity for hepatitis B surface antigen, but negativity for E antigen.
Abdominal contrast-enhanced MR showed lesions localized in the left lateral hepatic lobe, and in the gastric lumen along the lesser curvature. Immunohistochemical results showed: CK 8/18+, Hepar-1+, CK 20+, AFP-, CEA-, CD 34 vessel+, and CD 117-, which supported HCC invading the stomach. The patient underwent total gastrectomy and resection of the left lateral hepatic lobe followed by two episodes of preventive transhepatic arterial chemotherapy and embolization. Kimura and Maruyama have reported similar cases with different therapeutic strategy.
Reviewing the process of management of a case of GI bleeding, the initial diagnosis was gastric cancer with lymph node metastasis along the lesser curvature because the mass of liver was round and there was exophytic growth, which looks like an enlarged lymph node in the lesser curvature. Later, we will pay close attention to the cases of unknown cause of upper bleeding with liver mass, especially those located in the left lateral lobe and connected with gastric lumen, in which HCC invasion of the stomach should be taken into account.
Based on the reviewers’ comments, we maintain that our paper introduces a rare case of upper GI bleeding due to HCC direct invasion, expanding thinking of the diagnosis in this type of patient, especially when the lesion located in left lateral lobe. However, there were some flaws in the paper, such as immunohistochemical results not being shown by pictures and literary expression was not clear. These points have been revised according to reviewers’ comments.
P- Reviewer: Delladetsima IK, Liu EQ, Qin JM S- Editor: Qi Y L- Editor: O’Neill M E- Editor: Du P
| 1. | Courtney Townsend. Sabiston textbook of Surgery, 18th ed. Acute upper GI hemorrhage. Philadelphia, Pa: Saunders Elsevier 2007; . |
| 2. | Maruyama A, Murabayashi K, Hayashi M, Nakano H, Isaji S, Uehara S, Kusuda T, Miyahara S, Kondo A, Yabana T. Hepatocellular carcinoma complicated by gastrointestinal hemorrhage caused by direct tumor invasion of stomach. J Hepatobiliary Pancreat Surg. 1999;6:90-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Terracciano LM, Glatz K, Mhawech P, Vasei M, Lehmann FS, Vecchione R, Tornillo L. Hepatoid adenocarcinoma with liver metastasis mimicking hepatocellular carcinoma: an immunohistochemical and molecular study of eight cases. Am J Surg Pathol. 2003;27:1302-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Inagawa S, Shimazaki J, Hori M, Yoshimi F, Adachi S, Kawamoto T, Fukao K, Itabashi M. Hepatoid adenocarcinoma of the stomach. Gastric Cancer. 2001;4:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Nagai E, Ueyama T, Yao T, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach. A clinicopathologic and immunohistochemical analysis. Cancer. 1993;72:1827-1835. [PubMed] |
| 6. | Ishikura H, Kirimoto K, Shamoto M, Miyamoto Y, Yamagiwa H, Itoh T, Aizawa M. Hepatoid adenocarcinomas of the stomach. An analysis of seven cases. Cancer. 1986;58:119-126. [PubMed] |
| 7. | Gao YB, Zhang DF, Jin XL, Xiao JC. Preliminary study on the clinical and pathological relevance of gastric hepatoid adenocarcinoma. J Dig Dis. 2007;8:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 814] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 9. | Ishikura H, Kishimoto T, Andachi H, Kakuta Y, Yoshiki T. Gastrointestinal hepatoid adenocarcinoma: venous permeation and mimicry of hepatocellular carcinoma, a report of four cases. Histopathology. 1997;31:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 2.3] [Reference Citation Analysis (0)] |