Published online Sep 21, 2014. doi: 10.3748/wjg.v20.i35.12696
Revised: April 22, 2014
Accepted: July 24, 2014
Published online: September 21, 2014
Processing time: 294 Days and 5.8 Hours
Boerhaave’s syndrome refers to the spontaneous transmural rupture of the esophagus. Primary repair may be performed in patients who present within 24 h of perforation, and such cases have the best outcomes as most complications have not yet developed. However, the treatment of late perforations remains controversial. Various approaches and strategies to repair late perforations have been described in the literature, but there is no uniform approach. We present a case of Boerhaave’s syndrome in which the patient underwent surgical repair 48 h after the acute event and was subsequently treated successfully. The initial approach included direct esophageal repair, a drainage series, and nutritional support via a feeding jejunostomy. Although the repair site was subsequently disrupted, the patient showed complete healing of the perforation after three weeks. We consider that our surgical treatment strategy is safe and technically feasible, and appears to be a promising alternative approach for the treatment of patients with late Boerhaave’s perforation.
Core tip: Boerhaave’s syndrome refers to the spontaneous transmural rupture of the esophagus. The successful treatment of Boerhaave’s syndrome remains very challenging for surgeons, especially cases involving late perforations. Various strategies to repair late perforations have been previously described, but there is no uniform approach. We present a successful surgical strategy in a case of Boerhaave’s syndrome with late perforation, which involved surgical repair of the perforation and a series of drainage procedures. We consider that our strategy is safe and technically feasible, and appears to be a promising alternative approach for late Boerhaave’s perforation.
- Citation: Shen G, Chai Y, Zhang GF. Successful surgical strategy in a late case of Boerhaave’s syndrome. World J Gastroenterol 2014; 20(35): 12696-12700
- URL: https://www.wjgnet.com/1007-9327/full/v20/i35/12696.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i35.12696
Boerhaave’s syndrome, or spontaneous esophageal rupture, is a life-threatening condition characterized by the disruption of the distal esophagus due to a sudden forceful vomiting-induced increase in the internal esophageal pressure[1]. In such cases, there is often a significant delay between perforation and treatment, leading to chemical and bacterial mediastinitis followed by sepsis and multiorgan failure. Most studies describe a high mortality rate of 14%-40%[2,3]. Generally, prompt surgical repair and drainage are the gold standard treatments for cases of early perforation[4,5]. However, the treatment modalities for the cases of late perforation remain controversial[6]. In addition, when the appropriate drainage procedure is not followed in these cases of late perforation, severe complications may develop, which may lead to a high mortality rate. We present herein a successful strategy for the surgical treatment of a case of Boerhaave’s syndrome with late perforation, which involved surgical repair of the perforation and a series of drainage procedures.
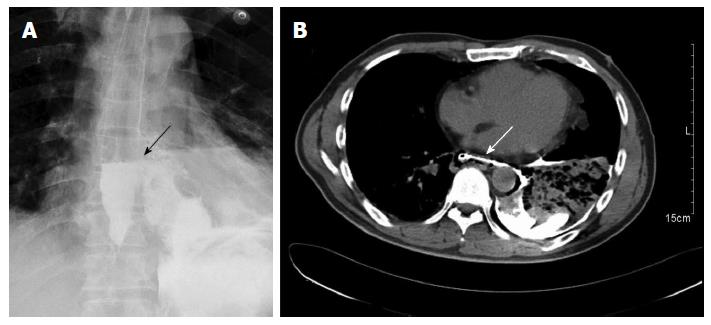
A 45-year-old man presented to our hospital after being treated at another institute. Forty hours previously, he had developed severe chest pain following violent vomiting after excessive alcohol intake and overeating. A chest X-ray at that time indicated a hydropneumothorax. Emergency intercostal drainage insertion was performed, and the patient was admitted to our hospital with septic shock. On initial presentation, his blood pressure was 86/50 mmHg, pulse rate was 113 beats/min, and respiratory rate was 22 breaths/min. The patient was febrile, with a temperature of 38.5 °C, and was mildly distressed. On chest examination, there was decreased air entry over the left hemithorax. Abdominal and cardiac examination results were normal; the extremities were cold without any edema. The leukocyte count on admission was 16 × 109/L. A gastrografin swallow demonstrated free extravasation of contrast from the left posterolateral aspect of the distal esophagus just above the level of the hiatus (Figure 1A). A subsequent computed tomography (CT) scan showed a free leak into the pleural space with a large collection, consistent with a diagnosis of esophageal perforation (Figure 1B). A CT scan of the abdomen was normal. Although a chest tube was inserted, it failed to clear the pleural space, because the pleural fluid was thick with food debris. After initial treatment, including aggressive volume resuscitation, commencement of broad-spectrum antibiotics, and nasogastric suction, the patient was in a stable condition. Thereafter, 6 h after admission, we decided to perform thoracotomy.
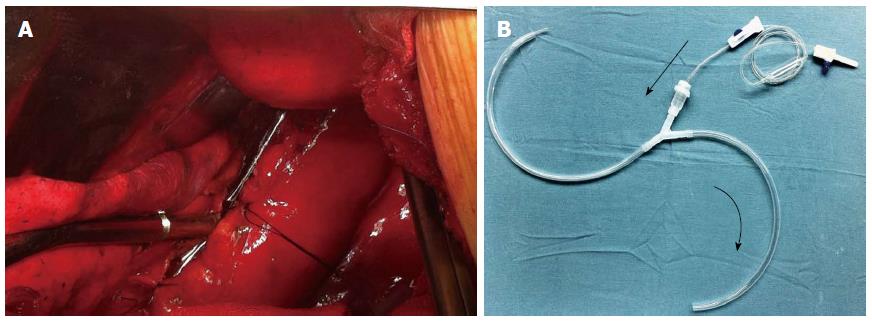
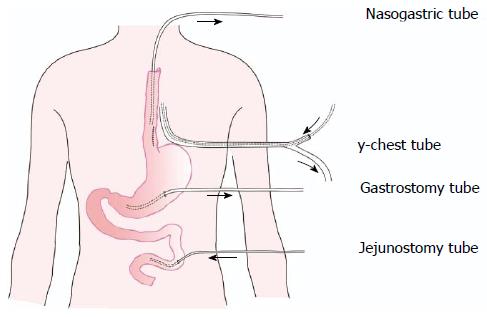
General anesthesia was administered with a double-lumen endotracheal tube. The patient was positioned in the right lateral decubitus position for thoracotomic exploration through an incision in the left seventh intercostal space. Exploration of the chest cavity revealed the presence of fibrin adhesions, necrotic tissue, and fluid collection. The posterior mediastinum was then opened. The esophageal longitudinal tear site was identified on the left side, having a length of 5 cm just above the esophageal hiatus. After wide opening and dissection of the posterior mediastinal tissues, meticulous irrigation with the evacuation of food debris and removal of necrotic tissue was accomplished. The nasogastric tube was replaced above the perforation. After removal of necrotic tissue, two-layer repair was performed using simple interrupted sutures and reinforced with a pleural flap using non-absorbable silk sutures (Mersilk, Ethicon, United Kingdom). For continuous postoperative irrigation, a custom-made y-chest tube was then positioned near and parallel to the repaired esophagus (Figure 2). One branch of the tube was used for irrigation at the perforation site with normal saline, and the other was used for passive drainage. The chest wall was closed in layers. The patient was then placed in a supine position and a laparoscopic exploration showed no intra-abdominal pathology. A laparoscopic gastrostomy and feeding jejunostomy were then performed, and the port sites were closed. The drainage is shown diagrammatically in Figure 3.
The patient was transferred to the intensive care unit. Ventilatory support was removed on the third postoperative day and the patient was discharged to the ward the next day. Postoperative treatment with broad-spectrum antibiotics was continued, and was subsequently changed to narrow-spectrum antibiotics according to the results of cultures from accumulated mediastinal or pleural fluid obtained during surgery or drainage or based on the results of blood cultures. On the seventh postoperative day, antibiotic treatment was discontinued.
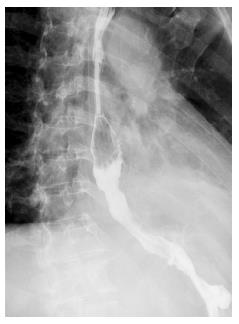
Chest irrigation via the y-chest tube was performed on each of the first 5 d after the operation. Gastric decompression though gastrostomy was started immediately after the operation and enteral nutrition through jejunostomy feeding was started on the second postoperative day. On the tenth postoperative day, a gastrografin swallow showed a small leak in the repair site. Full jejunostomy feeding and drainage were continued. The gastrografin swallow was repeated 10 d later and showed free flow of contrast from the esophagus into the stomach, without any leakage (Figure 4). The patient was then started on oral feeding. He was discharged to home on the 25th postoperative day, and was eating a normal diet. The 1-year follow-up has been uneventful.
Boerhaave’s syndrome refers to the spontaneous transmural rupture of the esophagus[1]. The successful treatment of Boerhaave’s syndrome remains very challenging for surgeons. The appropriate method for the management of esophageal perforation depends on a variety of factors, such as the location, severity, and duration of the perforation, and the age and condition of the patient. Data in the literature indicate that primary suturing of the esophagus complemented by mediastinal and chest drainage is associated with a success rate of approximately 90% in cases of complete esophageal rupture that are diagnosed within 24 h, if there is no accompanying esophageal disease (tumor, stricture, etc.)[4,7,8]. However, if the period between the occurrence of rupture and diagnosis is longer than 24 h, the outcome is significantly worse, because septic complications can rapidly develop and operative treatment is less successful[5,6]. At present, there is no uniform approach for the surgical treatment for late perforation. However, the key components in the management of all patients with perforation include aggressively delivering nutritional support and achieving the best possible local conditions for healing of the perforation, by adopting measures such as systemic broad-spectrum antibiotics, and local irrigation and drainage. In the present case, the treatment strategy followed the above fundamental principles. First, at the same time as antibiotic administration, a nasogastric tube placed above the perforation was used for drainage to achieve esophageal decompression, a gastric tube was used to decompress the stomach, and a y-chest tube was used to irrigate and drain the hemithorax and the mediastinum after primary closure of the perforation; in addition, feeding jejunostomy was performed by laparoscopy.
Since Eubanks et al[9] initially reported the case of a patient with Boerhaave’s syndrome who was successfully treated with a self-expandable metallic stent, there have been several reports of this condition; the results showed that endoscopic stent insertion seemed to offer a promising alternative for Boerhaave’s syndrome[10-13]. However, in the latest report by Schweigert et al[14], compared to the results of primary surgical therapy, the management of Boerhaave syndrome by means of endoscopic stent insertion offers no advantage in terms of morbidity and intensive care unit or hospital stay, and is associated with frequent treatment failure that eventually requires surgical intervention. Furthermore, endoscopic stenting shows a higher risk for fatal outcome than primary surgical therapy.
There have been several reports of the minimally invasive surgical management of spontaneous esophageal rupture via either laparoscopy or thoracoscopy, or both[15-17]. Avoidance of thoracotomy may result in considerable benefit for critically ill patients. However, open repair and drainage are still the gold standard treatments for this condition[4,8]. Moreover, a minimally invasive technique is used in cases with early perforation and stable hemodynamics, without signs of escalating sepsis, and without significant medical risk factors or contraindications for laparoscopy or thoracoscopy[16-18]. In the present case, thoracotomy was the approach for primary surgical repair, which allowed complete control of the perforation site and accurate clearing and drainage to treat the mediastinitis. However, laparoscopic gastrostomy and feeding jejunostomy may be of benefit for all perforations[19,20].
Control of the leak and eradication of the pleuromediastinal infection as expeditiously as possible are the goals of therapy, and can significantly improve the patient’s chances of survival[8]. Therefore, when managing this patient, we began with the thoracic procedures. Some authors consider that the reinforced primary suture recommended in cases of early perforation can also be applied successfully in cases of late perforation[4,7]. However, it is well known that, as in the present case, continuous esophageal leakage may occur despite careful surgical repair of an esophageal tear. In this phase, the perforation edges are always friable and edematous, with active bacterial infection, and final wound healing often requires the formation of surrounding fibrous tissue. Although many authors find external strengthening of the suture row desirable in cases of early perforation, including the intercostal muscle, pleura, omentum, and pericardium, the value and effectiveness of a pedicled flap in the cases of late perforation is still unclear[21,22].
Due to the high rate of esophageal leakage in the late perforations, adequate surgical drainage is the main treatment for Boerhaave’s syndrome. Some authors have used T-tube drainage or Jackson-Pratt drains, wherein a controlled fistula is created that closes spontaneously once the tube is removed[6,23]. In this patient, we created the best possible local conditions for the healing of the perforation. First, a custom-made y-chest tube was positioned near and parallel to the repaired esophagus for continuous postoperative irrigation and drainage. Second, the nasogastric tube was replaced above the perforation to drain the sputum and regurgitated gastric juices. Finally, laparoscopic gastrostomy was performed to relieve the suture row and keep the stomach decompressed to reduce gastroesophageal reflux. We believe that our drainage series could be a feasible surgical therapeutic option in all esophageal perforations.
Nutritional support was an important aspect of the postoperative management. In cases where we suspect that the patient may not be able to receive oral intake of food for a prolonged period of time, a secure method for the delivery of nutrition is optimal[8]. Although a nasogastric tube is an alternative approach, laparoscopic jejunostomy usually only adds 15-20 min to the operating time. This allows early institution of enteral feeding as well as a more reliable and comfortable method of enteral nutrition for the patient.
In conclusion, an aggressive surgical approach is still needed for late Boerhaave’s perforations. Adequate surgical drainage and nutritional support are the mainstay of treatment in all patients with Boerhaave’s perforation. We consider that our treatment strategy, which follows the above fundamental principles, is safe and technically feasible, and appears to be promising as an alternative approach for the treatment of patients with late Boerhaave’s perforation.
A 45-year-old man presented to their hospital with a history of severe chest pain following violent vomiting 40 h previously.
The patient was febrile, mildly distressed, and showed decreased air entry over the left hemithorax.
Hydropneumothorax, angina, and gastroduodenal perforation.
The leukocyte count on admission was 16 × 109/L.
A gastrografin swallow demonstrated free extravasation of contrast from the left posterolateral aspect of the distal esophagus just above the level of the hiatus.
The patient received an aggressive surgical treatment, including direct esophageal repair, a drainage series, and nutritional support via a feeding jejunostomy.
Various approaches and strategies to repair late perforations have been described in the literature, but there is no uniform approach.
Boerhaave’s syndrome is a spontaneous perforation of the esophagus that most commonly results from a sudden increase in intraesophageal pressure caused by straining or vomiting.
The treatment strategy presented in this case report is safe and technically feasible, and appears to be an alternative approach for the treatment of patients with late Boerhaave’s perforation.
The novelty of this case is the application of the suctioning-rinsing drainage (y-chest drain) in addition to primer suture.
P- Reviewer: Kozarek R, Lazar G, Muguruma N, Meshikhes AWN S- Editor: Ma N L- Editor: A E- Editor: Liu XM
| 1. | Derbes VJ, Mitchell RE. Hermann Boerhaave’s Atrocis, nec descripti prius, morbi historia, the first translation of the classic case report of rupture of the esophagus, with annotations. Bull Med Libr Assoc. 1955;43:217-240. [PubMed] |
| 2. | Richardson JD. Management of esophageal perforations: the value of aggressive surgical treatment. Am J Surg. 2005;190:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Chirica M, Champault A, Dray X, Sulpice L, Munoz-Bongrand N, Sarfati E, Cattan P. Esophageal perforations. J Visc Surg. 2010;147:e117-e128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Khan AZ, Strauss D, Mason RC. Boerhaave’s syndrome: diagnosis and surgical management. Surgeon. 2007;5:39-44. [PubMed] |
| 5. | Wolfson D, Barkin JS. Treatment of Boerhaave’s Syndrome. Curr Treat Options Gastroenterol. 2007;10:71-77. [PubMed] |
| 6. | Sulpice L, Dileon S, Rayar M, Badic B, Boudjema K, Bail JP, Meunier B. Conservative surgical management of Boerhaave’s syndrome: experience of two tertiary referral centers. Int J Surg. 2013;11:64-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Hill AG, Tiu AT, Martin IG. Boerhaave’s syndrome: 10 years experience and review of the literature. ANZ J Surg. 2003;73:1008-1010. [PubMed] |
| 8. | Lázár G, Paszt A, Simonka Z, Bársony A, Abrahám S, Horváth G. A successful strategy for surgical treatment of Boerhaave’s syndrome. Surg Endosc. 2011;25:3613-3619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Eubanks PJ, Hu E, Nguyen D, Procaccino F, Eysselein VE, Klein SR. Case of Boerhaave’s syndrome successfully treated with a self-expandable metallic stent. Gastrointest Endosc. 1999;49:780-783. [PubMed] |
| 10. | Tsunoda S, Shimada Y, Watanabe G, Nakau M, Imamura M. Covered metallic stent treatment of a patient with spontaneous rupture of the esophagus. Dis Esophagus. 2001;14:254-257. [PubMed] |
| 11. | Petruzziello L, Tringali A, Riccioni ME, Mutignani M, Margaritora S, Cesario A, Costamagna G. Successful early treatment of Boerhaave’s syndrome by endoscopic placement of a temporary self-expandable plastic stent without fluoroscopy. Gastrointest Endosc. 2003;58:608-612. [PubMed] |
| 12. | Davies AP, Vaughan R. Expanding mesh stent in the emergency treatment of Boerhaave’s syndrome. Ann Thorac Surg. 1999;67:1482-1483. [PubMed] |
| 13. | Yuasa N, Hattori T, Kobayashi Y, Miyata K, Hayashi Y, Seko H. Treatment of spontaneous esophageal rupture with a covered self-expanding metal stent. Gastrointest Endosc. 1999;49:777-780. [PubMed] |
| 14. | Schweigert M, Beattie R, Solymosi N, Booth K, Dubecz A, Muir A, Moskorz K, Stadlhuber RJ, Ofner D, McGuigan J. Endoscopic stent insertion versus primary operative management for spontaneous rupture of the esophagus (Boerhaave syndrome): an international study comparing the outcome. Am Surg. 2013;79:634-640. [PubMed] |
| 15. | Landen S, El Nakadi I. Minimally invasive approach to Boerhaave’s syndrome: a pilot study of three cases. Surg Endosc. 2002;16:1354-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Vaidya S, Prabhudessai S, Jhawar N, Patankar RV. Boerhaave’s syndrome: Thoracolaparoscopic approach. J Minim Access Surg. 2010;6:76-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Ashrafi AS, Awais O, Alvelo-Rivera M. Minimally invasive management of Boerhaave’s syndrome. Ann Thorac Surg. 2007;83:317-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Cho JS, Kim YD, Kim JW, I HS, Kim MS. Thoracoscopic primary esophageal repair in patients with Boerhaave’s syndrome. Ann Thorac Surg. 2011;91:1552-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Toelen C, Hendrickx L, Van Hee R. Laparoscopic treatment of Boerhaave’s syndrome: a case report and review of the literature. Acta Chir Belg. 2007;107:402-404. [PubMed] |
| 20. | Khan AZ, Forshaw MJ, Davies AR, Youngstein T, Mason RC, Botha AJ. Transabdominal approach for management of Boerhaave’s syndrome. Am Surg. 2007;73:511-513. [PubMed] |
| 21. | Yamashita S, Takeno S, Moroga T, Kamei M, Ono K, Takahashi Y, Yamamoto S, Kawahara K. Successful treatment of esophageal repair with omentum for the spontaneous rupture of the esophagus (Boerhaave’s syndrome). Hepatogastroenterology. 2012;59:745-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 22. | Matsuhashi N, Nagao N, Tanaka C, Nishina T, Kawai M, Kunieda K, Iwata H. A patient with spontaneous rupture of the esophagus and concomitant gastric cancer whose life was saved: case of report and review of the literature in Japan. World J Surg Oncol. 2011;9:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Lakshmanadoss U, Mogili S, Kothari T, Das V. Migration of the chest tube into the esophagus in a case of Boerhaave’s syndrome. Heart Lung. 2011;40:576-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |