Published online Sep 21, 2014. doi: 10.3748/wjg.v20.i35.12551
Revised: April 29, 2014
Accepted: June 12, 2014
Published online: September 21, 2014
Processing time: 199 Days and 19.5 Hours
AIM: To distinguish between the esophagus and adjacent organs using extraesophageal saline injection (ESI) in a canine model.
METHODS: ESI was performed through the esophagus under the guidance of linear-array endoscopic ultrasonography (EUS). Approximately 15 mL of methylene blue saline (0.5%) was then injected through each of the extraesophageal puncture points using a 22 G needle. Radial EUS examinations were conducted before and after ESI. EUS images of the trachea, tracheal bifurcation, arcus aortae and thoracic aorta were recorded. Vital signs were monitored during the ESI procedure and EUS examination. The dogs were then sacrificed for exploratory thoracotomy.
RESULTS: No obvious fluctuation in vital signs or serious adverse events occurred during the ESI procedure. On EUS imaging, an apparent hypoechoic area outside the esophagus, which separated the esophagus and adjacent organs, was visualized. The adventitious of the esophagus and adjacent organs were easily distinguished. The findings of subsequent exploratory thoracotomy confirmed the EUS findings: obvious accumulation of a blue liquid in the extraesophageal tissues, as well as in the esophageal-thoracic aorta space, esophageal-arcus aortae space and esophageal-tracheal space.
CONCLUSION: The esophagus and adjacent organs were successfully separated by ESI, and extraesophageal saline acted as an effective ultrasonic contrast agent.
Core tip: The esophagus and adjacent organs were distinguished by extraesophageal saline injection (ESI) in dogs to identify better the esophageal adventitia and adjacent organs by endoscopic ultrasonography (EUS). EUS showed that the esophagus and adjacent organs were separated clearly in the extraesophageal liquid dark area. The findings provide an adequate basis for combined ESI and EUS for accurate preoperative identification of Stage T3 and T4 esophageal cancer.
- Citation: Li JJ, Shan HB, He LJ, Wang TD, Xiong H, Chen LM, Li XH, Huang XX, Luo GY, Li Y, Xu GL. Extraesophageal saline enhances endoscopic ultrasonography to differentiate esophagus and adjacent organs. World J Gastroenterol 2014; 20(35): 12551-12558
- URL: https://www.wjgnet.com/1007-9327/full/v20/i35/12551.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i35.12551
Esophageal cancer (EC) is one of the most common and devastating malignant tumors worldwide, and China has the highest incidence of esophageal squamous cell carcinoma (ESCC)[1]. The main treatment modality for EC is esophagectomy[2]. According to the TNM staging system of the Union for International Cancer Control (7th Edition, 2012), EC invades the esophageal adventitia at the T3 stage; invades the pleura, pericardium and diaphragm at the T4a stage; and involves adjacent vital organs (mainly the thoracic aorta, aortic arch, trachea, tracheal bifurcation and spine) at the T4b stage[3]. T3- and T4a-stage tumors can be subjected to esophagectomy, whereas tumors at the T4b stage are not eligible for esophagectomy[4]. Therefore, it is important for physicians and patients to determine whether a tumor has metastasized to adjacent organs before considering resection.
The imaging methods used for preoperative staging of EC include endoscopic ultrasonography (EUS)[5-8], computed tomography (CT)[9,10], magnetic resonance imaging[11] and positron emission tomography (PET)[12,13]. EUS is most commonly used, especially for T- and N-stage tumors. One of its advantages is that the EUS probe can be directly placed into the esophageal lumen, so the involved esophageal mucosa, submucosa, muscularis propria and enlarged paraesophageal lymph nodes can be directly observed. Moreover, EUS has diagnostic accuracies of 75%, 39% and 88% for T1-, T2- and T3-stage ECs, respectively, which needs to be improved[9].
Our previous paper reported that submucosal saline injection can improve the accuracy of EUS in T1a- and T1b-stage ESCC because the injected saline forms a water cushion that acts as an ultrasonic contrast agent (negative) and separates the mucosal and submucosal layers[14,15]. Similar to the esophageal mucosa, the extraesophageal tissue also contains loose connective tissue; therefore, we envisaged that if saline or another liquid were directly injected extraesophageally, the esophageal adventitia would be distinguished from its adjacent organs (and especially the thoracic aorta, arcus aortae and tracheal bifurcation), and the changes could be visualized by EUS. If this hypothesis could be proven, the accuracy rate of the diagnosis of T3- and T4-stage EC could be improved. In this study, we attempted extraesophageal saline injection (ESI) and visualization of the esophagus and its adjacent organs by EUS in a canine model.
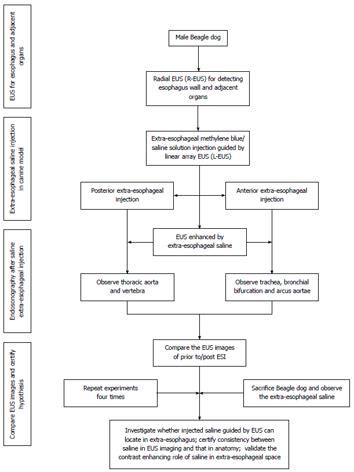
The experimental protocol was reviewed and approved by the animal welfare and ethics committees at Sun Yat-sen University Cancer Center, Guangzhou, China (approval number: GZR2012-114). Male beagle dogs (weighing an average of 10 kg) were provided by the medical animal center located on the north campus of Sun Yat-sen University. Five dogs were kept in separate cages and were given an absolute diet for 8 h and dehydrated for 6 h before the experiment. The dogs were injected intraperitoneally with 0.03 mg/kg pentobarbital sodium to induce anesthesia and then injected intraperitoneally with 0.03 mg/kg per hour pentobarbital sodium to maintain anesthesia. The flow diagram for the study is shown in Figure 1.
EUS examination was performed using a Fujinon 7000 endoscopy system (Fujinon, Japan) with a 7.5-MHz ultrasonic probe (EG-530UR) and a linear-array puncture probe (EG-530UT). Vital signs such as heart rate, respiratory rate, rectal temperature and peripheral oxygen saturation were monitored continuously using a multi-parameter monitor (UT4000A; Shenzhen Goldway Industrial Inc., China). To conduct ESI, we used 22 G EUS-guided fine-needle aspiration (EUS-FNA) needles (Endo-Flex, Germany).
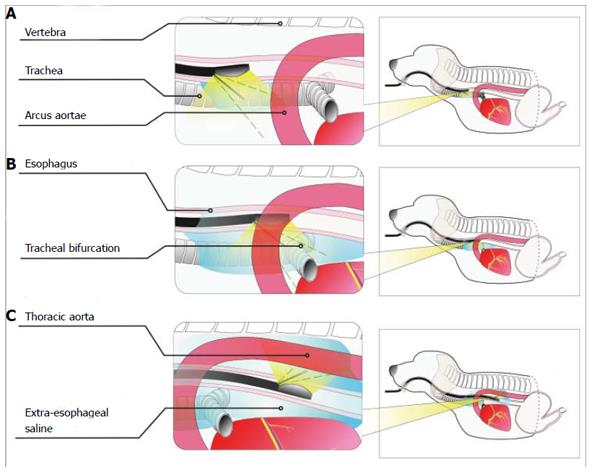
Puncture sites: The main purpose of ESI was to visualize the trachea, tracheal bifurcation, arcus aortae and thoracic aorta in relation to the esophagus on EUS images. To display the esophagus and trachea/large blood vessels in the upper thoracic segment, we injected saline through a puncture in the anterior esophageal wall (Figure 2A). To display the tracheal bifurcation, arcus aortae and thoracic aorta in the middle thoracic segment, we injected saline through a puncture in the anterior wall and the left lateral wall in the esophageal lumen (Figure 2B). Moreover, to display the thoracic aorta in the lower thoracic segment, we injected the saline via a puncture in the left posterior wall (Figure 2C). In addition, we added methylene blue to the saline at a concentration of 0.5% to display more clearly the esophagus and adjacent organs and the spaces between them when the animals were sacrificed.
Puncture procedure: The puncture was performed under the guidance of linear-array EUS (L-EUS). The location markers included the aorta/left atrium, trachea and tracheal bifurcation in the anterior wall and the thoracic aorta in the posterior wall. To display the esophagus and trachea/large blood vessels in the upper thoracic segment, a puncture was performed through the anterior wall. The puncture probe was placed in the center of the trachea or tracheal bifurcation, guided by white-light endoscopy and L-EUS, and gently rotated 5-10° to the left, until tracheal high-intensity linear echoes were displayed on the EUS images. A 22 G EUS-FNA needle was then pierced through the puncture point, and the movement of the needle tip was monitored by real-time sonography. The methylene blue saline was then injected when the needle was near the tracheal high-level linear echoes and when no blood or gas was observed upon reverse suction (excluding piercing of the needle into the blood or trachea). In addition, we performed ESI into the right side of the trachea or tracheal bifurcation by the procedures stated above. The amount of methylene blue saline injected depended on the amount required to show clearly the trachea, aorta and esophageal wall. Approximately 15 mL of saline was injected into each puncture site.
Attention had to be paid to the puncture depth because the saline was occasionally injected into the submucosal layer. The sign of submucosal injection was a dark blue mucosal bulge that was easily visualized by endoscopy (a water cushion formed by the methylene blue saline in the submucosal layer). When this sign occurred, we inserted the needle 3-5 mm forward, and the saline was then injected until the needle tip could be monitored outside the esophagus by real-time EUS. Similarly, the saline was reinjected when no gas or blood was observed upon reverse suction.
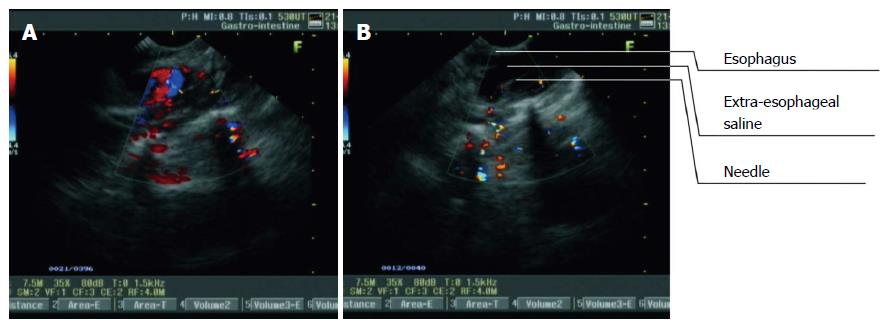
A puncture was also performed through the anterior wall and the left lateral wall to display the tracheal bifurcation, arcus aortae and thoracic aorta in the middle thoracic segment. The puncture site in the anterior wall was located about 3 mm to the left of the tracheal bifurcation in white-light endoscopy. In the left lateral wall, the arcus aortae or thoracic aorta were first visualized on L-EUS images [displayed as a long hypoechoic area and as an artery-like turbulent fluid in color Doppler flow imaging (CDFI)]. The probe was then rotated 5-10° to the left, until the point at which the long hypoechoic area (representing the aortic arch or thoracic aorta) disappeared. This point was used as the puncture site. The puncture and injection were then preformed as described above.
The thoracic aorta is mainly located on the posterior left side of the esophagus in the lower thoracic segment. Therefore, to display the thoracic aorta, we performed a puncture through the posterior and left lateral walls. The thoracic aorta was first found on L-EUS images (displayed as a hypoechoic area, and CDFI detected an artery-like turbulent fluid) (Figure 3A). The probe was then rotated 5-10° to the left, until the point at which the hypoechoic area that represented the thoracic aorta disappeared. This point was the puncture site. The puncture, needle insertion and saline injection were then preformed as described above (Figure 3B).
The followings were the signs of successful ESI: smooth injection of the saline, without obvious resistance; no mucosal bulge; the presence of a hypoechoic area between the esophageal adventitia (striped areas of medium-high echoes) and its adjacent organs; enlargement of the hypoechoic area with an increase in injection quantity; and intermittent striped areas of medium echogenicity in the extraesophageal saline, which represented the extraesophageal loose connective tissues.
Radial EUS (R-EUS) examination was conducted again after successful ESI. The esophageal wall and adjacent organs, such as the thoracic aorta, trachea, tracheal bifurcation and aortic arch, were visualized. If the hypoechoic area remained between the esophageal wall and the adjacent organs after ESI, the esophagus was considered to be separated from the adjacent organs at these sites during surgical resection.
The Beagle dogs were sacrificed by intravenous injection of 10% potassium chloride (2 mEq/kg) into the femoral vein after ESI and subsequent R-EUS examination. Exploratory thoracotomy was then performed. The intact esophagus was exposed gradually. The accumulated extraesophageal methylene blue saline, as well as the esophagus and adjacent organs, such as the thoracic aorta, trachea, tracheal bifurcation and aortic arch, were investigated.
Each of the procedures, including ESI and EUS (before and after ESI), were performed by a senior doctor with > 10 years of experience in EUS procedures. The exploratory thoracotomy was conducted by a senior surgeon with 10 years of experience in thoracic surgery.
Vital signs were stable during anesthesia, ESI procedure the EUS examination. No severe adverse events, such as esophageal perforation, bleeding or mediastinal emphysema, were found after ESI.
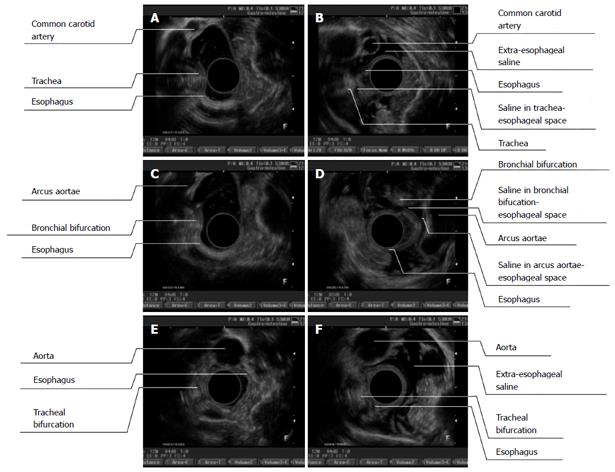
Before ESI, the esophageal wall showed a three-layer structure on the sonographic images taken with the 7.5-MHz ultrasonic probe; a high-echoic region in the first layer, which represented the esophageal mucosa and submucosa; a low-echoic region in the second layer, which represented the muscularis propria; and a high-echoic region in the third layer, which represented the esophageal adventitia. The extraesophageal connective tissue was hyperechoic. The esophageal adventitia and adjacent organs, such as the trachea, tracheal bifurcation, aortic arch and thoracic aorta, were difficult to identify (Figure 4A, C and E).
The esophageal adventitia was visualized clearly on the EUS images taken after ESI. The extraesophageal hyperechoic structure disappeared and was replaced by a hypoechoic area. Stripes of hyperechoic tissue were visible in the hypoechoic area, and the esophageal wall and adjacent organs were separated by an enlarged distance. Occasionally, several areas of the first esophageal layer were slightly thickened, which was due to submucosal saline injection at the puncture site when the needle tip was located in the submucosa (Figure 4B, D and F).
In the upper thoracic segment, a hypoechoic area was visualized in the extraesophageal space, and the esophagus could be distinguished from the large thoracic vessels and the trachea. The left subclavian artery and the left common carotid artery were parallel, appearing as round dark areas with distinguishable adventitias. A hypoechoic area was also visible in the esophageal-tracheal space; however, this area was not obvious because the tracheal impression on the esophagus was apparent due to the developed cricoid cartilage. The hypoechoic area was more apparent when the EUS probe was moved to the anterior side to reduce the influence of the tracheal impression on the esophagus (Figure 4B).
A hypoechoic area was also easily visualized in the middle thoracic segment; this dark area was found to extend from the anterior to the left lateral side of the extraesophageal space (as well as the rear side of the arcus aortae and bronchial bifurcation). CDFI showed turbulent blood flow through the arcus aortae, which could be observed from the anterior right to the posterior left side, with a distinguishable adventitia. The tracheal bifurcation with high echogenicity was representative of annular cartilage. The esophagus, arcus aortae and tracheal bifurcation were separated in this hypoechoic area (Figure 4D).
Extraesophageal saline was easily visualized outside the lower thoracic segment of the esophagus, and it expanded to the region of the anterior erector spinae. This observation meant that the saline had been deposited into the posterior esophagus (including the left lateral wall). Therefore, the esophageal adventitia and thoracic aortic adventitia could be distinguished (Figure 4F).
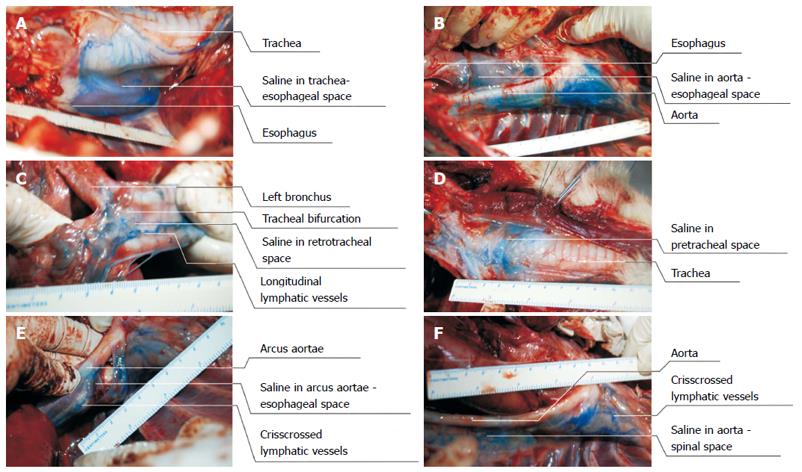
Exploratory thoracotomy revealed that a blue liquid was dispersed outside the esophageal adventitia (including its posterior, left lateral and anterior walls). This liquid was found to be dispersed in the esophageal-thoracic aorta space, esophageal-bronchial bifurcation space, and esophagus-arcus aortae space. This blue liquid was even found to be accumulated in the pretracheal space. The findings indicate that ESI was performed successfully under EUS guidance and that the saline was dispersed outside the esophagus. The esophageal wall and its adjacent organs were easily distinguished by saline, and the liquid had not only accumulated in the spaces at the puncture sites, but had also dispersed in the anterior and posterior spaces of the esophagus, which were composed of loose connective tissue and therefore allowed for easy expansion of saline (Figure 5).
The extraesophageal hypoechoic area increased in size with the increase in injection quantity on the EUS images, which indicated that the saline was between the esophagus and adjacent organs, such as the trachea (Figure 5A), thoracic aorta (Figure 5B), tracheal bifurcation (Figure 5C) and pretracheal space (Figure 5D). Subsequent exploratory thoracotomy not only detected accumulated liquid in the extraesophageal loose connective tissues, but also confirmed that these spaces were enlarged with methylene blue saline. Moreover, the extraesophageal liquid-related hypoechoic area was not only found to be consistent with the EUS images, but was also in accordance with the findings of the exploratory thoracotomy. The results revealed that the methylene blue saline dispersed in all directions, accumulated in all spaces, and even reached the lateral and anterior tracheal spaces.
After ESI and during exploratory thoracotomy, the amount of methylene-blue-dyed saline outside the esophagus decreased with time (as indicated by a reduced volume and a faded dye color) and was absorbed completely by about 30 min after exploratory thoracotomy (about 50 min from the end of ESI/animal sacrifice).
In the present study, we sought to answer the following questions: (1) can saline be delivered to extraesophageal areas under EUS guidance?; (2) can the saline effectively distinguish the esophageal wall from its adjacent organs?; and (3) can EUS detect these changes after ESI?
In this study, ESI procedures were performed while smoothly guided by L-EUS, and the thoracic aorta and aortic arch were avoided. Vital signs were stable during and after ESI, and no severe adverse events occurred. We therefore believe that ESI can be performed successfully under real-time L-EUS guidance. Saline can be fully absorbed in the body, with only a negligible impact on the internal environment of the animal. The ESI procedure seems safe and feasible. After ESI, we found a hypoechoic area between the esophageal adventitia and adjacent regions and extraesophageal saline among the connective tissues.
Moreover, the thoracic aorta and esophagus could be easily distinguished by ESI. The trachea and esophagus were also distinguished successfully by left and right lateral injections. Thus, the esophagus was easily distinguishable from the important adjacent organs. After ESI, the findings of the exploratory thoracotomy confirmed the results of the EUS images: obvious accumulation of a blue liquid was found in the extraesophageal tissues, including the esophageal-aortic space and esophageal-tracheal space. EUS is therefore reliable for detecting the changes that occur after ESI.
The extraesophageal saline accumulated and enlarged the distance between the esophagus and adjacent organs and also aided better visualization of the esophageal adventitia and the external layers of adjacent organs due to its negative contrast effect. This saline can therefore be used to examine whether advanced EC has invaded the esophageal adventitia, allowing physicians to judge whether esophagectomy is feasible.
There are some limitations to these techniques. (1) a major limitation is the lack of usefulness of this technique in humans. In particular, it remains to be demonstrated whether it is possible to inject saline outside large esophageal tumors; (2) in patients with esophagostenosis, it will be difficult to pass the EUS probe through the esophageal lumen; and (3) the insertion and injection methods need to be improved so that submucosal injection can be avoided and more accurate positioning, puncture and ESI can be performed successfully. Nevertheless, the present study proved the feasibility and effectiveness of the ESI technique. A series of in-depth studies should be conducted before formal clinical trials are initiated.
In conclusion, the esophagus and adjacent organs can be distinguished successfully by ESI. Saline serves as a contrast agent, so after ESI, the esophagus and adjacent organs are easily distinguishable on ultrasonography images.
It is of importance to explore a novel technique to distinguish better between esophagus and adjacent organs prior to esophagectomy in advanced esophageal cancer (EC) patients.
In this study, the authors used a new technique of extraesophageal saline injection (ESI) guide by endoscopic ultrasound to distinguish the esophagus from other adjacent organs.
The esophagus and adjacent organs were successfully separated by ESI, and the extraesophageal saline acted as an effective ultrasonic contrast agent.
The technique of ESI can be used prior to esophagectomy in advanced EC in order to identify resectable and unresectable advanced EC.
This was a well-designed study with some limitations, especially lack of practice in humans.
P- Reviewer: Fusaroli P S- Editor: Qi Y L- Editor: Kerr C E- Editor: Liu XM
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11831] [Article Influence: 845.1] [Reference Citation Analysis (4)] |
| 2. | Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol. 2007;8:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 388] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 3. | Sobin LH, Gospodarowicz MK, Wittekind Ch. TNM Classification of Malignant Tumors. 7th edn. New York: WileyBlackwell 2010; . |
| 4. | NCCN Practice Guidelines in Oncology-Esophageal Cancer. Version 2. 2011; Available from: http://www.jnccn.org/content/9/8/830.full.pdf. |
| 5. | Smith BR, Chang KJ, Lee JG, Nguyen NT. Staging accuracy of endoscopic ultrasound based on pathologic analysis after minimally invasive esophagectomy. Am Surg. 2010;76:1228-1231. [PubMed] |
| 6. | Lightdale CJ, Kulkarni KG. Role of endoscopic ultrasonography in the staging and follow-up of esophageal cancer. J Clin Oncol. 2005;23:4483-4489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Pech O, Günter E, Dusemund F, Origer J, Lorenz D, Ell C. Accuracy of endoscopic ultrasound in preoperative staging of esophageal cancer: results from a referral center for early esophageal cancer. Endoscopy. 2010;42:456-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Rampado S, Bocus P, Battaglia G, Ruol A, Portale G, Ancona E. Endoscopic ultrasound: accuracy in staging superficial carcinomas of the esophagus. Ann Thorac Surg. 2008;85:251-256. [PubMed] |
| 9. | Subasinghe D, Samarasekera DN. A study comparing endoscopic ultrasound (EUS) and computed tomography (CT) in staging oesophageal cancer and their role in clinical decision making. J Gastrointest Cancer. 2010;41:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Li H, Chen TW, Li ZL, Zhang XM, Chen XL, Wang LY, Zhou L, Li R, Li CP, Huang XH. Tumour size of resectable oesophageal squamous cell carcinoma measured with multidetector computed tomography for predicting regional lymph node metastasis and N stage. Eur Radiol. 2012;22:2487-2493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Quint LE, Bogot NR. Staging esophageal cancer. Cancer Imaging. 2008;8 Spec No A:S33-S42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Hiraki Y, Miyake M, Hayashi H, Takeda Y, Aono K. [Collagen diseases (author’s transl)]. Rinsho Hoshasen. 1981;26:1185-1203. [PubMed] |
| 13. | Barber TW, Duong CP, Leong T, Bressel M, Drummond EG, Hicks RJ. 18F-FDG PET/CT has a high impact on patient management and provides powerful prognostic stratification in the primary staging of esophageal cancer: a prospective study with mature survival data. J Nucl Med. 2012;53:864-871. [PubMed] |
| 14. | Li JJ, Shan HB, Xu GL, He LJ, Xia JC. Submucosal saline solution injection combined with endosonography for distinguishing between stages T1a and T1b of early esophageal cancer. Gastrointest Endosc. 2013;77:159-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |