Published online Sep 14, 2014. doi: 10.3748/wjg.v20.i34.12346
Revised: March 26, 2014
Accepted: May 23, 2014
Published online: September 14, 2014
Processing time: 220 Days and 3.6 Hours
MicroRNAs have been increasingly recognized as useful biomarkers for colorectal cancers (CRC). We have recently observed that microRNA-31 (miR-31) expression is associated with BRAF mutation and prognosis in CRC. Moreover, high miR-31 expression is frequently detected in sessile serrated adenomas compared with hyperplastic polyps (HPs). These results suggest that miR-31 may contribute to the progression of serrated lesions. At a follow-up colonoscopy, we observed the case of a 75-year-old man with a 7-mm flat-elevated lesion in the cecum and diagnosed the lesion as an early invasive carcinoma with serrated features. Tissue specimens were obtained from the representative areas to compare the molecular alterations in the carcinoma component with those in the HP component. Higher miR-31 expression was observed in the carcinoma component (57-fold increase) and the HP component (8-fold increase) compared with the paired normal mucosa, suggesting that miR-31 may be one of the key molecules in serrated pathway progression.
Core tip: At a follow-up colonoscopy, we observed the case of a 75-year-old man with a 7-mm flat-elevated lesion in the cecum. Because the flat-elevated area displayed serrated features, we diagnosed the lesion as an early invasive carcinoma with a hyperplastic polyp (HP) component. Higher microRNA-31 (miR-31) expression was observed in the carcinoma component (57-fold increase) and the HP component (8-fold increase) compared with the paired normal mucosa. This is the first case report of early invasive colorectal cancer with an HP component in which miR-31 expression was analyzed. Our results suggest that miR-31 may be an important molecule in serrated pathway progression.
- Citation: Aoki H, Nosho K, Igarashi H, Ito M, Mitsuhashi K, Naito T, Yamamoto E, Tanuma T, Nomura M, Maguchi H, Shinohara T, Suzuki H, Yamamoto H, Shinomura Y. MicroRNA-31 expression in colorectal serrated pathway progression. World J Gastroenterol 2014; 20(34): 12346-12349
- URL: https://www.wjgnet.com/1007-9327/full/v20/i34/12346.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i34.12346
The serrated pathway has attracted considerable attention as an alternative pathway of colorectal cancers (CRC) development[1,2]. In the World Health Organization (WHO) classification, CRCs with serrated morphology are recognized as a distinct subtype and three major categories of serrated lesion are currently used: hyperplastic polyp (HP), sessile serrated adenoma (SSA), and traditional serrated adenomas (TSA)[3]. It has been demonstrated that MLH1 silencing leading to microsatellite instability (MSI) is one of the important molecular events with regard to the carcinogenic mechanism of the serrated pathway[1,2].
In contrast, microRNAs constitute a class of small non-coding RNA molecules that function as post-transcriptional gene regulators and have been increasingly recognized as useful biomarkers for CRCs[4,5]. We have recently observed that high microRNA-31 (miR-31) expression is associated with BRAF mutation and prognosis in CRC[4]. In addition, high miR-31 expression is frequently detected in SSAs and TSAs compared with HPs[4]. These results suggest that miR-31 may contribute to the progression of serrated lesions. However, to the best of our knowledge, no studies have described its role in serrated pathway progression.
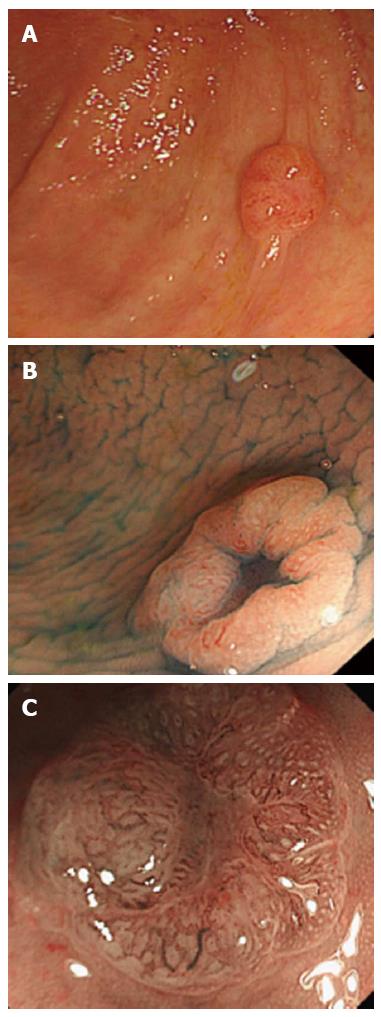
At a follow-up colonoscopy after a proctectomy, we observed the case of a 75-year-old man with a 7-mm flat-elevated lesion with a central depression in the cecum (Figure 1A and B). A magnifying colonoscopic examination by narrow-band imaging (NBI) of the depressed area revealed winding and prematurely terminating irregular blood vessels and sparse surface patterns (Figure 1C). Because the flat-elevated area displayed serrated features, we diagnosed the lesion as an early invasive carcinoma (T1) with an HP component. Ileocecal resection with regional lymph node dissection was performed without complications. The tumor measuring 3 mm × 3 mm consisted of a poorly-differentiated adenocarcinoma with serrated features. Some carcinoma cells had invaded the submucosal layer to a depth of 600 μm, but no lymphatic invasion, venous invasion or lymph node metastasis was observed. The final diagnosis was stage I (T1N0M0) in the TNM classification.
For molecular analysis in this case, genomic DNA and total RNA were extracted from formalin-fixed paraffin-embedded (FFPE) tissues as previously described[4]. Tissue specimens were obtained from the representative areas to compare the molecular alterations in the carcinoma component with those in the HP component. PCR and targeted pyrosequencing were performed for the BRAF (V600E) mutation using extracted genomic DNA[4]. The TP53 mutation was initially determined by PCR-single-strand conformation polymorphism (SSCP)[6]. We examined the MSI status as previously described[4]. In addition, bisulfite modification of genomic DNA and pyrosequencing for MLH1 methylation were performed[4]. miR-31 expression was analyzed using quantitative reverse transcription-PCR (qRT-PCR) as previously described[4].
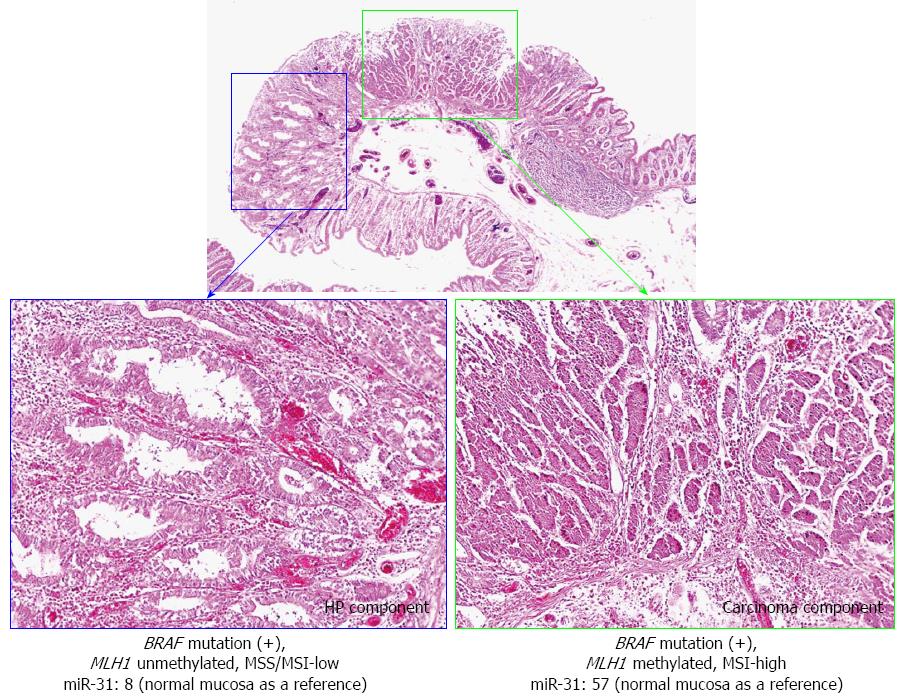
The molecular alterations of the carcinoma and HP components are presented in Figure 2. BRAF mutations were observed in both components. However, no TP53 mutation was observed in the carcinoma or HP component, whereas MLH1 methylation and MSI were only identified in the carcinoma component. Higher miR-31 expression was observed in the carcinoma component (57-fold increase) and the HP component (8-fold increase) compared with the paired normal mucosa (as a reference).
Various authors have reported that SSAs with a carcinoma component have genetic and epigenetic abnormalities and are at a high risk of progression to advanced CRCs[1,2,6-8]. A loss of staining for MLH1 (due to MLH1 methylation) leads to MSI, and repeat tract mutation in genes such as TGFβRII is restricted to SSA lesions with a carcinoma component[1,9,10]. In this case, MLH1 methylation and MSI were only identified in the carcinoma component. Because MSI by MLH1 methylation has been reported to play an important role in serrated pathway carcinogenesis[1,2], our data appear to be consistent with those of previous studies.
MiR-31 is located at 9p21.3 and is reportedly up-regulated in CRCs[4]. Using microRNA array analysis, we have recently discovered that miR-31 expression is significantly up-regulated in BRAF-mutated CRCs compared with wild-type CRCs[4]. Moreover, associations were identified between miR-31 expression, proximal tumor location and poor prognosis for CRCs. With regard to premalignant colorectal lesions, high miR-31 expression is frequently detected in cases with serrated lesions (SSAs and TSAs) when compared with cases with HPs, suggesting an association between miR-31 expression and the serrated pathway[4].
In this case, higher miR-31 expression was observed in the carcinoma component compared with the HP component, suggesting that not only accumulating epigenetic alterations but also miR-31 expression may contribute to the progression of HP (or SSA). In other words, these findings indicate that HPs (SSAs) with high miR-31 expression may be precursor lesions that progress to SSAs with high-grade dysplasia or CRCs.
In conclusion, this is the first case report of early invasive CRC with an HP component in which miR-31 expression was analyzed. Our results suggest that miR-31 may be one of the key molecules in serrated pathway progression. Further functional analysis is required to clarify the mechanism of miR-31 in the serrated pathway.
A 75-year-old man at a follow-up colonoscopy after a proctectomy.
Colonoscopic examination revealed a 7-mm flat-elevated lesion with a central depression in the cecum.
WBC 7100/μL; HGB 12.8 gm/dL; CEA 1.8 ng/mL; a metabolic panel and liver function test were within normal limits.
Esophagogastroduodenoscopy, abdominal ultrasonography, and computed tomography were normal.
The tumor measuring 3 mm × 3 mm consisted of a poorly-differentiated adenocarcinoma with serrated features. Some carcinoma cells had invaded the submucosal layer to a depth of 600 μm, but no lymphatic invasion, venous invasion or lymph node metastasis was observed. The final diagnosis was stage I (T1N0M0) in the TNM classification.
Ileocecal resection with regional lymph node dissection was performed without complications.
In this case, higher miR-31 expression was observed in the carcinoma component compared with the hyperplastic polyp (HP) component. Similarly, MLH1 methylation and microsatellite instability were only identified in the carcinoma component.
In this case report the authors describes the case of a 75-year-old man at a follow-up colonoscopy to have a 7-mm flat-elevated lesion in the cecum that was diagnosed as an early invasive carcinoma with a HP component. This short manuscript is well written and the case reported could be used as basis for further deepened studies aimed to clarify the role of miR-31 in colorectal carcinogenesis correlated to the serrated pathway. It is therefore acceptable for publication.
P- Reviewer: Huang ZH, Serafino A S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Bettington M, Walker N, Clouston A, Brown I, Leggett B, Whitehall V. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62:367-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 351] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 2. | Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, Goldblum JR, Guillem JG, Kahi CJ, Kalady MF. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315-129; quiz 1314, 1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 830] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 3. | Bosman FT; World Health Organization. International Agency for Research on Cancer. WHO classification of tumours of the digestive system. 4th ed. Lyon: International Agency for Research on Cancer 2010; . |
| 4. | Nosho K, Igarashi H, Nojima M, Ito M, Maruyama R, Yoshii S, Naito T, Sukawa Y, Mikami M, Sumioka W. Association of microRNA-31 with BRAF mutation, colorectal cancer survival and serrated pathway. Carcinogenesis. 2014;35:776-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Toiyama Y, Takahashi M, Hur K, Nagasaka T, Tanaka K, Inoue Y, Kusunoki M, Boland CR, Goel A. Serum miR-21 as a diagnostic and prognostic biomarker in colorectal cancer. J Natl Cancer Inst. 2013;105:849-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 402] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 6. | Yamamoto E, Suzuki H, Yamano HO, Maruyama R, Nojima M, Kamimae S, Sawada T, Ashida M, Yoshikawa K, Kimura T. Molecular dissection of premalignant colorectal lesions reveals early onset of the CpG island methylator phenotype. Am J Pathol. 2012;181:1847-1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Rosty C, Hewett DG, Brown IS, Leggett BA, Whitehall VL. Serrated polyps of the large intestine: current understanding of diagnosis, pathogenesis, and clinical management. J Gastroenterol. 2013;48:287-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 8. | Fujita K, Yamamoto H, Matsumoto T, Hirahashi M, Gushima M, Kishimoto J, Nishiyama K, Taguchi T, Yao T, Oda Y. Sessile serrated adenoma with early neoplastic progression: a clinicopathologic and molecular study. Am J Surg Pathol. 2011;35:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Ricciardiello L, Goel A, Mantovani V, Fiorini T, Fossi S, Chang DK, Lunedei V, Pozzato P, Zagari RM, De Luca L. Frequent loss of hMLH1 by promoter hypermethylation leads to microsatellite instability in adenomatous polyps of patients with a single first-degree member affected by colon cancer. Cancer Res. 2003;63:787-792. [PubMed] |
| 10. | Sheridan TB, Fenton H, Lewin MR, Burkart AL, Iacobuzio-Donahue CA, Frankel WL, Montgomery E. Sessile serrated adenomas with low- and high-grade dysplasia and early carcinomas: an immunohistochemical study of serrated lesions “caught in the act”. Am J Clin Pathol. 2006;126:564-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |