Published online Sep 14, 2014. doi: 10.3748/wjg.v20.i34.12308
Revised: April 14, 2014
Accepted: May 26, 2014
Published online: September 14, 2014
Processing time: 197 Days and 9.5 Hours
AIM: To investigate whether Helicobacter pylori (H. pylori) infection contributes to idiopathic thrombocytopenic purpura (ITP) or iron-deficiency anemia (IDA) onset in gerbils.
METHODS: A total of 135 Mongolian gerbils were randomly divided into two groups: an H. pylori infection group and a control group. Both groups were fed the same diet and the same amount of food. Each group was then divided into three subgroups, which were sacrificed at 6, 12, or 18 mo for analysis. At each time point, arterial blood was collected from the abdominal aorta and a complete blood cell count was analyzed in the clinical laboratory in the First Affiliated Hospital of Nanchang University.
RESULTS: There were no significant differences in platelet counts (938.00 ± 270.27/L vs 962.95 ± 162.56 × 109/L), red blood cell counts (8.11 ± 1.25/L vs 8.44 ± 1.48 × 1012/L), or hemoglobin levels (136.9 ± 8.76 g/L vs 123.21 ± 18.42 g/L) between the control and the H. pylori groups, respectively, at 18 mo. With the exception of the mean corpuscular volume (MCV), all other indicators, including white blood cell counts, hematocrit, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, red blood cell distribution width, mean platelet volume, platelet distribution width, lymphocyte count, and lymphocyte count percentage, showed no significant differences between the control and H. pylori infection groups at each time point. The MCV in the H. pylori infection group (52.32 f/L ± 2.86 f/L) was significantly lower than the control group (55.63 ± 1.89 f/L) at 18 mo (P = 0.005), though no significant differences were observed at 6 (54.40 ± 2.44 f/L vs 53.30 ± 1.86 f/L) or 12 mo (53.73 ± 2.31 f/L vs 54.80 ± 3.34 f/L).
CONCLUSION: A single H. pylori infection is insufficient to cause onset of ITP or IDA and other factors may be required for disease onset.
Core tip: This is the first study designed to investigate the relationship between Helicobacter pylori (H. pylori) infection and idiopathic thrombocytopenic purpura (ITP) and iron-deficiency anemia (IDA) hematological diseases in a Mongolian gerbil model. Although there were no significant differences between the H. pylori infection and control groups, this study may help us better understand the relationships between H. pylori and extragastric diseases. While a single H. pylori infection is not sufficient to cause ITP or IDA, the precise role of H. pylori infection in extragastric disease pathogenesis remains to be further elucidated.
-
Citation: Xie C, Xu LY, Li W, Yang Z, Lu NH.
Helicobacter pylori infection in Mongolian gerbils does not initiate hematological diseases. World J Gastroenterol 2014; 20(34): 12308-12312 - URL: https://www.wjgnet.com/1007-9327/full/v20/i34/12308.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i34.12308
Recently, several studies have demonstrated a close association between Helicobacter pylori (H. pylori) infection and hematological diseases, such as idiopathic thrombocytopenic purpura (ITP) and iron-deficiency anemia (IDA)[1]. In 1998, Gasbarrini et al[2] were the first to demonstrate that eradication of H. pylori may promote regression of ITP. Since that seminal study, many clinical studies have sought to identify the relationship between H. pylori and ITP. It is currently hypothesized that H. pylori may be involved in the pathogenesis of ITP[3], and ITP is listed as one of the indications for H. pylori eradication[4-6]. IDA, another hematological disease, also shows a close association H. pylori infection[7].
Although H. pylori infection appears to have a close association with both ITP and IDA, there are still many obstacles that exist in clinical practice. For example, exactly how H. pylori pathogenesis influences the onset of these diseases remains unclear. Moreover, eradication of H. pylori infection is not beneficial for all ITP and IDA patients[8-10]. Mongolian gerbils have been frequently used to study the pathogenesis of H. pylori infection as they are susceptible to colonization and develop gastric diseases as a result of infection[11,12]. To further investigate whether H. pylori infection is a contributing factor in the initiation of ITP and IDA, this study was designed to evaluate H. pylori infection in a Mongolian gerbil model.
A total of 135 Mongolian gerbils obtained from the Zhejiang Institute of Medical Sciences were randomly assigned to one of two groups: the control group (n = 45) or the H. pylori group (n = 90). Animals were individually housed in air isolation cages (IVC-II Suzhou Fengshi Animal Equipment Co., Suzhou, China). Gerbils in the H. pylori group were intragastrically administered a suspension of H. pylori, strain ATCC43504 (CagA+, VacA+; Chinese Center for Disease Control and Prevention, Beijing, China). The control group was administered Brucella broth. Each group was then divided into three subgroups, which were sacrificed at 6, 12, or 18 mo. Each control subgroup contained 15 Mongolian gerbils while each H. pylori subgroup contained 30 Mongolian gerbils. All Mongolian gerbils were fed the exact same diet and the same amount of food, and procedures were approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University.
All gastric tissues were harvested from the gastric antrum of the Mongolian gerbils. The tissues were then fixed in 10% formaldehyde overnight at 4 °C prior to paraffin embedding. Paraffin sections, 4 μm thick, were cut with a microtome and stored at room temperature. H. pylori infection was confirmed by Giemsa staining[13]. Each biopsy was assessed and diagnosed by two veteran pathologists.
Arterial blood was collected from the abdominal aorta at 6, 12, or 18 mo, and a complete blood count was obtained for each specimen in the clinical laboratory at the First Affiliated Hospital of Nanchang University.
All data are presented as mean ± SD. Statistical significance was evaluated using an unpaired Student’s t-test. Statistical analyses were performed with SPSS statistical software, version 19.0 (IBM, Chicago, IL, United States). A P value of < 0.05 was set as the threshold for statistical significance.
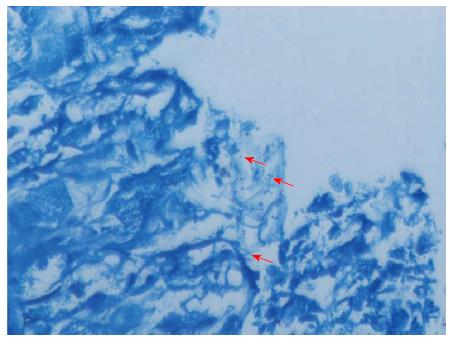
Due to a malfunction in the air supply system, 11 Mongolian gerbils from the 12-mo subgroup (H. pylori group: n = 8; control group: n = 6) and 14 Mongolian gerbils from the 18-mo subgroup (H. pylori group: n = 12; control group: n = 7) died. The remaining Mongolian gerbils were successfully infected with H. pylori, which was confirmed by pathology detection (Figure 1).
All of the blood cell count data are listed in Table 1. Platelet counts, which address the presence of ITP, were first analyzed. There were no significant differences observed between the control and H. pylori groups at any time point. Next, red blood cell counts and hemoglobin levels were assessed to determine the presence of IDA. Again, no significant differences were observed between the control and H. pylori groups. Leukocyte counts, hematocrits, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, mean corpuscular volume (MCV), red blood cell distribution width, mean platelet volume, platelet distribution width, lymphocyte count, and percentage of lymphocytes were also quantified. No significant differences were observed, with the exception of the MCV, which was significantly lower in the H. pylori group in comparison to the control group at 18 mo (52.32 ± 2.86 vs 55.63 ± 1.89 fL).
| Group | WBC (× 109/L) | RBC (× 1012/L) | HGB (g/L) | HCT (L/L) | MCV (fL) | MCH (pg) | MCHC (g/L) | RDW-SD (fL) | PLT (× 109/L) | MPV (fL) | PDW (%) | LYM (× 109/L) | LYM% |
| 6 mo | |||||||||||||
| Control (n = 15) | 3.92 ± 1.92 | 8.63 ± 0.68 | 136.9 ± 8.76 | 0.47 ± 0.03 | 53.30 ± 1.86 | 15.43 ± 0.81 | 276.78 ± 6.45 | 30.31 ± 1.43 | 1071.50 ± 246.91 | 6.82 ± 0.36 | 7.77 ± 0.47 | 1.97 ± 1.27 | 50.46 ± 19.10 |
| H. pylori (n = 30) | 4.43 ± 2.21 | 8.88 ± 0.59 | 131.9 ± 9.42 | 0.48 ± 0.03 | 54.40 ± 2.44 | 15.31 ± 0.47 | 283.68 ± 8.12 | 29.61 ± 1.07 | 962.95 ± 162.56 | 7.04 ± 0.32 | 7.63 ± 0.49 | 1.84 ± 0.88 | 43.29 ± 16.75 |
| 12 mo | |||||||||||||
| Control (n = 9) | 3.67 ± 1.82 | 8.25 ± 1.11 | 122.22 ± 10.16 | 0.43 ± 0.03 | 54.80 ± 3.34 | 15.47 ± 1.17 | 282.44 ± 4.90 | 29.95 ± 0.88 | 897.33 ± 157.61 | 7.30 ± 0.26 | 8.67 ± 0.31 | 1.98 ± 0.98 | 65.60 ± 10.35 |
| H. pylori (n = 27) | 2.93 ± 1.80 | 7.92 ± 0.78 | 124.61 ± 16.73 | 0.44 ± 0.06 | 53.73 ± 2.31 | 15.13 ± 0.97 | 281.23 ± 7.27 | 30.22 ± 2.21 | 1048.46 ± 289.42 | 7.19 ± 0.41 | 8.66 ± 0.84 | 1.81 ± 0.82 | 61.00 ± 13.38 |
| 18 mo | |||||||||||||
| Control (n = 8) | 2.60 ± 1.23 | 8.11 ± 1.25 | 136.50 ± 22.99 | 0.46 ± 0.07 | 55.63 ± 1.89 | 16.21 ± 0.84 | 291.37 ± 7.61 | 30.71 ± 1.31 | 938.00 ± 270.27 | 7.16 ± 0.37 | 8.60 ± 0.63 | 1.44 ± 0.57 | 52.97 ± 11.32 |
| H. pylori (n = 23) | 2.90 ± 2.08 | 8.44 ± 1.48 | 123.21 ± 18.42 | 0.42 ± 0.07 | 52.32 ± 2.86a | 15.24 ± 1.04 | 291.21 ± 12.24 | 29.18 ± 6.62 | 1011.43 ± 227.14 | 6.88 ± 1.55 | 8.42 ± 2.01 | 1.47 ± 0.76 | 51.14 ± 12.79 |
A majority of the studies on extragastric manifestations and H. pylori have predominantly focused on the epidemiology and clinical outcome after H. pylori eradication. It is currently believed that the prevalence of H. pylori infection in patients with ITP is significantly higher than that in healthy individuals, though H. pylori infection has not been attributed to severe forms of thrombocytopenia[14,15]. Previous research and systematic reviews have demonstrated that H. pylori eradication can increase the platelet count in adult ITP patients, especially in mild cases[16,17]. The cytotoxin-associated gene A (CagA), a virulence factor from H. pylori, has specifically been implicated in the pathogenesis of ITP[18,19]. Few studies, however, have focused on whether H. pylori infection is an initiating factor in ITP. Several hypotheses have been proposed to address how H. pylori may contribute to ITP disease onset, including molecular mimicry between H. pylori antigens and platelet glycoproteins, platelet aggregation, downregulation of the reticuloendothelial system, and induction of a Th1-mediated immune response[1,20-22].
Studies have also revealed that H. pylori infection is closely associated with IDA in both adults and children[23]. Active H. pylori infection has been detected in over 50% of patients with unexplained refractory IDA, and 64%-75% of such patients are permanently cured after H. pylori eradication[24]. A recent study conducted by Xia et al[25] showed that IDA is strongly correlated with H. pylori infection and that eradication promoted a more rapid response to oral iron therapy. Similarly, another study demonstrated that eradication of H. pylori increased the treatment efficacy in severe refractory IDA[26]. It is currently thought that H. pylori causes IDA by an increased iron loss through reduced iron absorption due to bacterial utilization of the iron[1,23].
Most of the previous studies were retrospective in nature, and carried out in a human patient population. Due to inherent individual differences between human subjects, the results from human studies have been difficult to interpret. Very few studies have used animal models to address the relationship between H. pylori and extragastric diseases. Huang et al[27] investigated the pathological changes occurring in the liver and gall bladder of H. pylori-colonized C57BL/6 mice over an eight-month period. Higgins and colleagues analyzed the influence of H. pylori infection on colitis and immune response in a mouse model[28].
In this study, a Mongolian gerbil animal model was used to study the association between H. pylori infection and ITP and IDA onset to eliminate many of the confounding factors present in human studies. Using this animal model, H. pylori infection was allowed to persist for up to 18 mo, a duration significantly longer than other comparable studies. No significant differences were observed between the H. pylori and control groups, with the exception of MCV levels, which were significantly lower in the H. pylori group. However, these lower MCV levels may be attributed to the small sample size. The results suggest that a single H. pylori infection is insufficient to cause either ITP or IDA. Thus, H. pylori may play an auxiliary role in ITP or IDA pathogenesis, but does not serve as an initiating factor.
This study also has some limitations. Only one bacterial strain of H. pylori was used in this study, which does not comprehensively account for the range of H. pylori infections that can occur. Relatively straightforward parameters were measured, which may not have detected more subtle changes in the immune system occurring before the appearance of abnormal blood cell counts. Furthermore, Mongolian gerbils may not acquire hematological diseases easily whereas mouse strains, which are prone to developing ITP or IDA, should be considered as an alternate model in the future[29,30].
In conclusion, H. pylori infection may require other pathogenic factors to contribute to ITP and IDA onset. Factors such as host polymorphisms may also contribute to disease development, a feature that should be examined in future studies.
Increasing evidence has demonstrated a strong correlation between Helicobacter pylori (H. pylori) infection and extragastric diseases, including hematological, cardiovascular, and nervous system diseases. Two diseases that show a robust association with H. pylori infection are idiopathic thrombocytopenic purpura (ITP) and unexplained iron-deficiency anemia (IDA).
Several epidemiological studies have demonstrated a higher rate of H. pylori infection in ITP and IDA patient populations in comparison to controls. Moreover, infection eradication is beneficial in most cases. The precise role of H. pylori in ITP and IDA disease onset, however, is still not understood and whether infection eradication is beneficial or necessary for all ITP or IDA patients remains controversial. This study was designed to evaluate whether a single H. pylori infection can trigger ITP or IDA onset.
Previous studies examining the role of H. pylori infection in ITP and IDA in patient populations were retrospective in nature, with several confounding factors affecting interpretation of study results. In the present study, Mongolian gerbils were used as an animal model of H. pylori infection to exclude many of the confounding factors present in the previous studies.
This study will further elucidate the roles played by H. pylori infection in the pathogenesis of hematological and other extragastric diseases.
Idiopathic thrombocytopenic purpura is an autoimmune hemorrhagic syndrome that manifests as isolated low platelet count due to immune destruction. Iron-deficiency anemia is a common form of anemia caused by iron deficiency due to several different factors, and may affect hemoglobin synthesis in blood cells.
This research report is an in vivo study evaluating the effect of H. pylori infection on ITP and IDA onset in a Mongolian gerbil model. Infection in gerbils is a suitable system to model human infection and the results obtained in this paper justify the conclusions drawn. The results of this study are clinically relevant and applicable to a broad audience.
P- Reviewer: Ananthakrishnan N, Day AS, D’Elios MM, Kim BW S- Editor: Ding Y L- Editor: O’Neill M E- Editor: Zhang DN
| 1. | Papagiannakis P, Michalopoulos C, Papalexi F, Dalampoura D, Diamantidis MD. The role of Helicobacter pylori infection in hematological disorders. Eur J Intern Med. 2013;24:685-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Gasbarrini A, Franceschi F, Tartaglione R, Landolfi R, Pola P, Gasbarrini G. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. Lancet. 1998;352:878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 300] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 3. | Roubaud Baudron C, Franceschi F, Salles N, Gasbarrini A. Extragastric diseases and Helicobacter pylori. Helicobacter. 2013;18 Suppl 1:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1591] [Article Influence: 122.4] [Reference Citation Analysis (5)] |
| 5. | Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 427] [Article Influence: 26.7] [Reference Citation Analysis (1)] |
| 6. | Liu WZ, Xie Y, Cheng H, Lu NH, Hu FL, Zhang WD, Zhou LY, Chen Y, Zeng ZR, Wang CW. Fourth Chinese National Consensus Report on the management of Helicobacter pylori infection. J Dig Dis. 2013;14:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Muhsen K, Cohen D. Helicobacter pylori infection and anemia. Am J Trop Med Hyg. 2013;89:398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Satoh T, Miyazaki K, Shimohira A, Amano N, Okazaki Y, Nishimoto T, Akahoshi T, Munekata S, Kanoh Y, Ikeda Y. Fcγ receptor IIB gene polymorphism in adult Japanese patients with primary immune thrombocytopenia. Blood. 2013;122:1991-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Scandellari R, Allemand E, Vettore S, Plebani M, Randi ML, Fabris F. Platelet response to Helicobacter pylori eradication therapy in adult chronic idiopathic thrombocytopenic purpura seems to be related to the presence of anticytotoxin-associated gene A antibodies. Blood Coagul Fibrinolysis. 2009;20:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Izzotti A, Durando P, Ansaldi F, Gianiorio F, Pulliero A. Interaction between Helicobacter pylori, diet, and genetic polymorphisms as related to non-cancer diseases. Mutat Res. 2009;667:142-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Yin YN, Wang CL, Liu XW, Cui Y, Xie N, Yu QF, Li FJ, Lu FG. Gastric and duodenum microflora analysis after long-term Helicobacter pylori infection in Mongolian Gerbils. Helicobacter. 2011;16:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Yokota K, Kurebayashi Y, Takayama Y, Hayashi S, Isogai H, Isogai E, Imai K, Yabana T, Yachi A, Oguma K. Colonization of Helicobacter pylori in the gastric mucosa of Mongolian gerbils. Microbiol Immunol. 1991;35:475-480. [PubMed] |
| 13. | Xie C, Xu LY, Yang Z, Cao XM, Li W, Lu NH. Expression of γH2AX in various gastric pathologies and its association with Helicobacter pylori infection. Oncol Lett. 2014;7:159-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Tan HJ, Goh KL. Extragastrointestinal manifestations of Helicobacter pylori infection: facts or myth? A critical review. J Dig Dis. 2012;13:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Payandeh M, Raeisi D, Sohrabi N, Zare ME, Kansestani AN, Keshavarz N, Gholami S, Hashemian AH. Poor platelet Count Response to Helicobacter Pylori Eradication in Patients with Severe Idiopathic Thrombocytopenic Purpura. Int J Hematol Oncol Stem Cell Res. 2013;7:9-14. [PubMed] |
| 16. | Kikuchi T, Kobayashi T, Yamashita T, Ohashi K, Sakamaki H, Akiyama H. Eight-year follow-up of patients with immune thrombocytopenic purpura related toH. pyloriinfection. Platelets. 2011;22:61-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Stasi R, Sarpatwari A, Segal JB, Osborn J, Evangelista ML, Cooper N, Provan D, Newland A, Amadori S, Bussel JB. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systematic review. Blood. 2009;113:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 18. | Takahashi T, Yujiri T, Shinohara K, Inoue Y, Sato Y, Fujii Y, Okubo M, Zaitsu Y, Ariyoshi K, Nakamura Y. Molecular mimicry by Helicobacter pylori CagA protein may be involved in the pathogenesis of H. pylori-associated chronic idiopathic thrombocytopenic purpura. Br J Haematol. 2004;124:91-96. [PubMed] |
| 19. | Franceschi F, Christodoulides N, Kroll MH, Genta RM. Helicobacter pylori and idiopathic thrombocytopenic purpura. Ann Intern Med. 2004;140:766-767. [PubMed] |
| 20. | D’Elios MM, Bergman MP, Amedei A, Appelmelk BJ, Del Prete G. Helicobacter pylori and gastric autoimmunity. Microbes Infect. 2004;6:1395-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Kuwana M. Helicobacter pylori-associated immune thrombocytopenia: clinical features and pathogenic mechanisms. World J Gastroenterol. 2014;20:714-723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Smyk DS, Koutsoumpas AL, Mytilinaiou MG, Rigopoulou EI, Sakkas LI, Bogdanos DP. Helicobacter pylori and autoimmune disease: cause or bystander. World J Gastroenterol. 2014;20:613-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 108] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 23. | Pacifico L, Osborn JF, Tromba V, Romaggioli S, Bascetta S, Chiesa C. Helicobacter pylori infection and extragastric disorders in children: a critical update. World J Gastroenterol. 2014;20:1379-1401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Hershko C, Camaschella C. How I treat unexplained refractory iron deficiency anemia. Blood. 2014;123:326-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Xia W, Zhang X, Wang J, Sun C, Wu L. Survey of anaemia and Helicobacter pylori infection in adolescent girls in Suihua, China and enhancement of iron intervention effects by H. pylori eradication. Br J Nutr. 2012;108:357-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Yamanouchi J, Azuma T, Yakushijin Y, Hato T, Yasukawa M. Dramatic and prompt efficacy of Helicobacter pylori eradication in the treatment of severe refractory iron deficiency anemia in adults. Ann Hematol. 2014;93:1779-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Huang Y, Tian XF, Fan XG, Fu CY, Zhu C. The pathological effect of Helicobacter pylori infection on liver tissues in mice. Clin Microbiol Infect. 2009;15:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Higgins PD, Johnson LA, Luther J, Zhang M, Sauder KL, Blanco LP, Kao JY. Prior Helicobacter pylori infection ameliorates Salmonella typhimurium-induced colitis: mucosal crosstalk between stomach and distal intestine. Inflamm Bowel Dis. 2011;17:1398-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Leontyev D, Katsman Y, Branch DR. Mouse background and IVIG dosage are critical in establishing the role of inhibitory Fcγ receptor for the amelioration of experimental ITP. Blood. 2012;119:5261-5264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Thomson MJ, Pritchard DM, Boxall SA, Abuderman AA, Williams JM, Varro A, Crabtree JE. Gastric Helicobacter infection induces iron deficiency in the INS-GAS mouse. PLoS One. 2012;7:e50194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |