Published online Sep 14, 2014. doi: 10.3748/wjg.v20.i34.12292
Revised: March 7, 2014
Accepted: April 28, 2014
Published online: September 14, 2014
Processing time: 302 Days and 14.8 Hours
AIM: To investigate whether amifostine contributes to the antioxidant and cytoprotective effects of histidine-tryptophan-ketoglutarate (HTK) and University of Wisconsin (UW) preservation solutions.
METHODS: Forty-eight Sprague Dawley male rats were equally divided into six groups: (1) ringer Lactate (RL) group; (2) RL + amifostine (RL + A) group; (3) HTK group; (4) HTK + A group; (5) UW group; and (6) UW + A group. Rats in the RL + A, HTK + A and UW + A groups were administered amifostine intraperitoneally at a dose of 200 mg/kg prior to laparotomy. The RL group was perfused with RL into the portal vein. The RL + A group were perfused with RL into the portal vein after amifostine administration. The HTK group received an HTK perfusion while the HTK + A group received an HTK perfusion after administration of amifostine. The UW group received a perfusion of UW, while the UW + A group received a UW perfusion after amifostine administration. Liver biopsy was performed to investigate histopathological, immunochemical [transferase mediated dUTP nick end labeling (TUNEL), inducible nitric oxide syntetase (iNOS)] and ultrastructural alterations. Biochemical alterations were determined by examining levels of alanine aminotransferase, alkaline phosphatase and nitric oxide in the perfusion fluid.
RESULTS: Pathological sinusoidal dilatation and centrilobular hydropic alteration were significantly lower in the groups that received amifostine prior to preservation solution perfusion. Although the best results were obtained in the UW + A group, we did not observe a statistically significant difference between the UW + A and HTK + A groups. iNOS grades were significantly lower in the amifostine groups 12 h after treatment. When the amifostine groups were compared against each other, the iNOS grades obtained from the UW + A and HTK + A groups were similar while the RL + A group had a much poorer score. TUNEL assays demonstrated a lower apoptosis ratio in the amifostine groups than in the non-amifostine groups 12 h after treatment. No statistically significant difference was observed between the UW + A and HTK + A groups for apoptosis. Cellular ultrastructure was best preserved in the UW + A and HTK + A groups.
CONCLUSION: Here, we show that preoperative administration of a single dose of amifostine is sufficient to minimize the preservation damage in hepatic cells.
Core tip: Preserving liver tissue obtained from a donor until implantation is a critical step that affects how the graft will function. Many different organ preservation solutions have been introduced over the last quarter century, and histidine-tryptophan-ketoglutarate (HTK) and University of Wisconsin (UW) are currently the most commonly used solutions in clinical practice. Because neither the HTK nor UW solution is ideal, many studies have focused on methods to enhance the antioxidant and cytoprotective effects of the preservation solutions. In this study, we investigated whether amifostine could boost the antioxidant and cytoprotective effects of HTK and UW preservation solutions.
- Citation: Akbulut S, Sevmis S, Karakayali H, Bayraktar N, Unlukaplan M, Oksuz E, Dagdeviren A. Amifostine enhances the antioxidant and hepatoprotective effects of UW and HTK preservation solutions. World J Gastroenterol 2014; 20(34): 12292-12300
- URL: https://www.wjgnet.com/1007-9327/full/v20/i34/12292.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i34.12292
Preserving liver tissue harvested from a living or deceased donor under ideal conditions until the time of transplantation a critical step that ultimately determines whether the graft will be functional in the host. Because a liver to be transplanted is often transported over a long distance, the prolonged cold ischemia process highlights the critical role played by the preservation process. In organ preservation, the primary goals are to establish hypothermia, prevent cellular swelling, and minimize free radical-induced ischemia-reperfusion injuries. Although many preservation solutions have been published over the last quarter century, the University of Wisconsin (UW) and histidine-tryptophan-ketoglutarate (HTK) solutions are most widely used clinically[1-5]. Preservations solutions contain a variety of substances to prevent cellular swelling, stabilize cell membranes, exert antioxidant and cytoprotective effects, and maintain intracellular electrolyte levels. The UW preservation solution uses adenosine, glutathione, and allopurinol as antioxidant and cytoprotective agents while the HTK solution utilizes histidine, tryptophan, ketoglutarate, and mannitol[5]. A current focus in the transplant field is to enhance the antioxidant and cytoprotective effects of preservation solutions.Amifostine (WR-2721) is an antioxidant and cytoprotective agent that can scavenge for and eliminate free oxygen radicals and reactive nucleophiles known to cause DNA damage and injury[6-8]. Like other published antioxidants, amifostine derives its antioxidant potential from a thiol molecular structure. The presence of intracellular free thiols has been shown to reduce oxidative stress and thus minimize apoptosis and DNA injury[6-8]. The aim of the present study was to determine whether preoperative administration of amifostine would enhance the antioxidant and cytoprotective effects of HTK and UW preservation solutions.
The Baskent University Ethics Committe for Animal Research approved this research (No. DA07/45). All experimental procedures within this manuscript were in compliance with the guidelines established by the National Institutes of Health within the “Guide for the Care and Use of Laboratory Animals”. Forty-eight adult male Sprague Dawley rats (mean weight: 300-350 g) were used for these experiments. The 48 rats were randomly assigned to one of six groups, with an equal number of rats contained within each group. The six groups were as follows: Group-I: Ringer Lactate (RL) group; Group-II: RL + amifostine (RL + A) group; Group-III: HTK group; Group-IV: HTK + A group; Group-V: UW group; Group-VI: UW + A group. Rats were given 50 mg/kg ketamine hydrochloride (Ketalar, Eczacibasi Warner-Lambert, Levent, İstanbul, Turkey) and 10 mg/kg xylazine hydrochloride (Rompon, Bayer, Sisli, İstanbul, Turkey) prior to all surgical procedures. Rats in groups II, IV, and VI were administered amifostine 200 mg/kg (Ethyol, Medimmune Pharma B.V., the Netherlands) intraperitoneally 30 min prior to the laparotomy. After confirming that the rats were adequately anesthetized, a laparotomy was performed via a midline incision. The distal part of the portal pedicle was ligated after a cannula was placed in the portal vein lumen. Group-I rats were perfused with RL preserved at 4 °C solution through the cannula into the portal vein. Group-II rats were administered amifostine before the laparotomy and then subsequently perfused with RL, like the rats in Group-I. Rats in Group-III were perfused with HTK (Custodiol; Odyssey Pharmaceutical Inc., East Hanover, NJ, United States) preserved at 4 °C. Group-IV rats were first infused with amifostine before laparotomy, followed by perfusion with HTK. Rats in Group-V were perfused with UW solution (Viaspan; DuPont Merck Pharmaceutical Company, Wilmington, DE, United States) preserved at 4 °C. Rats in Group-VI received amifostine prior to the laparotomy and were subsequently perfused with UW. During the perfusion process, the suprahepatic vena cava was cut to determine if the fluid draining from the liver was clear. The perfusion process was continued until the drained fluid became clear. After perfusion, a standard hepatectomy was performed. Isolated liver specimens were placed into bags containing RL, UW, or HTK solutions and stored in ice-filled storage boxes. At 0, 6, and 12 h post-hepatectomy (PH), biopsy samples were taken from the stored liver tissues for pathological and immunohistochemical evaluation, and fluid samples were taken for biochemical analysis. A liver biopsy was also taken 6 h PH for analysis by electron microscopy.
Evaluation of hepatocyte injury with hematoxylin and eosin: To determine if hepatocyte injury occurred during the preservation process, hematoxylin and eosin staining were used to assay four different parameters: fatty change (FC; absent or present: 1%-30%, 31%-60%, 61%-100%), hepatocyte ballooning degeneration (BD; absent or present: mild, moderate and severe), centrilobular hydropic change (CLHC; absent or present: focal or diffuse), and sinusoidal dilatation (SD; absent or present: focal or diffuse)[1,9].
Semi-quantitative evaluation of hepatocytes with inducible nitric oxide syntetase (iNOS) staining: Tissues were embedded in paraffin. Tissue cross-sections (3 μm thickness) were stained with iNOS and examined under microscope. Hepatocyte iNOS staining was scored as follows: Grade 0: less than 10% of hepatocytes stained; Grade I: 10%-25% of hepatocytes stained; Grade II: 25%-50% of hepatocytes stained; and Grade III: more than 50% of hepatocytes stained[10,11].
Evaluation of hepatocyte apoptosis: Three micron cross-sections from paraffin-embedded tissues were analyzed for apoptosis using terminal deoxynucleotidyl transferase mediated dUTP nick end labeling (TUNEL). TUNEL-processed tissues were analyzed in at least 10 different fields of view under a × 400 magnification, with at least 1000 hepatocytes counted. The number of cells with TUNEL-positive nuclei were recorded[12,13].
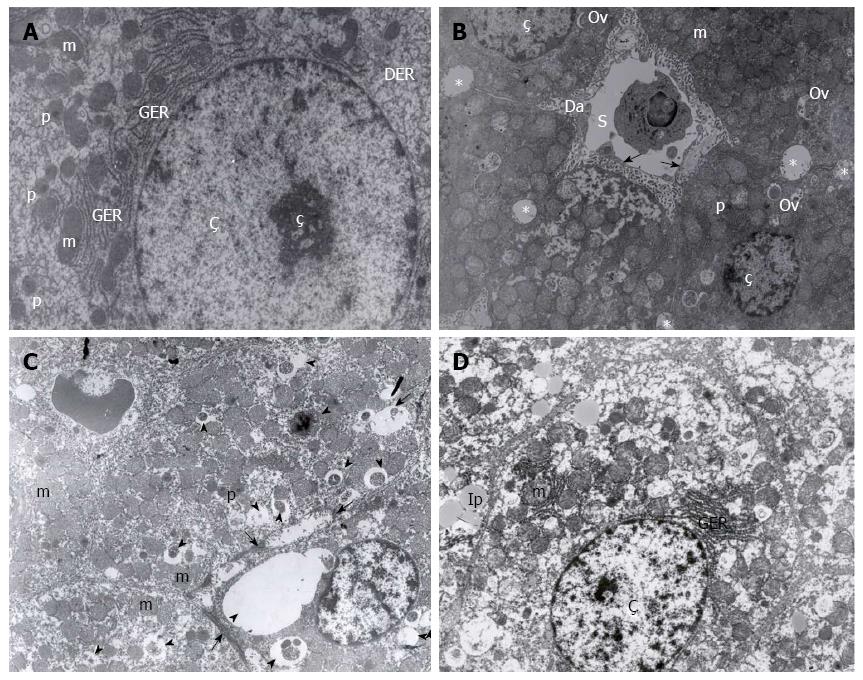
Liver specimens from four rats, randomly selected from each group, were treated with certain biochemical processes and examined under LEO 906E transmission electron microscopy. Changes in nuclei, endothelial cells, plasma membrane, mitochondria, and endoplasmic reticulum ultrastructure were examined by electron microscopy[1]. The results were evaluated by an experienced histologist.
Alanine aminotransferase (ALT) was analyzed by an Ultraviolet (UV) absorbance method that had been adapted to a standardized method while alkaline phosphatase (ALP) was analyzed using a colorimetric approach. The levels both enzyme were expressed in unit/liter (U/L). Nitric oxide (NO) concentration was analyzed using the nitrate/nitrite colorimetric test kit (Cayman chemical, Ann Arbor, MI, United States), with an absorbance of 550 nm. The results were presented as μm/mL[14].
All results from this study were analyzed with SPSS 11.5 statistical software (IBM, Chicago, IL, United States). Intergroup comparisons were performed with student’s t test, χ2 test, and ANOVA. A P value < 0.05 was set as the threshold for statistical significance. Where appropriate, the results presented in tables are displayed as the mean ± SD.
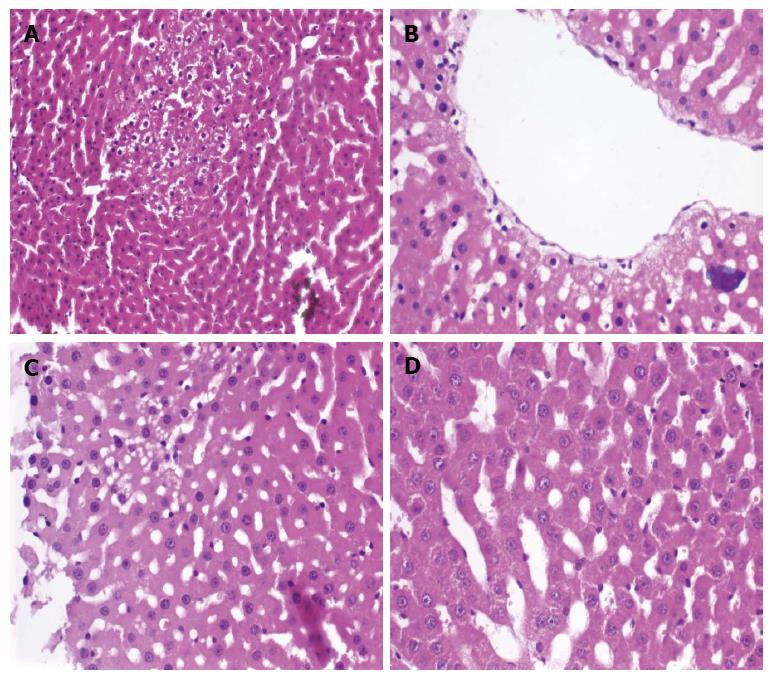
Liver biopsy samples stained with hematoxylin and eosin were examined to determine if FC, BD, CLHC, or SD pathological hepatocyte injuries occurred (Figure 1). A significant difference was observed between the RL and RL + A groups with respect to CLHC at 6 and 12 h PH (P < 0.05 for both time points). HTK and HTK + A groups were significantly different with regard to both SD (6 h PH: P = 0.05; 12 h PH: P < 0.05) and CLHC (6 h PH: P < 0.04; 12 h PH: P = 0.05). UW and UW + A groups had significantly different SD (P < 0.05 at both time points) and CLHC (P < 0.05 at both time points). When examining the amifostine-treated groups, the UW + A group had the best results although a statistically significant difference was not observed from the HTK + A group. Rats in the RL + A group displayed more prominent pathological changes in SD and CLHC when compared to the other amifostine-treated groups. FC and BD histology was unchanged between the groups. The results are summarized in Table 1.
| Groups | Time | n | SD | CLHC | BD | FC | ||||
| (+) | (-) | (+) | (-) | (+) | (-) | (+) | (-) | |||
| RL | 0 h | 8 | 1 | 7 | 2 | 6 | 1 | 7 | 0 | 8 |
| 6 h | 8 | 2 | 6 | 4 | 4 | 2 | 6 | 0 | 8 | |
| 12 h | 8 | 5 | 3 | 7 | 1 | 4 | 4 | 1 | 7 | |
| RL + A | 0 h | 8 | 2 | 6 | 2 | 6 | 0 | 8 | 0 | 8 |
| 6 h | 8 | 1 | 7 | 0 | 8 | 2 | 6 | 0 | 8 | |
| 12 h | 8 | 3 | 5 | 4 | 4 | 2 | 6 | 0 | 8 | |
| HTK | 0 h | 8 | 1 | 7 | 0 | 8 | 0 | 8 | 1 | 7 |
| 6 h | 8 | 4 | 4 | 4 | 4 | 1 | 7 | 0 | 8 | |
| 12 h | 8 | 3 | 5 | 4 | 4 | 2 | 6 | 1 | 7 | |
| HTK + A | 0 h | 8 | 0 | 8 | 0 | 8 | 0 | 8 | 0 | 8 |
| 6 h | 8 | 2 | 6 | 1 | 7 | 0 | 8 | 0 | 8 | |
| 12 h | 8 | 1 | 7 | 2 | 6 | 1 | 7 | 1 | 7 | |
| UW | 0 h | 8 | 1 | 7 | 0 | 8 | 0 | 8 | 0 | 8 |
| 6 h | 8 | 4 | 4 | 4 | 4 | 0 | 8 | 1 | 7 | |
| 12 h | 8 | 3 | 5 | 3 | 5 | 1 | 7 | 1 | 7 | |
| UW + A | 0 h | 8 | 0 | 8 | 0 | 8 | 0 | 8 | 0 | 8 |
| 6 h | 8 | 1 | 7 | 1 | 7 | 0 | 8 | 0 | 8 | |
| 12 h | 8 | 1 | 7 | 1 | 7 | 0 | 8 | 0 | 8 | |
The results of TUNEL assays used to evaluate cell death under the different preservation conditions are summarized in Table 2. During the first six hours PH, no significant differences in cell death were observed across any of the treatment groups. At 12 h PH, however, there was a significant reduction in apoptosis in groups that had been pre-treated with amifostine. When the non-amifostine groups were compared, the RL alone group had a significantly higher rate of apoptosis (P < 0.04), while HTK and UW groups did not significantly differ from each other (P < 0.25). Similarly, in the amifostine-treated groups, the RL+A had a significantly higher rate of apoptosis while there was no appreciable difference between the UW + A and HTK + A groups.
| Groups | n | 0 h | 6 h | 12 h |
| RL | 8 | 148 ± 26 | 185 ± 22 | 229 ± 41 |
| RL + A | 8 | 144 ± 25 | 166 ± 24 | 172 ± 24 |
| P value | 0.40 | 0.36 | 0.05 | |
| HTK | 8 | 111 ± 30 | 144 ± 46 | 169 ± 42 |
| HTK + A | 8 | 101 ± 19 | 126 ± 16 | 133 ± 14 |
| P value | 0.45 | 0.45 | 0.04 | |
| UW | 8 | 100 ± 23 | 115 ± 25 | 158 ± 24 |
| UW + A | 8 | 90 ± 16 | 100 ± 21 | 116 ± 18 |
| P value | 0.42 | 0.44 | 0.01 |
iNOS-stained samples were graded semi-quantitatively as 0, I, II, and III (described in the materials and methods section). The amifostine-treated groups received lower iNOS grades than the non-amifostine groups, a difference that became statistically significant at 12 h PH. Within the amifostine-treated groups, no significant differences were observed for the UW + A and HTK + A groups, while the RL + A group had a significantly worse grade. The findings are summarized in Table 3.
| Time | G | RL | RL + A | HTK | HTK + A | UW | UW + A |
| n = 8 | n = 8 | n = 8 | n = 8 | n = 8 | n = 8 | ||
| 0 h | 0 | 0 | 3 | 4 | 4 | 7 | 6 |
| I | 3 | 2 | 2 | 4 | 0 | 1 | |
| II | 3 | 2 | 2 | 0 | 1 | 1 | |
| III | 2 | 1 | 0 | 0 | 0 | 0 | |
| P value | 0.590 | 0.26 | 0.24 | ||||
| 6 h | 0 | 0 | 1 | 1 | 3 | 1 | 4 |
| I | 4 | 0 | 5 | 4 | 6 | 3 | |
| II | 4 | 6 | 2 | 1 | 1 | 1 | |
| III | 0 | 1 | 0 | 0 | 0 | 0 | |
| P value | 0.055 | 0.02 | 0.51 | ||||
| 12 h | 0 | 1 | 1 | 0 | 2 | 0 | 2 |
| I | 0 | 2 | 1 | 4 | 3 | 5 | |
| II | 1 | 4 | 5 | 1 | 3 | 1 | |
| III | 6 | 2 | 2 | 1 | 2 | 0 | |
| P value | 0.030 | 0.05 | 0.04 | ||||
NO levels from perfusion fluids were measured biochemically at 0, 6, and 12 h PH. Intergroup analyses showed that the UW+A group had the lowest NO levels while the RL group showed the highest NO levels at all time points examined. The amifostine-treated groups generally had lower NO levels than the other groups. The findings are summarized in Table 4.
| Groups | n | 0 h | 6 h | 12 h |
| RL | 8 | 0.79 ± 0.22 | 1.15 ± 0.24 | 1.78 ± 0.26 |
| RL + A | 8 | 0.73 ± 0.18 | 1.26 ± 0.18 | 1.34 ± 0.23 |
| P value | 0.690 | 0.61 | 0.022 | |
| HTK | 8 | 0.64 ± 0.19 | 1.09 ± 0.19 | 1.71 ± 0.15 |
| HTK + A | 8 | 0.71 ± 0.19 | 0.80 ± 0.10 | 1.04 ± 0.13 |
| P value | 0.590 | 0.06 | 0.016 | |
| UW | 8 | 0.81 ± 0.18 | 1.02 ± 0.34 | 1.31 ± 0.98 |
| UW + A | 8 | 0.46 ± 0.21 | 0.60 ± 0.14 | 0.69 ± 0.13 |
| P value | 0.033 | 0.01 | 0.020 |
The lowest ALT levels were observed in the UW + A group while the highest levels were observed in the RL group (RL vs RL + A: not significant; UW vs UW + A: P < 0.02; HTK vs HTK + A: P < 0.045). During the first 6 h PH, ALP levels were not statistically different between the groups. The lowest ALP levels were observed in the UW + A group while the highest levels were observed in the RL group. No significant differences existed between HTK + A and UW + A groups. The results are summarized in Table 5.
| Groups | 0 h | 6 h | 12 h | |||
| ALP | ALT | ALP | ALT | ALP | ALT | |
| RL | 2.6 ± 1.9 | 17.6 ± 9.8 | 55.9 ± 7.5 | 90.4 ± 16.2 | 82.1 ± 7.8 | 370.1 ± 73.8 |
| RL + A | 1.9 ± 0.8 | 15.8 ± 7.4 | 25.0 ± 3.3 | 84.4 ± 14.1 | 44.0 ± 6.0 | 309.5 ± 46.1 |
| HTK | 1.1 ± 0.3 | 19.4 ± 6.4 | 33.1 ± 6.2 | 60.6 ± 7.1 | 50.4 ± 6.5 | 172.6 ± 22.5 |
| HTK + A | 1.9 ± 1.3 | 23.4 ± 8.4 | 25.4 ± 3.5 | 41.4 ± 2.8 | 35.3 ± 4.2 | 110.9 ± 9.8 |
| UW | 1.3 ± 0.4 | 27.8 ± 7.5 | 32.0 ± 4.6 | 59.1 ± 5.9 | 46.9 ± 6.2 | 188.3 ± 76.5 |
| UW + A | 2.1 ± 2.2 | 16.6 ± 5.1 | 22.6 ± 5.3 | 41.6 ± 6.6 | 28.3 ± 5.6 | 80.0 ± 7.5 |
Evaluation of the cellular ultrastructure across the six groups revealed that the UW + A group were best preserved, resembling a control liver sample (Figure 2A and B). The HTK + A, UW and RL + A groups had an intermediate preservation phenotype, with some ultrastructural damage (Figure 2C), while the HTK and RL groups were poorly preserved (Figure 2D). Some hepatocytes in each group contained lipid droplets, though it may be attributed more too external factors such as diet.
To appreciate the substantial advances made in the field of organ transplantation, it is first necessary to briefly discuss its history. The first experimental liver transplantation in the world was performed by Welch in 1955 while the first human liver transplantation was performed by Starzl in 1963[15]. Starzl’s first several attempts at liver transplantation failed, until he achieved success in 1967. Bismuth et al[16] developed the first protocol for reduced-size liver transplantation in 1984. The first ex situ split liver transplantation was performed by Pichlmayr et al[17] in 1988 while the first living donor liver transplantation was successfully completed by Strong et al[18] in 1989. In Turkey, the first deceased donor liver transplantation was performed by Haberal et al in 1988. Karakayali et al[19] and Haberal et al[20,21] also successfully performed the first pediatric segmental living donor liver transplantation in Europe and the Middle East in 1990. That same year, the same team announced the first segmental adult living donor liver transplantation in the world.
After Busuttil et al[15] successfully performed a liver transplant, liver transplantation has become the standard therapy for end-stage liver disease and acute liver failure. Despite the recent advances in surgical techniques, intensive care unit facilities, and immunosuppressive therapies, graft failure still occurs during the postoperative period and, due to a shortage of donors, many patients are unable to survive long enough for retransplantation[22]. The term “Primary non-function” (PNF) has been developed to describe the clinical entity characterized by postoperative serum liver enzyme elevation, decreased bile production, and severe coagulopathy. Many factors such as poor surgical technique, poor graft quality, prolonged cold ischemia, ischemia-reperfusion injury, and preservation injury, have been implicated in inciting PNF during the postoperative period[1,5,23-25].
The process of organ preservation begins when a donor patient is diagnosed as brain dead or at a donor’s operation for organs from living donor origin. The process ends when vascular anastomosis is complete and the organ is fully functional. Once tissue blood flow ceases, oxidative phosphorylation mechanisms become impaired, intracellular ATP is reduced, and marked increases in AMP levels are observed in cells exposed to ischemia. The resultant increases in AMP follows the hypoxanthine, xanthine, and urea pathway in oxygenated medium, using molecular oxygen instead of NAD+ as the electron acceptor in non-oxygenated media, which ultimately leads to the production of anion radicals and hydrogen peroxide[26-28]. The fundamental goals of liver preservation from a deceased or a living donor are to keep the organ hypothermic, prevent cellular swelling, and minimize free radical-induced injury until the organ is functioning normally again. Although hundreds of preservation solutions have been developed over the last quarter century to facilitate a more successful transplant process, the HTK and UW solutions are most commonly used in clinical practice. Both solutions contain electrolytes that help maintain the osmotic equilibrium of the cell as well as membrane-stabilizing agents and antioxidants that scavenge free oxygen radicals produced during the ischemic period[1-5,29,30].
UW solution was developed by Belzer et al at the University of Wisconsin. It contains glutathione, which prevents free radical formation, allopurinol, raffinose, adenosine for ATP production, hydroxyethyl starch to prevent interstitial edema, and phosphate ions for pH stabilization. Because of the high potassium content, however, UW solution can lead to hyperkalemia onset. Compared to HTK solution, UW preservation solution has a higher viscosity and more prominent endothelium-protective properties. In contrast, its ischemic biliary complication rates are much higher than the HTK solution. UW solution also increases hepatic artery resistance and enhances erythrocyte aggregation, which are undesirable features in a preservation solution[1,5,30].
HTK solution, an extracellular protective solution, was initially developed to treat cardioplegia in the 1980s. In this solution, the mannitol and histidine components exert both antioxidant and osmotic effects. Ketoglutarate and tryptophan, two other primary components in HTK, are both membrane-protective agents; ketoglutarate also acts as a substrate in the Krebs cycle in an oxygen-deprived cell. Because HTK contains low amounts of K+ and Na++, it was initially widely used during cardiac transplantation. The HTK solution has a lower viscosity and promotes more rapid cooling of organs, with a flow rate three times higher than other preservation solutions. Compared to UW, HTK is more affordable and poses no risk for hyperkalemia when used in flushing mode. In general, the HTK solution reduces leukocyte adhesion, capillary permeability, and ATP consumption while increasing tissue oxygenation and LDH levels. Previous studies have demonstrated that HTK is as efficient as UW in liver preservation[2,3,5,30]. Mangus et al[3] reported no difference between liver transplantation groups that received HTK or UW with respect to graft survival, patient survival, PNF development, and postoperative complications. The same research group also reported that HTK, when compared to UW, allowed for more rapid cooling and cleared blood from the organ more rapidly and more efficiently. UW, on the other hand, was shown to more efficiently preserve grafts with longer cold ischemia time. The same authors reported in a separate study that biliary complications and overall costs were lower with HTK[4]. Moray et al[2] reported no difference between the HTK and UW groups with respect to complication rates but added that HTK was more cost-efficient. In the present study, ALP was examined biochemically and bile canaliculi were examined histopathologically to evaluate the biliary system. No significant difference was detected between the amifostine-treated groups with regard to ALP level. Histopathology of the samples, however, demonstrated that cell frame (i.e., structure of bile canaliculi) was better preserved in the UW + A group.
Amifostine (WR-2721) is an inorganic thiophosphate compound weighing 214 kDa. Amifostine is dephosphorylated by the membrane-bound enzyme alkaline phosphatase and internalized through the cell membrane. Once inside the cell, amifostine is converted to its active form, the metabolite WR-1065, which stabilizes the membrane of intracellular organelles and protects cells against cytotoxic agents. Amifostine eliminates free oxygen radicals formed during ionizing radiation, or by chemicals such as anthracycline and bleomycin. Thus, amifostine is an antioxidant and cytoprotective agent that is capable of eliminating free oxygen radicals. Healthy cells have higher alkaline phosphatase activity while hypoxic and cancer cells, which have altered membrane lipid structure, have substantially lower enzymatic activity. Thus, amifostine administration is required prior to the development of cellular injury. If amifostine is administered after cellular damage has already occurred, it is unable enter the cell or be converted to its active metabolite, WR-1065. Clinical studies with amifostine have administered it intravenously 30 min prior to radiotherapy. For rats, the recommended dose is 200 mg/kg given subcutaneously, though studies have demonstrated that intravenous and subcutaneous administration result in the same drug efficacy[6-8]. In our study, three amifostine-treated groups were compared to three non-amifostine-treated groups. Biochemical analyses revealed that all three amifostine-treated groups had lower ALT and ALP levels, as well as less vacuolization and reduced bile canaliculi injury and epithelial degeneration in the sinusoidal epithelium. Immunohistochemical analyses revealed less overall cellular injury in groups that received amifostine. Taken together, these results demonstrate the robust protective effect exerted by amifostine.
Hepatic injury is readily observed through biochemical, histopathological, immunohistochemical or ultrastructural approaches. Atık et al[10] observed a strong correlation between the extent of cellular injury and the induced expression of iNOS. Further, Romero et al[11] demonstrated a correlation between the severity of cellular injury in transplant rejection and the iNOS staining grade. In our study, less iNOS staining (and thus a lower grade) was evident in groups treated with amifostine, especially 12 h PH. These results support the hypothesis that amifostine reduces hepatocyte injury during cold preservation.
Kuo et al[31], using a TUNEL approach to examine cell death, showed that cold preservation-induced cell damage caused cell death, primarily through apoptosis. Natori et al[32] reported that although caspase-dependent apoptosis was evident in sinusoidal epithelial cells during the cold ischemia period, hepatocyte damage was rarely observed. Using a TUNEL approach, Natori et al[32] also showed that provision of caspase inhibitors protected endothelial cells against apoptosis. In our study, administration of amifostine resulted in significantly lower apoptosis rates when compared to the non-amifostine groups.
In conclusion, studies to identify the best preservation solution and technique to minimize cold ischemia injury and preserve cadaveric livers are essential for better success in transplantation procedures. In the present study, we showed that a single dose of amifostine during the preoperative period minimized preservation injury in hepatic cells.
After the first successful liver transplantation by Starzl et al, liver transplantation has become the gold standard for treatment of end-stage liver disease and acute liver failure. Despite several recent advances in surgical techniques, intensive care unit facilities, and immunosuppressive therapies, there is still an appreciable rate of graft failure during the postoperative period. Many factors, such as prolonged cold ischemia, preservation injury and ischemia-reperfusion injury, have been implicated in graft failure.
The process of organ preservation begins when a donor patient is diagnosed as brain dead or at a donor’s operation for organs from living donor origin. The process ends when vascular anastomosis is complete and the organ is fully functional. Organ preservation is a critical step in determining successful engraftment. Although many organ preservation solutions have been introduced in the last quarter century, the University of Wisconsin (UW) and histidine-tryptophan-ketoglutarate (HTK) solutions are most widely used clinically. Despite these advances, the search for ideal preservation conditions is still ongoing. One way to overcome this obstacle is to provide antioxidant agents to the HTK and UW solutions.
The primary aim of this study was to investigate whether amifostine contributed to the antioxidant and cytoprotective effects of HTK and UW preservation solutions. No studies exist on the combined therapy of amifostine and UW or HTK solutions.
UW solution was developed for graft preservation from either a living or deceased donor. UW solution contains components such as glutathione to prevent free radical formation, adenosine for ATP production, hydroxyethyl starch for preventing interstitial edema, and phosphate ions for pH stabilization. The HTK solution was initially developed for cardioplegia in the 1980s. In this solution, mannitol and histidine exert antioxidant and osmotic effects. The ketoglutarate and tryptophan components, on the other hand, are membrane-protective agents while ketoglutarate additionally acts as a substrate of the Krebs cycle in an oxygen-deprived cell. Amifostine is an antioxidant and cytoprotective agent that can scavenge for and eliminate free oxygen radicals and reactive nucleophiles, which are known to cause DNA injury. The antioxidant properties of amifostine are due to its thiol structure.
In the manuscript: Antioxiadant and cytoprotective effect of amifostine, Akbulut et al have examined the protective effect of amifostine in preservation of liver in combination with known protective agents. The results are well represented and define the suitability of amifostine along with HTK and UW preservation solutions.
P- Reviewer: Buell JF, Ramani K, Zielinski J S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Başaran O, Emiroğlu R, Karakayali H, Moray G, Bilgin N, Yağmurdur MC, Ozdemir H, Haberal M. Comparison of two different liver storage techniques in rat liver model. Transplant Proc. 2003;35:2816-2820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 2. | Moray G, Sevmis S, Karakayali FY, Gorur SK, Haberal M. Comparison of histidine-tryptophan-ketoglutarate and University of Wisconsin in living-donor liver transplantation. Transplant Proc. 2006;38:3572-3575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Mangus RS, Tector AJ, Agarwal A, Vianna R, Murdock P, Fridell JA. Comparison of histidine-tryptophan-ketoglutarate solution (HTK) and University of Wisconsin solution (UW) in adult liver transplantation. Liver Transpl. 2006;12:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Mangus RS, Fridell JA, Vianna RM, Milgrom MA, Chestovich P, Chihara RK, Tector AJ. Comparison of histidine-tryptophan-ketoglutarate solution and University of Wisconsin solution in extended criteria liver donors. Liver Transpl. 2008;14:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Feng L, Zhao N, Yao X, Sun X, Du L, Diao X, Li S, Li Y. Histidine-tryptophan-ketoglutarate solution vs. University of Wisconsin solution for liver transplantation: a systematic review. Liver Transpl. 2007;13:1125-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | McKibbin T, Panetta JC, Fouladi M, Gajjar A, Bai F, Okcu MF, Stewart CF. Clinical pharmacokinetics of amifostine and WR1065 in pediatric patients with medulloblastoma. Clin Cancer Res. 2010;16:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Koukourakis MI. Amifostine in clinical oncology: current use and future applications. Anticancer Drugs. 2002;13:181-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Kanat O, Kurt E, Yalcinkaya U, Evrensel T, Manavoglu O. Comparison of uroprotective efficacy of mesna and amifostine in Cyclophosphamide- induced hemorrhagic cystitis in rats. Indian J Cancer. 2006;43:12-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Lim AY, Segarra I, Chakravarthi S, Akram S, Judson JP. Histopathology and biochemistry analysis of the interaction between sunitinib and paracetamol in mice. BMC Pharmacol. 2010;10:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Atik E, Onlen Y, Savas L, Doran F. Inducible nitric oxide synthase and histopathological correlation in chronic viral hepatitis. Int J Infect Dis. 2008;12:12-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Romero M, García-Monzón C, Clemente G, Salcedo M, Alvarez E, Majano PL, Moreno-Otero R. Intrahepatic expression of inducible nitric oxide synthase in acute liver allograft rejection: evidence of modulation by corticosteroids. Liver Transpl. 2001;7:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Raina K, Rajamanickam S, Singh RP, Agarwal R. Chemopreventive efficacy of inositol hexaphosphate against prostate tumor growth and progression in TRAMP mice. Clin Cancer Res. 2008;14:3177-3184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Ma ZY, Qian JM, Rui XH, Wang FR, Wang QW, Cui YY, Peng ZH. Inhibition of matrix metalloproteinase-9 attenuates acute small-for-size liver graft injury in rats. Am J Transplant. 2010;10:784-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Menaka KB, Ramesh A, Thomas B, Kumari NS. Estimation of nitric oxide as an inflammatory marker in periodontitis. J Indian Soc Periodontol. 2009;13:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Busuttil RW, De Carlis LG, Mihaylov PV, Gridelli B, Fassati LR, Starzl TE. The first report of orthotopic liver transplantation in the Western world. Am J Transplant. 2012;12:1385-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Bismuth H, Houssin D. Reduced-sized orthotopic liver graft in hepatic transplantation in children. Surgery. 1984;95:367-370. [PubMed] |
| 17. | Pichlmayr R, Ringe B, Gubernatis G, Hauss J, Bunzendahl H. [Transplantation of a donor liver to 2 recipients (splitting transplantation)--a new method in the further development of segmental liver transplantation]. Langenbecks Arch Chir. 1988;373:127-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 347] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Strong RW, Lynch SV, Ong TH, Matsunami H, Koido Y, Balderson GA. Successful liver transplantation from a living donor to her son. N Engl J Med. 1990;322:1505-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 545] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 19. | Karakayali H, Haberal M. The history and activities of transplantation in Turkey. Transplant Proc. 2005;37:2905-2908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Haberal M, Tokyay R, Telatar H, Buyukpamukcu N, Simsek H, Kayhan B, Arslan G, Bilgin N, Ekici E, Uzunalimoglu B. Living related and cadaver donor liver transplantation. Transplant Proc. 1992;24:1967-1969. [PubMed] |
| 21. | Haberal M, Buyukpamukcu N, Telatar H, Bilgin N, Arslan G, Simsek H, Ekici E, Karamehmetoglu M. Segmental living liver transplantation in children and adults. Transplant Proc. 1992;24:2687-2689. [PubMed] |
| 22. | Müller SA, Mehrabi A, Schmied BM, Welsch T, Fonouni H, Engelmann G, Schemmer P, Weitz J, Schmidt J. Partial liver transplantation-living donor liver transplantation and split liver transplantation. Nephrol Dial Transplant. 2007;22 Suppl 8:viii13-viii22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Chen H, Peng CH, Shen BY, Deng XX, Shen C, Xie JJ, Dong W, Li HW. Multi-factor analysis of initial poor graft function after orthotopic liver transplantation. Hepatobiliary Pancreat Dis Int. 2007;6:141-146. [PubMed] |
| 24. | Li W, Meng Z, Liu Y, Patel RP, Lang JD. The hepatoprotective effect of sodium nitrite on cold ischemia-reperfusion injury. J Transplant. 2012;2012:635179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Stahl JE, Kreke JE, Malek FA, Schaefer AJ, Vacanti J. Consequences of cold-ischemia time on primary nonfunction and patient and graft survival in liver transplantation: a meta-analysis. PLoS One. 2008;3:e2468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Weigand K, Brost S, Steinebrunner N, Büchler M, Schemmer P, Müller M. Ischemia/Reperfusion injury in liver surgery and transplantation: pathophysiology. HPB Surg. 2012;2012:176723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 27. | Siemionow M, Arslan E. Ischemia/reperfusion injury: a review in relation to free tissue transfers. Microsurgery. 2004;24:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 28. | Kayyali US, Donaldson C, Huang H, Abdelnour R, Hassoun PM. Phosphorylation of xanthine dehydrogenase/oxidase in hypoxia. J Biol Chem. 2001;276:14359-14365. [PubMed] |
| 29. | Jain A, Mohanka R, Orloff M, Abt P, Kashyap R, Cullen J, Lansing K, Bozorgzadeh A. University of Wisconsin versus histidine-tryptophan-ketoglutarate for tissue preservation in live-donor liver transplantation. Exp Clin Transplant. 2006;4:451-457. [PubMed] |
| 30. | Avolio AW, Agnes S, Nure E, Maria G, Barbarino R, Pepe G, Castagneto M. Comparative evaluation of two perfusion solutions for liver preservation and transplantation. Transplant Proc. 2006;38:1066-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Kuo PC, Drachenberg CI, de la Torre A, Bartlett ST, Lim JW, Plotkin JS, Johnson LB. Apoptosis and hepatic allograft reperfusion injury. Clin Transplant. 1998;12:219-223. [PubMed] |
| 32. | Natori S, Selzner M, Valentino KL, Fritz LC, Srinivasan A, Clavien PA, Gores GJ. Apoptosis of sinusoidal endothelial cells occurs during liver preservation injury by a caspase-dependent mechanism. Transplantation. 1999;68:89-96. [PubMed] |