Published online Sep 14, 2014. doi: 10.3748/wjg.v20.i34.12283
Revised: April 29, 2014
Accepted: June 2, 2014
Published online: September 14, 2014
Processing time: 220 Days and 2 Hours
AIM: To examine the efficacy and safety of otilonium bromide (OB) in treatment-sensitive functional irritable bowel syndrome (IBS) clinical parameters.
METHODS: Ninety-three patients (44.8 ± 12.6 years, 69% female) with IBS symptoms complying with Rome II criteria participated in this double-blind, placebo-controlled, randomised, dose-ranging phase I/II study. Patients were administered OB 20 mg (n = 24), 40mg (n = 23) and 80 mg (n = 23) tid or placebo (n = 23) in 4 parallel groups for 4 wk. Primary efficacy variables included abdominal discomfort, intestinal habits, number of daily evacuations and stool consistency. Secondary efficacy measures included return to regular intestinal habits and global discomfort. Safety was also assessed.
RESULTS: Baseline clinical characteristics were similar among the 4 groups. Although individual parameters such as intensity and frequency of abdominal discomfort, bloating or pain were reduced by OB over the 4 wk, no significant differences were observed between groups. Similarly, no difference was observed between OB treatment or placebo for mucus in stool and incomplete or difficulty of evacuation. However, evacuation frequency was significantly reduced after 4 wk by 80 mg OB compared to placebo (-8.36% for placebo vs -41.9% for 80 mg OB, P < 0.01). While 21.7% of patients in the placebo group experienced regular intestinal habits after 4 wk, this improvement was greater for patients treated with 40 mg OB (P < 0.01 vs placebo). Furthermore, a dose-dependent reduction in frequency of diarrhoea (χ2-test for trend = 11.5, P < 0.001) and an increase in normal stool frequency was observed. Combining individual variables into a global discomfort index revealed significant improvement among increasing OB doses, favouring 40 mg (P = 0.013) and 80mg OB (P = 0.001) over placebo. No difference was observed between frequency of adverse events for placebo vs OB.
CONCLUSION: This dose-ranging study demonstrates that OB at 40 and 80 mg can improve individual and global clinical symptoms of IBS compared to placebo over a 4-wk period.
Core tip: Although previous trials have confirmed the efficacy of a single dose of otilonium bromide (OB) on well-defined endpoints in patients with irritable bowel syndrome (IBS), no study has specifically defined the optimal dosage of OB on standard IBS efficacy measures in a controlled cross-over design. Findings from this dose-ranging study demonstrate that OB at 40 and 80 mg can improve both individual and global clinical IBS symptoms compared to placebo over 4 wk. All doses of OB were well tolerated compared to placebo. Future long-term controlled trials on global efficacy measures will help reinforce findings from the present trial.
- Citation: Chmielewska-Wilkoń D, Reggiardo G, Egan CG. Otilonium bromide in irritable bowel syndrome: A dose-ranging randomized double-blind placebo-controlled trial. World J Gastroenterol 2014; 20(34): 12283-12291
- URL: https://www.wjgnet.com/1007-9327/full/v20/i34/12283.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i34.12283
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder characterized by chronic or recurrent abdominal pain or discomfort, and disturbed defecation[1]. The severity of the disorder ranges from mild to severe and intractable symptoms. The prevalence of IBS ranges from 5%-20%, depending upon the criteria employed and population evaluated[1-3]. The Rome diagnostic criteria for IBS requires the presence of at least 3 mo of continuous or recurrent symptoms of abdominal pain or discomfort relieved with defecation, or associated with a change in frequency or consistency of stool[4]. While the prevalence of IBS according to Rome I and III criteria is approximately 12%, it is considerably lower (about 3%) for patients diagnosed according to Rome II criteria, since Rome II criteria requires higher symptom frequency (> 10% vs > 25% of the time)[2,5].
Clinical symptoms of IBS relate to abnormalities in motility and visceral sensation and are influenced by psychosocial factors via the brain-gut axis[6]. Treatment is based on a combined pharmacological and behavioral approach. In light of the evidence of enhanced visceral perception in IBS and the frequent occurrence of pain as a key symptom, it is generally accepted that any agent considered for the treatment of IBS should demonstrate effective pain relief. Antispasmodics are often used to treat IBS, particularly for symptoms such as abdominal pain and bloating[7,8]. Otilonium bromide (OB) is an antispasmodic that exerts its mechanism of action by reducing hypermotility and modulating visceral sensation, factors thought to be responsible for pain in IBS[9,10]. It is a quaternary ammonium derivative with selective spasmolytic action on the gastrointestinal tract, in particular on the colon[11,12]. The efficacy of OB in IBS has been confirmed in 3 large randomized double-blind clinical trials[13-15]. Some of these trials were subjected to either an extended analysis or to meta-analysis[7,16]. Other studies taken into consideration by this meta-analysis[7] were smaller[17-19] and did not meet current standards for IBS trial design[20]. Although these large trials have confirmed the efficacy of a single dose of OB on well-defined endpoints over 15 wk[14-16] no study has specifically defined the optimal dosage of OB on standard IBS efficacy measures in a controlled cross-over design. Therefore, the objective of this trial was to evaluate the dose-response relationship of 20 mg, 40 mg, 80 mg OB and placebo administered tid for 4 wk on functional and/or clinical efficacy IBS variables.
The study population was drawn from male and female out-patients with IBS according to the Rome II Criteria[1], and conducted in 4 Centers in Krakow, Poland from December 2007 to May 2008. Inclusion criteria included: (1) male or female Caucasian patients ≥ 18 years and ≤ 65 years of age with IBS diagnosed according to the Rome II Criteria; (2) patients who have had at least a 6-mo history of IBS, with at least moderate abdominal pain or discomfort occurring on at least 4 d in each of the 4 wk prior to the study; (3) accurate anamnesis to exclude in particular the lactase deficiency syndrome (requiring H2-breath tests, when strongly suspected clinically), bowel inflammatory disease, diets or drugs that may cause gastrointestinal symptoms and alvus disturbances; (4) written informed consent; (5) the use of oral contraceptives or intrauterine devices or previous sterilization required for women of child-bearing potential and (6) negative urine pregnancy test required for pre- or premenopausal women (at baseline visit).
Exclusion criteria included: (1) patients who could not be definitely diagnosed as IBS; (2) history of intolerance or hypersensitivity to OB; (3) alimentary intolerance; (4) pregnant or nursing females; (5) previous severe abdominal surgery; (6) other concomitant diseases which could affect results of the study; (7) any malignancy; (8) any concomitant treatment that could affect gastrointestinal motility and function or medication that cannot be stopped; (9) participation in another clinical study within 2 mo prior to enrolment; and (10) insufficient patient comprehension. This study was approved by the Bioethics Committee at the District Chamber of Physicians in Krakow, Poland and conducted in accordance with International Conference on Harmonization (ICH) guidelines for Good Clinical Practice (GCP) and the Declaration of Helsinki. Written informed consent was obtained from each patient prior to screening and before any study procedures were performed.
This was a double-blind, placebo-controlled, randomized dose-ranging study in 4 parallel groups (EudraCT number 2007-001679-12; trial number MeIn/06/OB-20/80/001 available on http://www.menarini.com/clinical_studies/clinical_trial_registry). Following a screening visit, all patients entered a 2-wk treatment-free run-in period. Baseline responses to ano-rectal manometry and rectal distension, using a manometric device, were investigated and baseline assessment of IBS was recorded. Patients who reported at least moderate symptoms of IBS and demonstrated a clinically relevant degree of colonic hypersensitivity were stratified by gender and randomly allocated to either 20 mg OB tid, 40 mg OB tid, 80 mg OB tid or placebo for a treatment period of 4 wk. All tablets were film-coated for oral administration and immediate release. The inactive placebo had the same appearance as the active medication but filled with an inactive placebo mixture. A computer-generated randomization list was used to assign treatment group. The blinding of the different dosing regimens was maintained by double dummy technique. Patients recorded their assessment of IBS, adverse events and study drug intake in diaries. At every weekly visit (Visit-1, 0; baseline, 1, 2, 3 and 4) the patient’s diary data was checked and documented by the investigator. Immediately after the end of the treatment period the investigation of responses to distal colonic distension with a rectal balloon device was repeated.
Patients filled a weekly diary card at the end of each week in the 2-wk run-in period to record baseline values, and in the 4-wk treatment period for efficacy assessment. During the treatment period, patients provided a weekly global efficacy assessment by answering the question whether (or not) they had obtained adequate relief of their IBS pain and discomfort during the previous 7 d compared to the baseline period. Twelve efficacy measures of IBS were evaluated by coded scales, ranked 0-3 or 0-4; the only exception being intestinal habits, in which categorical assessment was applied. The scoring system for these standard IBS variables has been previously described in detail[14,16]. Briefly, intestinal habits were identified by the following features: regular (no constipation and/or diarrhoea); constipation (less than three evacuations during the week); diarrhoea (three or more evacuations per day, for at least 5 d); or alternating (more than 2 d without evacuation, together with some days containing three or more evacuations). The average daily number of evacuations was scored as “1 or less”, “2”, “3-4” or “5 or more”. The days without evacuation during the week were ranked as “0-1”, “2-3”, “4-5” or “6-7”[16]. In addition, in order to evaluate the “consistency and the shape of the stool”, the Bristol classification was used and the quantitative index termed regular stool rate (RSR) was created. RSR was calculated according to the formula: RSR = number of days with regular stool/total number of day × 100. Furthermore, a global discomfort index was generated and this was calculated as follows: (daily frequency of abdominal discomfort, bloating or pain × number of evacuations)/GDI mean × 100, where GDI Mean = mean efficacy in the 14 d prior to randomization (Screening Period).
All patients enrolled were considered for tolerability assessment (Safety Population). Safety was assessed by comparing differences between treatment groups in: (1) frequency of adverse events; (2) presence of serious adverse events/hospitalizations; and (3) withdrawals due to any adverse event.
The primary endpoint in the present study was the change in intensity or frequency of abdominal discomfort, bloating or pain (expressed on a 5-point scale), in addition to change in stool/defecation (evacuation) frequency (expressed on a 4-point scale). Based on previous studies[14-16], and expecting a relationship between the standard deviation of the responses and the slope of the dose-response curve not to exceed 250, a sample size of 21 evaluable patients per treatment group (total of 84 evaluable patients) was deemed sufficient to attain significance (using Wilcoxon Rank-Sum test) of the slope at a (one-sided) alpha of 0.025 with 81% power. Assuming a maximum dropout rate of 10%, the total number of patients to be recruited was estimated at 96. Data are presented as mean ± SD or number and percent. Statistical analysis was performed using SAS Software (version 9.1.3, SAS Institute, Cary, NC, United States). The effect of treatment over time for continuous efficacy variables was analyzed by ANOVA followed by Bonferroni-post hoc. Comparisons between two groups (e.g., treatment at specific time point) with normally distributed variables were analyzed by independent samples t-test. Categorical variables were analyzed by χ2 test. Comparisons between three or more groups were performed by one-way ANOVA, followed by Bonferroni post-hoc test. Nonparametric continuous variables (e.g., global discomfort index) were compared by the Mann-Whitney U or Wilcoxon Rank-Sum test, for independent samples comparisons, and Wilcoxon Signed-Rank test, for related sample comparisons. Where comparisons were made, quoted P values are two-tailed; n values refer to the number of patients examined. A value of P < 0.05 was considered statistically significant.
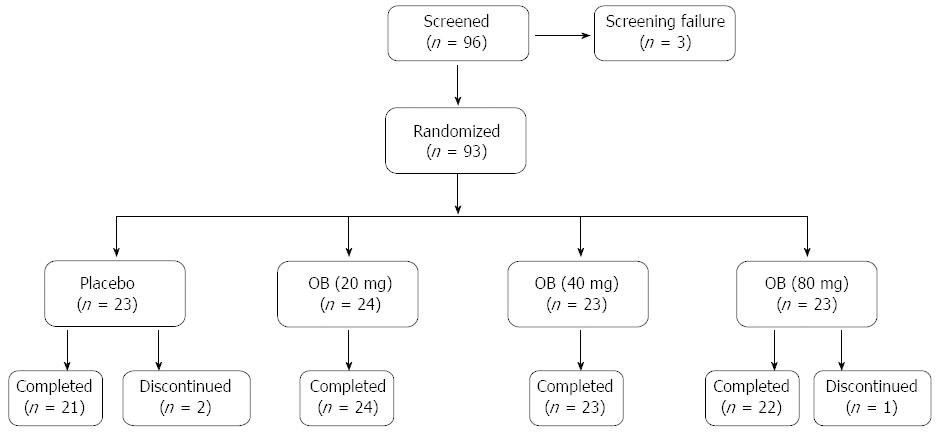
A total of 96 patients with IBS symptoms complying with Rome II criteria were screened and 93 were randomized for double-blind treatment with placebo or 3 doses of OB (Figure 1). Two patients discontinued from the study in the placebo group due to withdrawal of consent and one patient discontinued from the 80 mg OB arm due to abnormal laboratory values. Placebo and treatment arms had comparable demographic and baseline clinical characteristics (Table 1). Mean age for all patients was 44.8 ± 12.6 years and 69% were female. All subjects were Caucasian. Mean compliance for placebo and study medication was high in all groups, ranging from 91.3% to 93.8%.
| Characteristic | Placebo | OB 20 mg | OB 40 mg | OB 80 mg | Total |
| (n = 23) | (n = 24) | (n = 23) | (n = 23) | (n = 93) | |
| Demographic | |||||
| Age (yr) | 47.8 ± 13.1 | 44 ± 12.71 | 44.6 ± 13.66 | 42.9 ± 11.12 | 44.8 ± 12.6 |
| Female patients | 16 (69.6) | 16 (66.7) | 16 (69.6) | 16 (69.6) | 64 (68.8) |
| Height (cm) | 166.4 ± 7.9 | 170.4 ± 9.7 | 166.1 ± 7.8 | 170.2 ± 10.9 | 168 ± 9.24 |
| Weight (kg) | 69.8 ± 15.9 | 72.9 ± 16.6 | 68.9 ± 12.9 | 74.4 ± 16.9 | 71.5 ± 15.6 |
| Baseline ano-rectal parameters | |||||
| Anal canal length (mm) | 33.7 ± 1.6 | 34.6 ± 8.9 | 33.4 ± 9.8 | 33.6 ± 8.1 | 33.8 ± 9.4 |
| Depth of insertion probe (mm) | 24.8 ± 18.8 | 24.9 ± 14.3 | 24.2 ± 16.7 | 28.8 ± 18.1 | 25.6 ± 16.7 |
| Sphincter pressure (mmHg) | 51.9 ± 19.2 | 50.7 ± 16.4 | 54.4 ± 21.2 | 49.4 ± 20.2 | 51.6 ± 19 |
| Baseline symptom severity | |||||
| Intensity of abdominal pain1 | 2.7 ± 0.9 | 2.04 ± 0.94 | 2.19 ± 0.98 | 2.19 ± 0.8 | 2.2 ± 0.91 |
| Frequency of abdominal pain1 | 3.09 ± 1.83 | 3.55 ± 3.72 | 2.79 ± 2.14 | 3.35 ± 2.76 | 3.2 ± 2.6 |
| Evacuation frequency (daily) | 1.86 ± 1.72 | 1.54 ± 1.69 | 2 ± 1.69 | 1.77 ± 1.66 | 1.8 ± 1.69 |
| Intestinal habits2 | 2.56 ± 1 | 2.22 ± 1.1 | 2.45 ± 1.05 | 2.33 ± 0.97 | 2.4 ± 1.03 |
| Regular stool rate (%) | 32.2 | 25.8 | 35.8 | 30 | 31 |
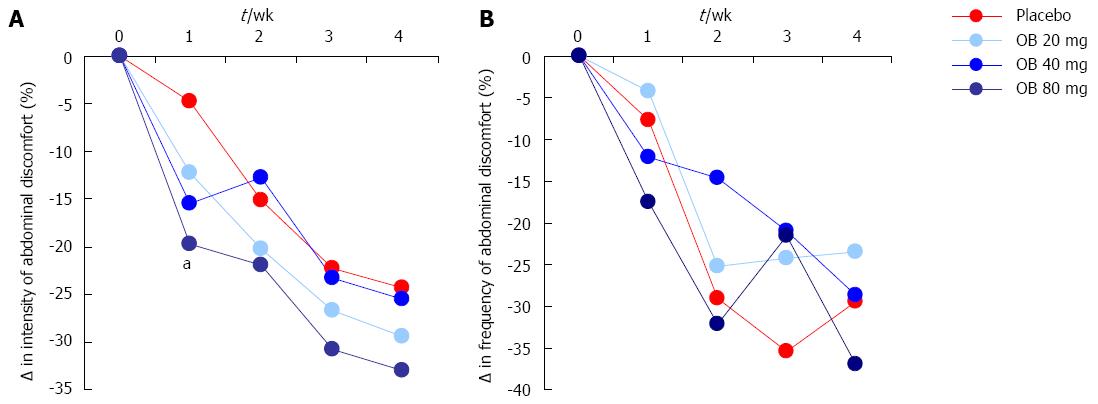
Intensity of abdominal discomfort, bloating or pain: The effect of OB compared to placebo on the change in intensity of abdominal discomfort, bloating or pain is shown in Figure 2A. Although the intensity of abdominal discomfort, bloating or pain were reduced by OB and placebo over the 4-wk period (1-way ANOVA, P = 0.015), no significant difference was observed between OB dose or between OB and placebo after 4 wk (Figure 2A). However, after just 1 wk of treatment with OB, a significant difference was noted in patients treated with 80 mg OB compared to those receiving placebo (-19.7% vs -4.8%, P < 0.05) (Figure 2A).
Frequency of abdominal discomfort, bloating or pain: The effect of OB compared to placebo on the change in frequency of abdominal discomfort, bloating or pain was also evaluated (Figure 2B). Similar to intensity, the frequency of abdominal discomfort, bloating or pain was also reduced (by approximately 30%) by OB and placebo groups over the study period, with no difference between treatments at 4 wk (Figure 2B).
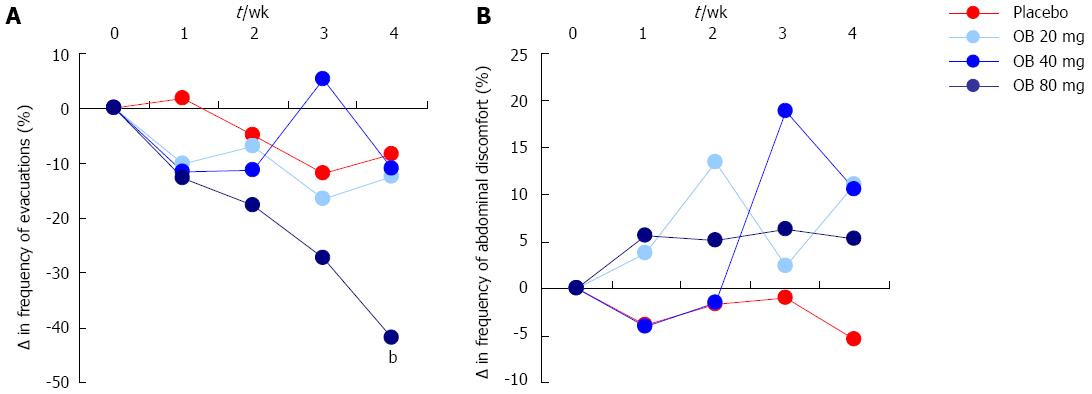
Frequency of evacuations: Although both 20 and 40 mg OB did not significantly alter evacuation frequency compared to placebo, a pronounced and statistically significant reduction in the frequency of stool evacuations was observed at 4 wk for patients treated with 80 mg OB compared to placebo (-41.9% vs -8.4%, P < 0.01) (Figure 3A).
Sensation of mucus in stool, incomplete evacuation or evacuation difficulty: There was no discernible effect of OB treatment or placebo on the change in sensation of mucus in stool, incomplete evacuation or evacuation difficulty over the 4 wk (Figure 3B).
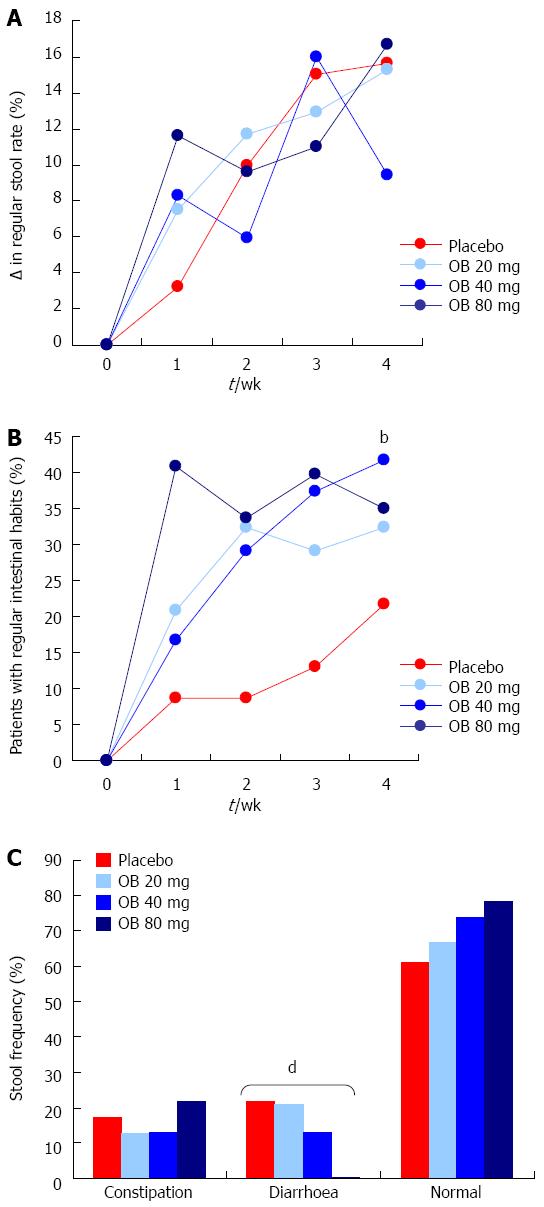
Regular stool rate: Regular stool rate was evaluated to measure the shape and consistency of the stool. In all 4 treatment arms, the regular stool rate increased over the 4 wk by approximately 16%, with no difference observed between treatments (Figure 4A).
Regular intestinal habits: Patients treated with OB (all 3 doses) experienced a marked improvement (30%-40%) in regular intestinal habits over the 4 wk, the 40 mg dose of OB attaining statistical significance compared to the placebo group (41.7% vs 21.7%, P < 0.01) (Figure 4B).
Stool frequency: The proportion of patients by treatment type experiencing constipation, diarrhoea or normal stool was also recorded at 4 wk (Figure 4C). Although the proportion of patients who experienced constipation did not significantly alter following placebo or treatment with OB, a statistically significant dose-dependent reduction in the frequency of diarrhoea was observed (χ2-test for trend = 11.5, P < 0.001). As expected, the proportion of patients experiencing normal stools increased inversely but this increase did not attain statistical significance (Figure 4C).
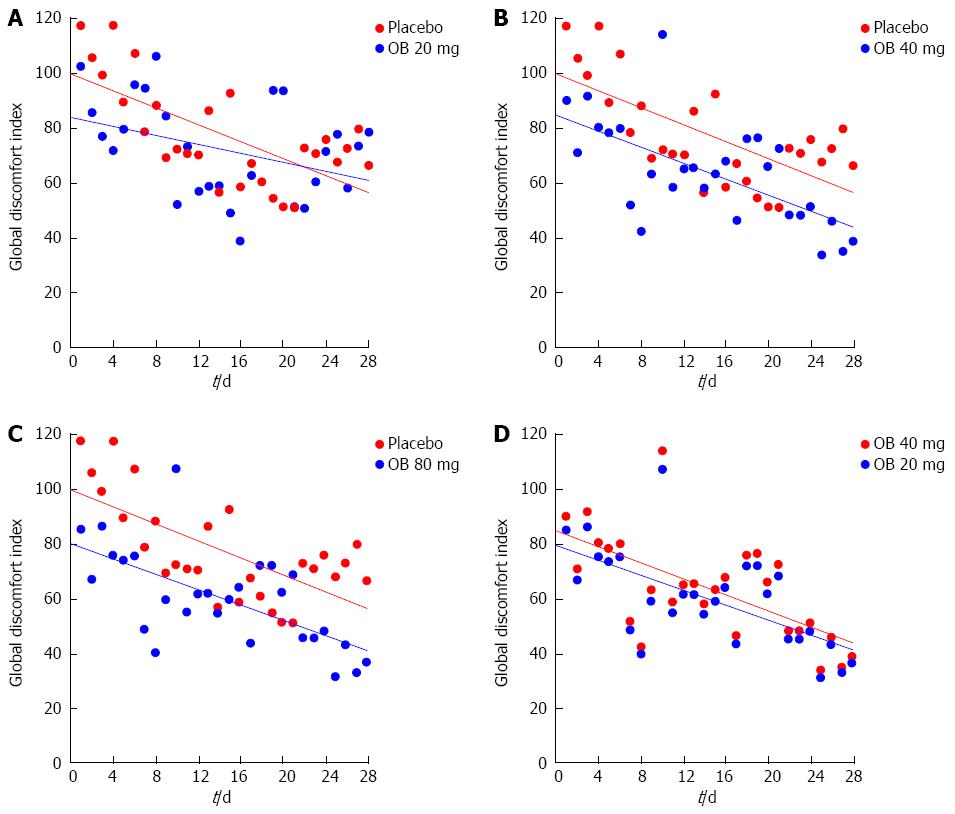
Global discomfort index: Combining individual efficacy variables into a global discomfort index revealed significant reduction over the 4 wk in all treatment groups (Figure 5). Comparing treatment groups revealed an improvement among increasing OB doses, favouring 40 mg (P = 0.013) and 80 mg OB (P = 0.001) over placebo (Table 2).
| Treatment | Ranks | Non-parametric tests | ||||
| Mean rank | Sum of ranks | Mann Whitney U | Wilcoxon Rank-Sum | Z | P-value | |
| 30.2 | 845 | |||||
| Placebo vs OB 20 mg | 26.8 | 751 | 345 | 751 | -0.77 | 0.440 |
| 33.9 | 949 | |||||
| Placebo vs OB 40 mg | 23.1 | 647 | 241 | 647 | -2.47 | 0.013 |
| 35.6 | 997 | |||||
| Placebo vs OB 80 mg | 21.4 | 599 | 193 | 599 | -3.26 | 0.001 |
| 30.4 | 851 | |||||
| OB 40 mg vs OB 80 mg | 26.6 | 745 | 339 | 745 | -0.87 | 0.390 |
Safety: No deaths or serious adverse events were reported for the entire study period. A total of 14 adverse events were reported, 6 being rated as moderate. The number of adverse events were similarly distributed among the 4 study groups, the most frequent being headache or migraine (n = 4) followed by flu/common cold (n = 3) and nausea (n = 2) (Table 3). During the treatment period, only 3 adverse events in the OB groups (one case of headache, dry mouth and a case of nausea) and one in the placebo group (one case of headache) were judged as related to the treatment by the Investigator.
| Treatment | Adverse event | Duration (d) | Intensity1 | Serious | Outcome | Relation |
| Placebo | Flu/fever | 12 | 1 | No | Resolved | Not related |
| Placebo | Severe headache | 3 | 2 | No | Resolved | Not related |
| Placebo | Headache | 14 | 2 | No | Resolved | Probably related2 |
| 20 mg | Headache | 5 | 1 | No | Resolved | Possible related2 |
| 20 mg | Xerostomia | 6 | 2 | No | Resolved | Unlikely related2 |
| 20 mg | High fever | 1 | 1 | No | Resolved | Not related |
| 20 mg | Pharyngitis | 7 | 2 | No | Resolved | Not related |
| 20 mg | Pneumonia | 17 | 2 | No | Resolved | Not related |
| 40 mg | Flu/fever | 4 | 1 | No | Resolved | Not related |
| 40 mg | Asthma exacerbation | 1 | 1 | No | Resolved | Not related |
| 40 mg | GI infection | 6 | 2 | No | Resolved | Not related |
| 80 mg | Cystitis | 19 | 1 | No | Resolved | Not related |
| 80 mg | Migraine attack1 nausea | 1 | 1 | No | Resolved | Not related |
| 80 mg | Nausea | 4 | 1 | No | Resolved | Possible related2 |
The main findings from this dose-ranging study demonstrate that OB at 40 and 80 mg can improve individual and global clinical symptoms of IBS compared to placebo over a 4-wk period. Furthermore, all 3 doses of OB, including the higher 80 mg dose were well tolerated compared to placebo control. Although the efficacy of the recommended dose of 40 mg OB in IBS has already been confirmed in 3 previous large studies[13-15], the present report is the first to examine different doses of OB in a double-blind placebo-controlled design.
Several limitations of the older IBS trials were addressed in the more recent Otilonium Bromide in Irritable Bowel Syndrome (OBIS) trial in which 356 patients were randomized to 40 mg OB (tid) or placebo for 15 wk[15]. The OBIS trial had similar inclusion criteria to that used in the present study. The main findings that emerged from this trial were that OB was superior to placebo in reducing the number of abdominal pain episodes and improving abdominal bloating and patient-assessed global efficacy. It is worth noting that the severity of abdominal bloating at baseline was moderate, which decreased to mild intensity following treatment. Other efficacy measures (e.g., abdominal pain severity, stool frequency, stool consistency, safety measures, etc.) did not differ between OB and placebo. Findings from OBIS have confirmed the clinical efficacy and tolerability of OB and demonstrated superiority of OB vs placebo in the reduction of abdominal pain frequency and bloating severity and in protecting patients from relapse. However, due to the long study duration (15-wk treatment period and 10-wk follow-up) and single-blind placebo run-in period, only patients with chronic, stable symptoms were included (according to Rome II criteria), therefore these findings have limited value to patients with wax and wane type symptoms.
Likewise, in the present trial, although we did not demonstrate improvement in endpoints used in the OBIS trial, we did note a significant reduction in evacuation frequency and an increase in regular intestinal habits by OB compared to placebo. Moreover, and similar to OBIS, we also observed a significant improvement by OB in global discomfort index, which is comparable to the patient global efficacy score used in OBIS. Further studies examining the efficacy of OB or similar spasmolytic should give more importance to global scores as opposed to individual measures, particularly in short-term studies, where differences in therapy or dose can be difficult to detect.
One of the key differences in the present dose-finding study is the short study duration of 4 wk compared to 15 wk and greater in the majority of previous efficacy trials[14-16]. The lack of significance between OB doses and placebo for several endpoints at 4 wk may well be attributed to the short study duration and to the low number of patients. However, another earlier trial by Baldi and colleagues[13] showed a significant decrease in patient-reported abdominal pain and bloating following treatment with 40 mg OB (tid) over 4 wk compared to placebo. It is well established (and noted in our findings) that a strong placebo effect persists in all therapeutic trials in IBS[21]. In our trial, the frequent number of follow-up visits that favours patient expectation as well as the patient-practitioner relationship may have been a contributing factor that we attempted to address, in part, by conducting a 2-wk run-in period to establish baseline levels. It is likely that differences in actual endpoints measured, baseline disease severity and use of concomitant medication may be relevant explanatory variables.
As with any pilot study, the aim of this trial was not to definitely examine the efficacy of a single dose of OB in patients with IBS, which has already been established[12-15]. Instead, this study was specifically designed to assess whether IBS symptoms could be improved in a dose-dependent manner compared to placebo control. Using individual efficacy measures as well as a global discomfort score, evidence of a dose-dependent improvement in IBS symptoms was detectable. However, due to the small sample size, strong placebo effect and variation in patient characteristics due to the natural history of IBS with cyclical variation of symptom intensity over time[22], data for several individual endpoints possessed a degree of variability that resulted in lack of statistical significance. We addressed this, in part, by establishing a baseline after a 2-wk run in period.
Regardless, an integrated symptom assessment such as global discomfort index is more proper to evaluate the efficacy in IBS and in our trial it is clearly demonstrated a dose-dependent improvement with OB compared with placebo for this parameter. Several endpoint measures used in the present trial differ from binary endpoints used in recent IBS trials resulting in potential difficulty with cross-comparison of results.
In summary, results from this dose-ranging cross-over placebo-controlled trial demonstrate that different doses of OB are safe, well tolerated and superior to placebo for specific endpoint measures over a period of 4 wk. Although inherent variability in results for specific endpoint measures were likely attributable to the low sample size, short study duration, and strong placebo effect, we were still able to demonstrate a dose-response improvement in some IBS symptoms. Future long-term (approximately 10-15 wk) controlled trials with long-term run-in periods on global efficacy measures will help reinforce findings in the present trial.
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder characterized by chronic or recurrent abdominal pain or discomfort, and disturbed defecation. Antispasmodics, (e.g., otilonium bromide, OB) are often used to treat IBS, particularly for symptoms such as abdominal pain and bloating.
Although previous trials have confirmed the efficacy of a single dose of OB on well-defined endpoints in patients with IBS, no study has specifically defined the optimal dosage of OB on standard IBS efficacy measures in a controlled cross-over design.
This dose-ranging study demonstrated that OB at 40 and 80 mg can improve individual and global clinical symptoms of IBS compared to placebo over a 4-wk period. Furthermore, all 3 doses of OB, including the higher 80 mg dose were well tolerated compared to placebo control.
These findings reinforce previous findings on the efficacy and safety of not only the 40 mg dose of OB but also the higher 80 mg dose. Global efficacy measures should be considered over individual efficacy measures to assess short-term therapeutic effect in the treatment of IBS.
Otilonium bromide is an antispasmodic that exerts its mechanism of action by reducing hypermotility and modulating visceral sensation, factors thought to be responsible for pain in IBS.
This is well done randomized, double-blind, placebo controlled clinical investigating efficacy and tolerability of oiliniu OB in IBS.
P- Reviewer: Lembo AJ, Rahimi R S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3413] [Cited by in RCA: 3381] [Article Influence: 177.9] [Reference Citation Analysis (1)] |
| 2. | Mearin F, Badía X, Balboa A, Baró E, Caldwell E, Cucala M, Díaz-Rubio M, Fueyo A, Ponce J, Roset M. Irritable bowel syndrome prevalence varies enormously depending on the employed diagnostic criteria: comparison of Rome II versus previous criteria in a general population. Scand J Gastroenterol. 2001;36:1155-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 164] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Saito YA, Schoenfeld P, Locke GR. The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97:1910-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 157] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45 Suppl 2:II43-II47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 830] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 5. | Sperber AD, Shvartzman P, Friger M, Fich A. A comparative reappraisal of the Rome II and Rome III diagnostic criteria: are we getting closer to the ‘true’ prevalence of irritable bowel syndrome? Eur J Gastroenterol Hepatol. 2007;19:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Drossman DA. Review article: an integrated approach to the irritable bowel syndrome. Aliment Pharmacol Ther. 1999;13 Suppl 2:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Ford AC, Talley NJ, Spiegel BM, Foxx-Orenstein AE, Schiller L, Quigley EM, Moayyedi P. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ. 2008;337:a2313. [PubMed] |
| 8. | Boeckxstaens G, Corazziari ES, Mearin F, Tack J. IBS and the role of otilonium bromide. Int J Colorectal Dis. 2013;28:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Martínez-Cutillas M, Gil V, Gallego D, Mañé N, Martín MT, Jiménez M. Mechanisms of action of otilonium bromide (OB) in human cultured smooth muscle cells and rat colonic strips. Neurogastroenterol Motil. 2013;25:e803-e812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Evangelista S. Otilonium bromide: a selective spasmolytic for the gastrointestinal tract. J Int Med Res. 1999;27:207-222. [PubMed] |
| 11. | Signorini C, Tosoni S, Ballerini R, Chinol M, Mannucci C. A study of the absorption of octylonium bromide following oral administration in man. Drugs Exp Clin Res. 1984;10:273-276. |
| 12. | Amenta F, Baroldi P, Ferrante F, Napoleone P, Meli A. Autoradiographic localization of octylonium bromide binding sites in the rat gastrointestinal tract. Arch Int Pharmacodyn Ther. 1991;311:5-19. [PubMed] |
| 13. | Baldi F, Longanesi A, Blasi A, Monello S, Cestari R, Missale G, Corazziari E, Badiali G, Pescatori M, Anastasio G. Clinical and functional evaluation of the efficacy of otilonium bromide: a multicenter study in Italy. Ital J Gastroenterol. 1991;23:60-63. [PubMed] |
| 14. | Battaglia G, Morselli-Labate AM, Camarri E, Francavilla A, De Marco F, Mastropaolo G, Naccarato R. Otilonium bromide in irritable bowel syndrome: a double-blind, placebo-controlled, 15-week study. Aliment Pharmacol Ther. 1998;12:1003-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Clavé P, Acalovschi M, Triantafillidis JK, Uspensky YP, Kalayci C, Shee V, Tack J. Randomised clinical trial: otilonium bromide improves frequency of abdominal pain, severity of distention and time to relapse in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2011;34:432-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 16. | Glende M, Morselli-Labate AM, Battaglia G, Evangelista S. Extended analysis of a double-blind, placebo-controlled, 15-week study with otilonium bromide in irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2002;14:1331-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Baldi F, Corinaldesi R, Ferrarini F, Balestra R, Brunetti G, Cassan M, Barbara L. Clinical and functional evaluation of octylonium bromide in the treatment of irritable bowel syndrome. A double-blind controlled trial. Clin Trials J. 1983;20:77-88. |
| 18. | Castiglione F, Daniele B, Mazzacca G. Therapeutic strategy for the irritable bowel syndrome. Ital J Gastroenterol. 1991;23:53-55. [PubMed] |
| 19. | D’Arienzo A, D’Agostino L. L’ottilonio bromuro nel trattamento della sindrome del colon irritabile. Rass Int Clin Ter. 1980;60:649-656. |
| 20. | Tack J, Fried M, Houghton LA, Spicak J, Fisher G. Systematic review: the efficacy of treatments for irritable bowel syndrome--a European perspective. Aliment Pharmacol Ther. 2006;24:183-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Musial F, Klosterhalfen S, Enck P. Placebo responses in patients with gastrointestinal disorders. World J Gastroenterol. 2007;13:3425-3429. [PubMed] |
| 22. | Evangelista S. Benefits from long-term treatment in irritable bowel syndrome. Gastroenterol Res Pract. 2012;2012:936960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |