Published online Sep 14, 2014. doi: 10.3748/wjg.v20.i34.12249
Revised: February 14, 2014
Accepted: April 30, 2014
Published online: September 14, 2014
Processing time: 329 Days and 23.9 Hours
AIM: To investigate Toll-like receptor (TLR) signaling regulators in microscopic and ulcerative colitis patients.
METHODS: Total RNA and microRNA were isolated from fresh frozen colonic biopsies of non-inflamed controls and patients with active or in-remission collagenous colitis (CC), lymphocytic colitis (LC), or ulcerative colitis (UC). We compared expressions of interleukin-1 receptor-associated kinase (IRAK)-2, IRAK-M, interleukin (IL)-37, microRNA (miR)-146a, miR-155, and miR-21 using quantitative real time reverse transcription polymerase chain reaction.
RESULTS: IRAK-M expression was increased in LC patients with active disease in histopathological remission (LC-HR; P = 0.02) and UC patients (P = 0.01), but no differences in IRAK-2 expression were detected compared to controls. miR-146a, -155 and -21 expressions were increased in LC-HR (P = 0.04, 0.07, and 0.004) and UC (P = 0.02, 0.04 and 0.03) patients. miR-146a and miR-21 expressions were significantly enhanced in UC patients compared to UC remission (UC-R; P = 0.01 and 0.04). Likewise, active CC patients showed significantly increased expression of miR-155 (P = 0.003) and miR-21 (P = 0.006). IL-37 expression was decreased in both CC (P = 0.03) and LC (P = 0.04) patients with a similar trend in UC patients but not statistically significant, whilst it was increased in UC-R patients compared to controls (P = 0.02) and active UC (P = 0.001).
CONCLUSION: The identification of differentially expressed miRNAs, IL-37, and IRAK-M suggests different pathophysiologic mechanisms in various disease stages in LC, CC, and UC.
Core tip: Epithelial destruction is observed in both microscopic and ulcerative colitis, likely resulting in enhanced contact between gut microbiota and Toll-like receptors (TLRs), which do not normally face the lumen. Insufficiently regulated TLR signaling may result in chronic inflammation. In this study, we analyzed several important regulatory molecules of TLR signaling: interleukin-1 receptor-associated kinase (IRAK)-2, IRAK-M, interleukin (IL)-37, microRNA (miR)-146a, miR-155, and miR-21. A possible association between the regulatory effects of IRAK-M and miR-146a was revealed in both diseases. This novel analysis of miR-155 and miR-21 in microscopic and ulcerative colitis revealed different expressions in active disease and remission.
- Citation: Günaltay S, Nyhlin N, Kumawat AK, Tysk C, Bohr J, Hultgren O, Hörnquist EH. Differential expression of interleukin-1/Toll-like receptor signaling regulators in microscopic and ulcerative colitis. World J Gastroenterol 2014; 20(34): 12249-12259
- URL: https://www.wjgnet.com/1007-9327/full/v20/i34/12249.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i34.12249
Microscopic colitis (MC), comprising collagenous colitis (CC) and lymphocytic colitis (LC), is characterized clinically by chronic watery diarrhea, abdominal pain, and weight loss. In contrast to ulcerative colitis (UC), the colonic mucosa in MC is endoscopically normal or near-normal. Diagnosis of MC thus relies on typical histopathological features observed upon microscopic examination[1-3]. Chronic inflammation is seen in both CC and LC, including lymphocytic infiltration of the epithelium and lamina propria, and damaged, flattened, and detached epithelial cells with loss of mucin[4]. In CC, a thickened subepithelial collagen layer is observed beneath the basal membrane, whereas the characteristic feature of LC is a more pronounced increase of intraepithelial lymphocytes[5]. Although the etiology remains unclear, barrier dysfunction, increased numbers of intraepithelial T lymphocytes, and/or immune responses to luminal agents can cause dysregulated immune responses and chronic inflammation[1].
The recognition of microbial products by Toll-like receptors (TLRs) is an early and important step in activation of the innate immune system, resulting in expression of co-stimulatory molecules and inflammatory cytokines such as type I interferons (IFNs) and chemokines[6]. However, inappropriate activation, duration, or magnitude of the TLR signaling may mediate the detrimental effects seen in chronic diseases[7]. Interleukin (IL)-1 receptor-associated kinase-2 (IRAK-2) has been suggested to be a critical molecule in sustaining late-phase TLR responses as its kinase activity is sustained for longer than that of IRAK-1 upon TLR stimulation[8]. Its role in inflammatory bowel diseases (IBD) is so far undiscovered. Upon lipopolysaccharide (LPS) challenge, expression of IRAK-M is increased through TLR-mediated NF-κB activation, but then feeds back negatively on TLR signaling by acting on other IRAKs[9-12]. In the dextran sodium sulfate (DSS) induced mouse model of colitis, IRAK-M-deficient mice had exacerbated colitis and significantly increased colonic expression of TNF, IL-1β, IL-6, and IL-17[13]. In a previous study, we observed increased mRNA expressions of IL-1β, IL-6, IL-17, IFN-γ and protein expression of TNF in MC patients, which led us to investigate the role of the innate immune regulator IRAK-M in MC[14]. In addition, about 19-25 nucleotide-containing non-coding microRNAs (miRNAs) whose expressions depend on NF-κB activity have been identified as post-transcriptional regulators of multiple TLR signaling components at the mRNA level[15]. Whereas miR-146a and miR-21 feed back negatively in TLR signaling, miR-155 is a pro-inflammatory miRNA[16]. Hence, continuous stimulation of TLRs by gut microbiota may affect microRNA levels and thus curb or induce chronic inflammation; this could be an important factor in MC immunopathology, as previously observed in IBD[17-21].
IL-37 (IL-1F7) is a newly described member of the IL-1 family. It is found in small amounts under steady-state conditions but is markedly increased during inflammatory challenges to prevent excessive inflammation[22-24]. Enhanced epithelial IL-37b protein expression has been observed in the inflamed mucosa of IBD patients[25]. Since the role of IL-37 is still not fully clarified in different inflammatory conditions, we investigated its expression levels in both MC and UC.
The inflammation in MC is more subtle than in UC and Crohn’s disease, and so this condition is likely an excellent “model” to study the role of basal differences in immune regulation and gain knowledge on basic pathophysiology in chronic gut inflammation. In the current study, we examined mRNA expression profiles of IRAK-2, IRAK-M, IL-37, miR-146a, miR-155, and miR-21 in colonic biopsies of MC and UC patients in active disease and in remission, compared to non-inflamed controls. Our findings suggest that alterations in expression of miR-146a, miR-155, and miR-21, IL-37, and IRAK-M reveal different immunopathogenic mechanisms in various disease stages of LC, CC, and UC.
Colonic mucosal biopsies were collected from a total of 12 CC, 13 LC, and 16 UC patients as well as 11 controls. Patients with a previous history of Crohn’s disease, gastrointestinal infection, ischemic colitis, colorectal cancer, or treatment with immunosuppressive drugs or antibiotics were excluded following endoscopic observations, small bowel imaging, and histopathological examinations. Biopsy specimens from MC patients and controls were taken from the hepatic flexure, whereas biopsies from UC patients were collected from endoscopically affected areas of the distal colon.
The MC patients underwent colonoscopy because of watery diarrhea; some of them also had abdominal pain and/or weight loss. In addition to the biopsy collection for this study, routine biopsy specimens were obtained from the proximal, transverse, and distal colon for confirmation of diagnosis through histopathological examination of paraffin embedded slides by an experienced gastropathologist. Histopathological criteria for CC were a diffusely distributed and thickened subepithelial collagen layer (≥ 10 μm), epithelial damage such as flattening and detachment, inflammation in the lamina propria with mainly mononuclear cells, and increased numbers of intraepithelial lymphocytes (IELs). Histopathological criteria for LC were, in addition to epithelial damage and inflammation in the lamina propria, ≥ 20 IELs per 100 surface epithelial cells but with a normal collagen layer.
Three patients with an established diagnosis of CC and six patients with LC no longer fulfilled the histopathological criteria for MC despite clinical symptoms of active disease. These patients were therefore categorized as clinically active but histopathologically in remission (CC-HR/LC-HR)[26] and were analyzed separately.
One of the CC patients had Sjögren’s syndrome. One CC and one LC patient had hypothyroid disease. Two patients with CC and two patients with LC were treated with budesonide at the time of colonoscopy, but one of the CC patients was on budesonide treatment only for the last three days before the investigation. The patients on budesonide treatment are indicated in the figures with circled symbols.
The diagnosis of UC relied on well-established diagnostic criteria[27]. Endoscopic activity was assessed at colonoscopy and graded according to the Mayo endoscopic subscore[28]. Based on this score, patients were categorized in two groups, with grade 0 representing endoscopic remission and grades 1-3 active disease. Six patients had active UC, and were treated with 5-aminosalicylic acid (5-ASA) at the time of colonoscopy. Ten patients, undergoing colonoscopy for colitis cancer surveillance, were asymptomatic and had a Mayo score of 0. Six of them were treated with 5-ASA, and the remaining 4 had no treatment at the time of colonoscopy. There were no patients with conversion between LC and UC.
Eleven control individuals underwent colonoscopy due to changes in bowel habits (n = 3), iron deficiency anemia (n = 3), rectal bleeding (n = 3), follow up after diverticulitis (n = 1), or colon cancer screening (n = 1). All colonoscopies were endoscopically normal except for occasional diverticula in the left colon, and routine biopsy specimens from the ascending, transverse, and distal colon revealed no pathological changes.
Biopsy specimens for this immunologic study were obtained with standard biopsy forceps from the proximal colon in both cases and controls, and were immediately immersed in RNAlater™ (Ambion, Life Technologies, Foster City, CA) and then stored at -80 °C until analysis.
Ethical approval: Patients and controls were informed of the study protocol before endoscopy, and gave their written consent to donate tissue samples for research purposes. The study was approved by the ethics committee of Örebro-Uppsala County (#2008/278).
Total RNA and miRNA isolation: Total RNA and miRNA were isolated with miRNeasy Kits (Qiagen, GmbH, Hilden, Germany) according to the manufacturer’s protocol and quantified using a NanoDrop ND 1000 spectrophotometer (Nano Drop Technologies, Inc, Wilmington, DE).
Reverse-transcription and quantitative real time reverse transcription polymerase chain reaction: All products used in the reverse transcription and quantitative real time reverse transcription polymerase chain reaction (qRT-PCRs) were ordered from Applied Biosystems, Life Technologies (Foster City, CA). cDNA transcription of 1 μg/μL of total RNA was performed with High-Capacity cDNA Reverse Transcription Kits according to the manufacturer’s protocol. For analysis of miRNA levels, 10 ng/μL of RNA was reverse transcribed using TaqMan microRNA Reverse Transcription Kits. The following primer-probe sets were used for analysis of gene expressions: IRAK-2 (Hs00176394_m1), IRAK-M (Hs00200502_m1), and IL-37 (Hs00367201_m1). Primer-probe sets used for human miRNAs were: miR-146a-5p (000468), miR-155-5p (002623), and miR-21-5p (00397). Normalization of qRT-PCR results was performed using the three housekeeping genes GAPDH (Hs99999905_m1), GUSB (Hs99999908_m1), and 18S (Hs99999901_s1); for miRNA normalization, RNU6B (001093), RNU44 (001094), and RNU48 (001006) were used. PCR amplification of miRNA and gene expressions was performed according to the manufacturers’ protocols. For gene expression, TaqMan Fast Universal Master Mix was used with the thermal cycling parameters suggested in the manufacturer’s protocol. For miRNA expression profiles, TaqMan 2X Universal Master Mix (No AmpErase UNG) and specific TaqMan probes for each miRNA were used to quantify miRNA expressions according to the manufacturer’s suggestions. The samples were run in the Applied Biosystems 7900HT Fast Real-Time PCR System (Life Technologies). Gene and miRNA expression were expressed relative to the average of the housekeeping genes and non-coding small RNAs, respectively. The comparative threshold cycle method was used to compare results between controls and patients[29].
Data values were compared using the non-parametric Mann-Whitney test with statistical significance set at P≤ 0.05 (GraphPad Prism 4, San Diego, CA). Statistical outliers were detected by the Tukey test and excluded from the statistical analysis; these values are marked as crosses (X) in the figures. The different patient groups including those with active disease (CC/LC/UC) and those in histopathological remission (CC-HR/LC-HR) or UC remission (UC-R), and the pooled active and histopathological remission CC and LC patients, respectively, were compared to non-inflamed control tissues. Patients with active disease or in (histological) remission within the same disease groups were also compared to each other.
We compared expressions of the TLR signaling regulators IRAK-2, IRAK-M, IL-37, and miR-146a, miR-155, and miR-21 in microscopic and ulcerative colitis patients. Characteristics of the patients are given in Table 1.
| Control | Active CC | CC-HR | Active LC | LC-HR | Active UC | UC-R | |
| Number of patients | 11 | 9 | 3 | 7 | 6 | 6 | 10 |
| Male/female | 6/5 | 1/8 | 0/3 | 0/7 | 0/6 | 6/0 | 8/2 |
| Mean age (yr) (range) | 58.6 (29-88) | 67.3 (54-84) | 55 (50-64) | 69.4 (49-86) | 61 (24-80) | 52 (33-70) | 59 (42 -79) |
| Mean duration of disease (yr) (range) | NA | 6.8 (0-17) | 5 (1-9) | 2.3 (0-7) | 1.2 (0-3) | 14.3 (0-53) | 29 (6-43) |
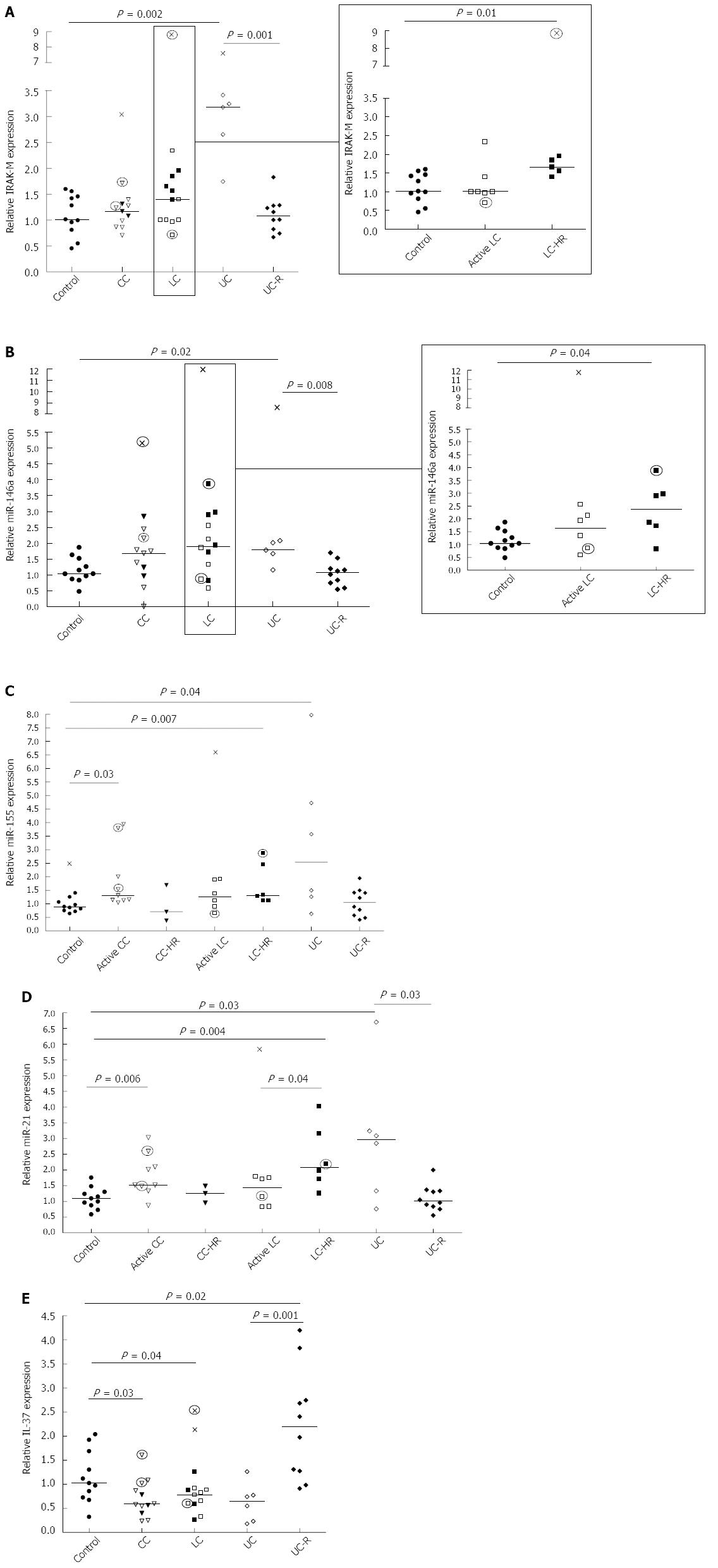
IRAK-M acts as a negative feedback regulator of TLR signaling via blocking of IRAK-1 and -4[9-11]. Significantly enhanced IRAK-M expression was observed in LC-HR patients compared to controls (P = 0.01; Figure 1A, insert), and these patients also showed a trend towards an increase compared to active LC (P = 0.07). In contrast, CC patients did not differ significantly from controls. A highly significant increase in IRAK-M expression was observed in the UC patients with active disease (P = 0.002; Figure 1A). There was a significantly reduced expression in UC-R patients compared to UC patients with active disease (P = 0.001; Figure 1A).We also investigated the expression of IRAK-2, involved in up regulation of NF-ĸB. There was a trend towards increased IRAK-2 expression in the active UC patients compared to controls (P = 0.05; data not shown) but no significant changes in either MC or UC-R patients compared to non-inflamed controls (data not shown). Likewise, there were no significant differences in IRAK-2 expression between CC patients and those in histopathological remissions (CC-HR) or between active UC and UC patients in remission.
The results of relative mRNA expression of IRAK-M are described in Figure 1A. Open symbols represent patients with active disease. Filled symbols represent patients with clinically active disease but histopathologically in remission, or UC patients in remission. Patients on budesonide treatment are indicated by circled symbols.
miR-146a has an important regulatory role in TLR signaling through inhibition of IRAKs at the mRNA level. Significantly increased expression of miR-146a was observed in LC-HR patients compared to non-inflamed controls (P = 0.04; Figure 1B, insert). In contrast, there were no significant changes in active LC, active CC, or CC-HR patients compared to controls. Moreover, significantly increased expression was detected in UC patients with active disease compared to controls (P = 0.02) and to UC-R patients (P = 0.008; Figure 1B). Again, in contrast to UC, there were no significant differences between CC or LC patients with active disease and those in histopathological remission.
The results of relative expression of miR146a are described in Figure 1B. Each symbol represents one patient. Open symbols represent patients with active disease, whereas filled symbols represent patients with clinically active disease but histopathologically in remission, or UC patients in remission. Patients on budesonide treatment are indicated by circled symbols.
Whereas miR-146a has a regulatory role in TLR signaling, miR-155 increases the inflammatory response. Significantly increased expression of miR-155 was observed in active CC and LC-HR patients compared to non-inflamed controls (P = 0.003 and P = 0.007, respectively), but not in CC-HR and LC patients (Figure 1C). Similarly, miR-155 expression was significantly increased in UC patients compared to controls (P = 0.04; Figure 1C). There was also a trend towards increased miR-155 expression in active UC patients compared to UC-R patients (P = 0.06).
The results of relative expression of miR155 are described in Figure 1C. Each symbol represents one patient. Open symbols represent patients with active disease. Filled symbols represent patients with clinically active disease but histopathologically in remission, or UC patients in remission. Patients on budesonide treatment are indicated by circled symbols.
The anti-inflammatory miR-21 is upregulated in order to decrease miR-155 expression which in turn dampens TLR signaling[30]. Expression of miR-21 was significantly enhanced in both CC and LC-HR patients (P = 0.006 and P = 0.004, respectively; Figure 1D). A significantly lower expression was detected in active LC patients compared to LC-HR patients (P = 0.04; Figure 1D). In contrast, there were no significant differences between CC patients with active disease compared to those in histopathological remission (Figure 1D). Similarly to the other parameters investigated, UC patients also showed significantly increased miR-21 expression compared to non-inflamed controls and UC-R patients (P = 0.03 and P = 0.03, respectively; Figure 1D).
The results of relative expression of miR-21 are described in Figure 1D. Each symbol represents one patient. Open symbols represent patients with active disease. Filled symbols represent patients with clinically active disease but histopathologically in remission, or UC patients in remission. Patients on budesonide treatment are indicated by circled symbols.
Finally, we investigated the newly described IL-1 family cytokine IL-37, previously demonstrated to inhibit the production of pro-inflammatory mediators without affecting the production of anti-inflammatory mediators[23]. In both CC and LC patients, there was a significant decrease in IL-37 expression compared to non-inflamed controls (P = 0.03 and P = 0.04, respectively; Figure 1E). There were no significant differences between CC and LC patients with active disease compared to those in histopathological remission. Similarly, active UC patients showed a trend towards decreased IL-37 expression (P = 0.06), whereas UC remission patients demonstrated significantly increased IL-37 expression (P = 0.02; Figure 1E) compared to controls. Comparison of active and in remission UC patients also revealed a highly significant increased expression in UC patients in remission (P = 0.001; Figure 1E).
The results of relative mRNA expression of IL-37are described in Figure 1E. Each symbol represents one patient. Open symbols represent patients with active disease. Filled symbols represent patients with clinically active disease but histopathologically in remission, or UC patients in remission. Patients on budesonide treatment are indicated by circled symbols.
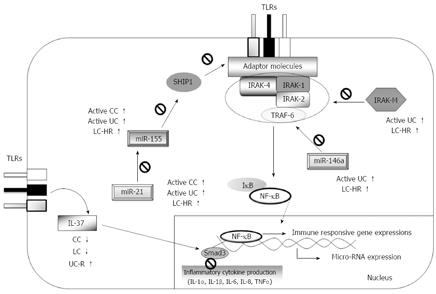
Although clinical and epidemiological data on MC continue to emerge[1-3], the pathophysiology of MC is still unclear, and the search for triggering factors and underlying dysfunctions in the immune system is still at an early stage. In this study, we explored expression patterns of molecules involved in TLR signaling pathways as potentially important regulators of inflammatory responses. Expressions of IRAK-2, IRAK-M, and IL-37, and three microRNAs (miR-146a, -155 and -21) in MC and UC patients were compared to that in controls, and several important differences were found between CC and LC patients, as well as between MC and UC patients in different stages of the disease (summarized in Figure 2). Previous studies have frequently suggested that MC shows associations with other autoimmune diseases and with non-steroidal anti-inflammatory drugs[3]. The present study included four patients on budesonide treatment. Overall, we did not detect any significant correlations between the TLR signaling regulators investigated and other autoimmune diseases, nor did we detect any correlations between these regulators and current medications including budesonide. Nevertheless, we cannot exclude the possibility that low numbers of patients may have disguised possible effects of the drugs on the parameters investigated.
IRAK-2 is suggested to be an important molecule in sustaining TLR responses[31]. Our finding of upregulated IRAK-2 expression in UC patients with active disease but not those in remission suggest that it is a marker for sustained colonic inflammation. Lack of significant changes in IRAK-2 expression in MC patients is probably due to the subtler inflammation in MC.
IRAK-M has varying roles in immunopathology, depending on the disease context. In chronic inflammation, IRAK-M expression is likely desirable in order to limit excessive immune responses, though it may prevent proper innate immune clearance of pathogens. Moreover, a possible impaired IRAK-M production has been suggested as a risk factor for UC patients carrying three Crohn’s disease-associated mutations in the caspase recruitment domain 15 (CARD15) compared to patients without mutations, but this has not been demonstrated experimentally[32]. IRAK-M expression is also induced in response to the activation of TLRs, and regulates the inflammatory response by inhibiting IRAK-1 and IRAK-4 dissociations[12], whereas miR-146a regulates mRNA expression of IRAK-1, IRAK-2, and TNF receptor-associated factor-6 (TRAF6) in the TLR signaling pathway[33], thereby inhibiting downstream signaling. By negatively regulating TLR signaling, IRAK-M and miR-146a inhibit production of pro-inflammatory mediators and contribute to the induction of endotoxin tolerance[12,16]. We here report for the first time on significantly increased IRAK-M and miR-146a expressions in UC patients with active disease, whereas no increased expression was observed in UC patients in remission. This might be due to enhanced exposure of gut microbiota through ulcerations[34], which is not observed in MC; if so, healing of the mucosa is likely to decrease IRAK-M expression to levels found in MC patients. IRAK-M expression levels in UC or MC patients have not previously been reported. In addition a previous study was unable to detect significant changes in miR-146a profiles in inflamed vs non-inflamed colon tissues[20]. However, that study relied on comparison between tissues from the same patient, whereas we investigated patients with active disease or in remission. The mechanisms behind regulation of expression of IRAK-M and miR-146a likely have common denominators, as increased expressions were observed in both active UC and LC-HR patients, but decreased expressions were noted in UC remission and active LC patients compared to controls. The finding of reciprocal expression levels in patients with active UC or LC compared to patients in remission is intriguing, and at present unexplained. Nevertheless, it is important to note that although the LC patients did not fulfill the histological criteria for LC, they still had clinically active disease, whereas the UC patients in remission had no clinical symptoms. The reason for the lack of significant differences in IRAK-M and miR-146a expressions in CC patients compared to controls remains unknown, but it is noteworthy that the median values in CC patients were higher than those in controls, although this difference did not reach statistical significance. We have previously reported on increased IRAK-M mRNA expression in parallel to increased severity of colitis in the DSS-induced mouse model of colitis[13]. Ulcerations similar to those in human IBD are seen in this model, likely due to cytotoxic effects of DSS on the epithelium.
miR-155 is regarded as a pro-inflammatory miRNA, and hence could contribute to disease activity. It enhances the TLR signaling pathway by repression of mRNAs encoding the TLR signaling inhibitors SHIP1 and SOCS1[35,36], whereas miR-21 leads to an anti-inflammatory response in TLR signaling[30]. Our finding of increased miR-155 expression in the active UC patients compared to controls supports the previous findings[21]. We also observed decreased expression in UC remission patients.
We found increased expression of miR-21 in UC patients with active disease compared to healthy controls, similarly to Wu et al[17]. We also found increased miR-21 expression in patients with active CC, whereas Wu et al[17] were unable to detect differences in MC patients. This was most likely due to the low number of patients in their study, which included only 2 patients with CC and 1 with LC. In addition, we detected a significant increase in miR-21 expression in LC-HR patients compared to controls.
An anti-inflammatory mechanism is usually initiated to decrease a pro-inflammatory response and maintain homeostasis, and therefore one might expect to see both pro-inflammatory and anti-inflammatory responses being increased simultaneously. Since active CC, LC-HR, and active UC patients had increased expressions of both miR-155 and miR-21 in our study, it is possible that miR-21 expression is increased in order to decrease miR-155 expression through IL-10, which in turn dampens TLR signaling[30]. However, we were unable to observe changes in protein levels of IL-10[14]. Thus, our recorded up regulated miR-155 and miR-21 expressions may have stemmed from other cell types, such as activated T cells and/or dendritic cells[37].
Overall, the increased expressions of IRAK-M, miR-146a, miR-155 and miR-21 in LC-HR but not in active LC patients might indicate a role in the mucosal healing; but it could also indicate that these changes may not be sufficient to cure clinical symptoms. This suggests different mechanism(s) for complete recovery of the symptoms, compared to CC. In addition, differences in IRAK-M, miR-146a, miR-155, and miR-21 expressions in different stages of CC and LC suggest different types of immunopathogenesis between the two types of MC.
IL-37 is a newly described member of the IL-1 family. It is suggested to be a fundamental inhibitor of innate immunity that has so far only been identified in humans[23]. Here, we report for the first time a decreased IL-37 expression in a chronic inflammatory illness. The increased IL-37 mRNA levels in UC remission patients compared to patients with active UC indicate that IL-37 may curb the inflammation and thereby contribute to remission. Conversely, the low IL-37 expression in MC and UC may be one reason for the chronicity of the colonic inflammation in these patients.
Studies in mice expressing human IL-37 have shown ameliorated DSS-induced colitis and LPS-induced shock[23,38]. Recently, increased levels of IL-37b protein were detected by immunohistochemistry in tissues obtained from inflamed lesions of UC patients[25]. There are however several differences between this study and ours. Firstly, in the Imaeda study tissues were collected during surgery due to resistance to medication or other complications, and the patients were on corticosteroid treatment. Secondly, inflamed and non-inflamed tissues from the same patient were compared. Thirdly, whereas we investigated mRNA expression of IL-37, the Imaeda study investigated protein levels semi-quantitatively.
Taken together, our results demonstrate different expression patterns of IRAK-M, IL-37, and microRNAs -146a, -155 and -21 in CC, LC, and UC patients in either active disease or in remission. A potential limitation of this study is the small cohort of MC patients. There are several difficulties in recruiting MC patients: MC can only be diagnosed by histopathological examination, and patients with a confirmed diagnosis do not usually undergo repeated colonoscopy. It will therefore take time to accumulate appropriate patients. Nevertheless, to be able to cure the disease rather than simply relieve the symptoms, studies like this one are necessary to understand the immunopathogenesis despite the low number of patients available. Our study shows that CC and LC have different immunopathogenic behaviors in different disease stages, which further supports the hypothesis that CC and LC are two different entities[26,39].
The level of mucosal destruction in UC and MC affects the magnitude of innate immune responses both in active and in remission stages; and the subtler inflammation in MC compared to UC is reflected in relatively smaller changes in the immune parameters investigated in the present study. In addition to revealing differentially expressed regulators of TLR signaling in the colonic mucosa of MC patients, this study further supports the importance of MC as a “model” to study the role of changes in immune regulation and basic pathophysiology of IBD, where MC patients may reveal important immunoregulatory mechanisms.
We are very grateful to the nursing staff (especially Ulla Vidmark) and the gastroenterologists at the Division of Gastroenterology, Örebro University Hospital, for their excellent support in collecting the mucosal biopsies.
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the intestine that consists of ulcerative colitis (UC) and Crohn’s disease, as well as a subtler type of disease; microscopic colitis (MC). MC, comprising collagenous colitis (CC) and lymphocytic colitis (LC), is characterized clinically by chronic watery diarrhea, abdominal pain, and weight loss. In contrast to UC, the colonic mucosa in MC is endoscopically normal or near-normal. MC can influence any age group but it commonly affects middle-aged and elderly individuals with a female dominance. Although clinical and epidemiological data on MC continue to emerge, the pathophysiology of MC is still unclear, and the search for triggering factors and underlying dysfunctions in the immune system is still at an early stage. Currently, there is no cure for MC; the patients are given different medications only to relieve their symptoms. Therefore, it is important to find new avenues for treatment and eventually cure the disease.
Previous studies have shown that upon stimulation of Toll-like receptors (TLRs) with bacterial products, interleukin-1 receptor-associated kinase (IRAK)-2, IRAK-M, interleukin-37 (IL-37), and microRNAs (miR-146a, -155, and -21) become upregulated. IRAK-2 has been suggested to be a critical molecule in sustaining late-phase TLR responses. IRAK-M, miR-146a, and miR-21 inhibit production of pro-inflammatory mediators by negatively regulating TLR signaling. miR-155 is regarded as a pro-inflammatory microRNA and hence could contribute to disease activity. Increased miR-155 and miR-21 expressions have been shown in UC patients with active disease; however, alteration in IRAK-2, IRAK-M or miR-146a expression has not been observed in IBD. IL-37 is suggested to be a fundamental inhibitor of innate immunity, which has hitherto been shown to be upregulated in different human chronic inflammations.
In this study, for the first time, the authors have shown increased IRAK-M expression in MC and UC patients. Moreover, miR-146a, miR-155, and miR-21 expressions were increased in MC and UC patients. Remarkably, miR-146a and miR-21 expressions were enhanced in UC patients with active disease compared to UC patients in remission (UC-R). Decreased IL-37 expression in MC, as well as in UC patients with a similar trend was detected for the first time in human chronic inflammatory diseases. On the other hand, increased IL-37 expression in UC-R patients compared to controls and UC patients with active disease was observed, which may reveal an important role of IL-37 in remission stage of UC immunopathology.
In this study, the authors analyzed several important regulatory molecules of TLR signaling: IRAK-2, IRAK-M, IL-37, miR-146a, miR-155, and miR-21. A possible association between the regulatory effects of IRAK-M and miR-146a was indicated in both MC and UC immunopathology. The identification of differentially expressed miRNAs, IL-37, and IRAK-M suggests different pathophysiologic mechanisms in various disease stages in LC, CC, and UC. Moreover, the level of mucosal destruction in UC and MC affects the magnitude of innate immune responses both in active and remission stages. The subtler inflammation in MC compared to UC is reflected in relatively smaller changes of the immune parameters investigated in the present study. This study further supports the importance of MC as a “model” to study the role of changes in immune regulation and basic pathophysiology of IBD.
In this study, certain MC patients with an established diagnosis of CC and LC did not fulfill the histopathological criteria for MC despite having clinical symptoms of active disease, such as watery diarrhea, abdominal pain and/or weight loss. They were therefore categorized as clinically active but histopathologically in remission (CC-HR/LC-HR) and analyzed separately.
The authors compared the expressions of IL-1/TLR signaling regulators, IRAK-2, IRAK-M, IL-37, and microRNAs (miR-146, -155, and -21) in colonic biopsies obtained from patients with MC and UC. The identification of differentially expressed IRAK-M, IL-37, and miRNAs, suggests that collagenous colitis and lymphocytic colitis are different clinical entities, which is an interesting and very original finding. This study sheds a new light on the pathophysiology of MC.
P- Reviewer: Day AS, Homan M, Konturek PC, Tanaka T, Torres MI S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Tysk C, Wickbom A, Nyhlin N, Eriksson S, Bohr J. Recent advances in diagnosis and treatment of microscopic colitis. Ann Gastroenterol. 2011;24:253-262. [PubMed] |
| 2. | Pardi DS, Kelly CP. Microscopic colitis. Gastroenterology. 2011;140:1155-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Münch A, Aust D, Bohr J, Bonderup O, Fernández Bañares F, Hjortswang H, Madisch A, Munck LK, Ström M, Tysk C. Microscopic colitis: Current status, present and future challenges: statements of the European Microscopic Colitis Group. J Crohns Colitis. 2012;6:932-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 4. | Mahajan D, Goldblum JR, Xiao SY, Shen B, Liu X. Lymphocytic colitis and collagenous colitis: a review of clinicopathologic features and immunologic abnormalities. Adv Anat Pathol. 2012;19:28-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Warren BF, Edwards CM, Travis SP. ‘Microscopic colitis’: classification and terminology. Histopathology. 2002;40:374-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1380] [Cited by in RCA: 1545] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 7. | Drexler SK, Foxwell BM. The role of toll-like receptors in chronic inflammation. Int J Biochem Cell Biol. 2010;42:506-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 8. | Kawagoe T, Sato S, Matsushita K, Kato H, Matsui K, Kumagai Y, Saitoh T, Kawai T, Takeuchi O, Akira S. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat Immunol. 2008;9:684-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 334] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 9. | Oshima N, Ishihara S, Rumi MA, Aziz MM, Mishima Y, Kadota C, Moriyama I, Ishimura N, Amano Y, Kinoshita Y. A20 is an early responding negative regulator of Toll-like receptor 5 signalling in intestinal epithelial cells during inflammation. Clin Exp Immunol. 2010;159:185-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Wesche H, Gao X, Li X, Kirschning CJ, Stark GR, Cao Z. IRAK-M is a novel member of the Pelle/interleukin-1 receptor-associated kinase (IRAK) family. J Biol Chem. 1999;274:19403-19410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 326] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 11. | Balaci L, Spada MC, Olla N, Sole G, Loddo L, Anedda F, Naitza S, Zuncheddu MA, Maschio A, Altea D. IRAK-M is involved in the pathogenesis of early-onset persistent asthma. Am J Hum Genet. 2007;80:1103-1114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Kobayashi K, Hernandez LD, Galán JE, Janeway CA, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1055] [Cited by in RCA: 1096] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 13. | Berglund M, Melgar S, Kobayashi KS, Flavell RA, Hörnquist EH, Hultgren OH. IL-1 receptor-associated kinase M downregulates DSS-induced colitis. Inflamm Bowel Dis. 2010;16:1778-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Kumawat AK, Strid H, Tysk C, Bohr J, Hörnquist EH. Microscopic colitis patients demonstrate a mixed Th17/Tc17 and Th1/Tc1 mucosal cytokine profile. Mol Immunol. 2013;55:355-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | O’Neill LA, Sheedy FJ, McCoy CE. MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat Rev Immunol. 2011;11:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 707] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 16. | O’Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Annu Rev Immunol. 2012;30:295-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 712] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 17. | Wu F, Zikusoka M, Trindade A, Dassopoulos T, Harris ML, Bayless TM, Brant SR, Chakravarti S, Kwon JH. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology. 2008;135:1624-1635.e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 404] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 18. | Wu F, Zhang S, Dassopoulos T, Harris ML, Bayless TM, Meltzer SJ, Brant SR, Kwon JH. Identification of microRNAs associated with ileal and colonic Crohn’s disease. Inflamm Bowel Dis. 2010;16:1729-1738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 19. | Wu F, Guo NJ, Tian H, Marohn M, Gearhart S, Bayless TM, Brant SR, Kwon JH. Peripheral blood microRNAs distinguish active ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2011;17:241-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 20. | Fasseu M, Tréton X, Guichard C, Pedruzzi E, Cazals-Hatem D, Richard C, Aparicio T, Daniel F, Soulé JC, Moreau R. Identification of restricted subsets of mature microRNA abnormally expressed in inactive colonic mucosa of patients with inflammatory bowel disease. PLoS One. 2010;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 21. | Takagi T, Naito Y, Mizushima K, Hirata I, Yagi N, Tomatsuri N, Ando T, Oyamada Y, Isozaki Y, Hongo H. Increased expression of microRNA in the inflamed colonic mucosa of patients with active ulcerative colitis. J Gastroenterol Hepatol. 2010;25 Suppl 1:S129-S133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 22. | Bufler P, Gamboni-Robertson F, Azam T, Kim SH, Dinarello CA. Interleukin-1 homologues IL-1F7b and IL-18 contain functional mRNA instability elements within the coding region responsive to lipopolysaccharide. Biochem J. 2004;381:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11:1014-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 704] [Cited by in RCA: 683] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 24. | Boraschi D, Lucchesi D, Hainzl S, Leitner M, Maier E, Mangelberger D, Oostingh GJ, Pfaller T, Pixner C, Posselt G. IL-37: a new anti-inflammatory cytokine of the IL-1 family. Eur Cytokine Netw. 2011;22:127-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 286] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 25. | Imaeda H, Takahashi K, Fujimoto T, Kasumi E, Ban H, Bamba S, Sonoda H, Shimizu T, Fujiyama Y, Andoh A. Epithelial expression of interleukin-37b in inflammatory bowel disease. Clin Exp Immunol. 2013;172:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 26. | Kumawat AK, Strid H, Elgbratt K, Tysk C, Bohr J, Hultgren Hörnquist E. Microscopic colitis patients have increased proportions of Ki67(+) proliferating and CD45RO(+) active/memory CD8(+) and CD4(+)8(+) mucosal T cells. J Crohns Colitis. 2013;7:694-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Dignass A, Eliakim R, Magro F, Maaser C, Chowers Y, Geboes K, Mantzaris G, Reinisch W, Colombel JF, Vermeire S. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 641] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 28. | Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1958] [Cited by in RCA: 2252] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 29. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149116] [Cited by in RCA: 133995] [Article Influence: 5583.1] [Reference Citation Analysis (1)] |
| 30. | Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O’Leary JJ, Ruan Q, Johnson DS, Chen Y, O’Neill LA. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 787] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 31. | Keating SE, Maloney GM, Moran EM, Bowie AG. IRAK-2 participates in multiple toll-like receptor signaling pathways to NFkappaB via activation of TRAF6 ubiquitination. J Biol Chem. 2007;282:33435-33443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 168] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 32. | Weersma RK, Oostenbrug LE, Nolte IM, Van Der Steege G, Oosterom E, Van Dullemen HM, Kleibeuker JH, Dijkstra G. Association of interleukin-1 receptor-associated kinase M (IRAK-M) and inflammatory bowel diseases. Scand J Gastroenterol. 2007;42:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481-12486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3121] [Cited by in RCA: 3550] [Article Influence: 186.8] [Reference Citation Analysis (0)] |
| 34. | Gitter AH, Wullstein F, Fromm M, Schulzke JD. Epithelial barrier defects in ulcerative colitis: characterization and quantification by electrophysiological imaging. Gastroenterology. 2001;121:1320-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 186] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Wang P, Hou J, Lin L, Wang C, Liu X, Li D, Ma F, Wang Z, Cao X. Inducible microRNA-155 feedback promotes type I IFN signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1. J Immunol. 2010;185:6226-6233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 357] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 36. | O’Connell RM, Chaudhuri AA, Rao DS, Baltimore D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc Natl Acad Sci USA. 2009;106:7113-7118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 676] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 37. | Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608-611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1554] [Cited by in RCA: 1552] [Article Influence: 86.2] [Reference Citation Analysis (0)] |
| 38. | McNamee EN, Masterson JC, Jedlicka P, McManus M, Grenz A, Collins CB, Nold MF, Nold-Petry C, Bufler P, Dinarello CA. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci USA. 2011;108:16711-16716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 276] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 39. | Rasmussen MA, Munck LK. Systematic review: are lymphocytic colitis and collagenous colitis two subtypes of the same disease - microscopic colitis? Aliment Pharmacol Ther. 2012;36:79-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |