Published online Sep 14, 2014. doi: 10.3748/wjg.v20.i34.12226
Revised: February 10, 2014
Accepted: May 28, 2014
Published online: September 14, 2014
Processing time: 317 Days and 8.9 Hours
AIM: To evaluate the ability of the McGill Brisbane Symptom Score (MBSS) to predict survival in resectable pancreatic head adenocarcinoma (PHA) patients.
METHODS: All PHA patients (n = 83) undergoing pancreaticoduodenectomy at the McGill University Health Center, Quebec between 1/2001-1/2010 were evaluated. Data related to patient and cancer characteristics, MBSS variables, and treatment were collected; univariable and multivariable survival analyses were performed. We obtained complete follow-up until February 2011 in all patients through the database of the provincial health insurance plan of Quebec. The unique health insurance numbers of these patients were used to retrieve information from this database which captures all billable clinical encounters, and ensures 100% actual survival data.
RESULTS: Median survival was 23 mo overall: 45 mo for patients with low MBSS, 17 mo for high MBSS (P = 0.005). At twelve months survival was 83.3% (95%CI: 66.6-92.1) vs 58.1% (95%CI: 42.1-71.2) in those with low vs high MBSS, and24 mo survival was 63.8% (95%CI: 45.9-77.1) and 34.0% (95%CI: 20.2-48.2) respectively. In the multivariate Cox model (stratified by chemotherapy), after addition of clinically meaningful covariates, MBSS was the variable with the strongest association with survival (HR = 2.63; P = 0.001). Adjuvant chemotherapy interacted with MBSS category such that only high MBSS patients accrued a benefit. In univariate analysis we found a lower mortality in high MBSS but not low MBSS patients receiving adjuvant chemotherapy. This interaction variable, on Cox model, resulted in an adjusted mortality HR for the high MBSS (compared to low MBSS) of 4.14 (95%CI: 1.48-11.64) without chemotherapy and 2.11 (95%CI: 1.06-4.17) with chemotherapy.
CONCLUSION: The MBSS is a simple prognostic tool for resectable PHA. Preoperative categorization of patients according to the MBSS allows effective stratification of patients to guide therapy.
Core tip: The McGill Brisbane Symptom Score (MBSS) was described and validated as a score to predict survival in patients with unresectable pancreatic adenocarcinoma. In this paper we validate it in a cohort of patients with resectable pancreatic head adenocarcinoma. It is a simple yet powerful preoperative tool that can potentially guide therapy and stratify patients for neoadjuvant or adjuvant chemotherapy trials. We also found in this study that adjuvant chemotherapy seems to be effective only in patients in the high MBSS group but adds no additional survival benefit in patients in the low MBSS group. The MBSS seems to provide a better discrimination in survival than any conventional preoperative method.
- Citation: Jamal MH, Doi SAR, Moser AJ, Dumitra S, Khalil JA, Simoneau E, Chaudhury P, Onitilo AA, Metrakos P, Barkun JS. McGill Brisbane Symptom Score for patients with resectable pancreatic head adenocarcinoma. World J Gastroenterol 2014; 20(34): 12226-12232
- URL: https://www.wjgnet.com/1007-9327/full/v20/i34/12226.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i34.12226
In spite of significant progress in treating many gastrointestinal cancers, pancreatic adenocarcinoma remains a particularly lethal disease[1]. Resectable patients are a minority, and the median survival of this selected, “favorable” group remains poor[2-9]. This clinical reality persists in spite of numerous attempts at adjuvant, and more recently neoadjuvant therapy[10]. One reason may be the significant heterogeneity of pancreatic cancer inherent at the genetic level[11]. However, ill-documented heterogeneity can also be seen in the clinical presentations, at the time of the evaluation of patients who may undergo pancreaticoduodenectomy[12]. To our knowledge, beyond commonly used imaging results and CA19.9, there is no published preoperative scheme which can predict survival, and thus allow for a stratification of possible treatment strategies. We have reported on the McGill Brisbane Symptom Score (MBSS), which is a fully clinical score shown to be an excellent predictor of survival in patients with unresectable pancreatic cancer[13]. It is based on the preoperative presence of weight loss, jaundice, abdominal pain, and smoking (Table 1). The association of this score with survival in unresectable patients was found to be greater than that of conventional imaging studies and was validated in two independent cohorts of unresectable patients[13]. The goal of this paper is to evaluate the ability of the MBSS to predict survival in patients with resectable pancreatic head adenocarcinoma (PHA) undergoing pancreaticoduodenectomy.
| Symptom | Points |
| Weight loss greater than 10% | 8 |
| Pain | 5 |
| Jaundice | 4 |
| Smoker | 4 |
| Total possible | 21 |
We evaluated a cohort of patients with resectable PHA who underwent a pancreaticoduodenectomy at the McGill University Health Center (MUHC) from January-2001 to January-2010 (n = 83). We obtained complete follow-up until February 2011 in all patients through the database of the provincial health insurance plan of Quebec. The unique health insurance numbers of these patients were used to retrieve information from the this database which captures all billable clinical encounters, and ensures 100% actual survival data.
The preoperative evaluation of all patients included routine preoperative lab tests and CA19-9 tumor marker measurement in patients after 2006. Imaging consisted of computed tomography scans with a triphasic pancreas protocol. Magnetic resonance imaging (MRI) was only obtained in selected cases if preoperative anatomical evaluation was deemed equivocal on CT scan. Preoperative endoscopic retrograde cholangiopancreatography (ERCP) with a view to biliary stenting was performed in patients who were jaundiced, because of the estimated time lag between diagnosis and scheduled surgery. Positron emission tomography was used in only a handful of patients. Endobiliary stent insertion was performed at ERCP by an experienced endoscopist where appropriate. A plastic stent was usually inserted although a self-expandable metal stent may have been used if the resectability of the patient had not yet been determined. All patients were seen by a clinical nutritionist, a hepatobiliary surgeon and a medical oncologist. The nutritional consultation provided valuable information in determining the amount of weight loss preoperatively. All patients were discussed at a multidisciplinary tumor board.
All patients underwent a pancreaticoduodenectomy with a curative intent after having been found to have a lesion which was classified as localized and resectable[12]. We excluded patients who were taken to the operating room with an intent to resect and had findings of unresectable pancreatic adenocarcinoma at laparotomy, either due to local invasion of major arterial vessels (Celiac artery or SMA) or distant metastases. In all others, a pancreaticoduodenectomy was performed with gastric transection just proximal to the pylorus[14]. Some patients had a pancreaticojejunal anastomosis, while others had a pancreaticogastrostomy according to surgeon preference. The surgical team consisted in all cases of an experienced hepatobiliary surgeon and a fellow or a chief resident. Perioperative management included the temporary use of pancreatic drainage[15]; perioperative prophylactic octreotide administration was not used.
Serial slicing of the entire pancreatic head specimen was performed in a single axial plane according to the guidelines of the Royal College of Pathologists and the Leeds Pathology Protocol[16,17]. Thus, large slices were obtained, allowing a precise study of each inked margin in increments of 0.5 mm from 0 mm to 2.0 mm. Margin involvement (R1) was defined for the 0-mm margin if tumour cells were present at the inked margin; R1 was also defined for each margin width if tumour cells were present within the margin, independently of the mode of tumour spread. The resection was considered as curative (R0) if no tumour cells were identified in the peripancreatic fat or at any of the resection margins (bile duct, pancreatic neck and uncinate resection margins).
The pathological protocol also included the maximal transverse diameter of the tumour, the tumour-node-metastasis (TNM) classification according to the 6th Ed of the AJCC staging manual classification, the grade of differentiation, the presence or absence of perineural, lymphatic and/or vascular spread, and the number of lymph nodes retrieved from the specimen. The presence and grading of pre-neoplastic lesions (PIN) was also recorded.
The postoperative treatment includes adjuvant therapy as per protocol in the form of gemcitabine for 6 mo for the study period. Although there is an intent to administer chemotherapy to all patients, the quantity and length of treatment are tailored according to patient tolerance. Postoperative follow-up abided by institutional protocol which included 3-monthly CT scan imaging and CA-19-9 (after 2006) for the first two years. Postoperative follow-up was performed by both oncologists and surgeons.
Data on operative technique and findings, endoscopic procedures, preoperative symptoms, and radiological investigations were collected using the MUHC prospective hepatobiliary tumor database and by chart review (MUHC). Admission notes, attending staff letters, and nutrition consultations were used to determine the preoperative symptoms. These included jaundice (clinical, confirmed by bilirubin > 50 mmol/L), weight loss > 10% from baseline actual weight, persistent abdominal or back pain (as reported by the patient), onset of diabetes mellitus within a year of diagnosis, and a history of cigarette smoking for more than 5 years within 5 years of the current diagnosis of cancer. Note that determination of > 10% weight loss was mostly performed from preoperative nutritional assessments because of their greater accuracy. Table 1 illustrates how these symptoms are used to calculate the value of the MBSS[13].
Univariate analyses were conducted to describe the distributions of baseline variables. Survival curves, median survival, and estimated survival estimates at fixed time points were based on the Kaplan-Meier method. In patients surviving to the end of the study, the date of last contact was used to estimate survival and censored via the Kaplan Meier method. Survival was calculated from the date of first imaging diagnosis (the date of the first radiological evidence of PA). Additional univariate analyses were carried out to determine probabilities of survival for the MBSS stratified by chemotherapy. Finally, Cox’s model was used to evaluate various combinations and interactions of prognostic variables (in addition to MBSS) in a multivariate manner. The additional variables included in the analyses were five variables that are known to impact on survival (age, gender, margin status, size of tumor, and chemotherapy)[18]. Graphical assessment of proportional hazards based on Schoenfeld residuals or -ln[-ln(survival)] vs ln(time) plots showed no important departure from proportionality (no association with time) for any of the other variables included in the model except for chemotherapy; this was dealt with via an interaction term. Significance statements refer to P values of two-tailed tests that were < 0.05. We used Stata version 11 (StataCorp LP, College Station, TX, United States) for all statistical analyses.
Patient and tumor characteristics are shown in Table 2. Median patient age was 65 years with a slight male predominance; the median radiological preoperative tumor size was 3.1 cm. A majority of patients presented with jaundice (79%) followed by abdominal or back pain (57%) and weight loss of more than 10% (42%). Only a third of the subjects were smokers as defined in the methods. MBSS scores across all patients (range 0-21) were stratified into two categories with a score of > 9 as a cut off (Table 1). This grouped patients according to low and high MBSS. In the low MBSS group, median scores were 5 (IQR: 4-9; range: 0-9), and in the high MBSS group median scores were 13 (IQR: 12-17; range: 12-21). A majority (67%) of patients had R0 resections, 15% had a portal vein resection and the median follow-up was 18.5 mo. There were three missing survival times, one in the low and two in the high MBSS categories. One subject in the low MBSS category died earlier than 1 mo after surgery.
| Variable | Median, (range) (n = 83) |
| Age, yr | 65 (23– 88) |
| Gender | |
| Men | 39 (53) |
| Women | 44 (47) |
| Tumor size, cm | 3.1 (0.5-10.8) |
| Missing | n = 4 |
| Presenting symptoms | |
| Weight loss > 10% | 40 (48) |
| Smoking | 21 (25) |
| Pain | 47 (57) |
| Jaundice | 65 (78) |
| Margin status | |
| R0 | 56 (67) |
| R1 | 22 (27) |
| R2 | 5 (6) |
| 1 yr overall survival | |
| R0 | 72.2% |
| R1/2 | 64.0% |
| Lymph Node Status | |
| Positive | 30 (36) |
| Negative | 23 (29) |
| Missing | 30 (35) |
| Average lymph node ratio | 16.0% |
| Portal vein resection | 13 (15) |
| Median follow-up in months (IQR) | 18.5 (11-48.5) |
| Median survival | 23 mo |
| 5 yr survival | 29.4% |
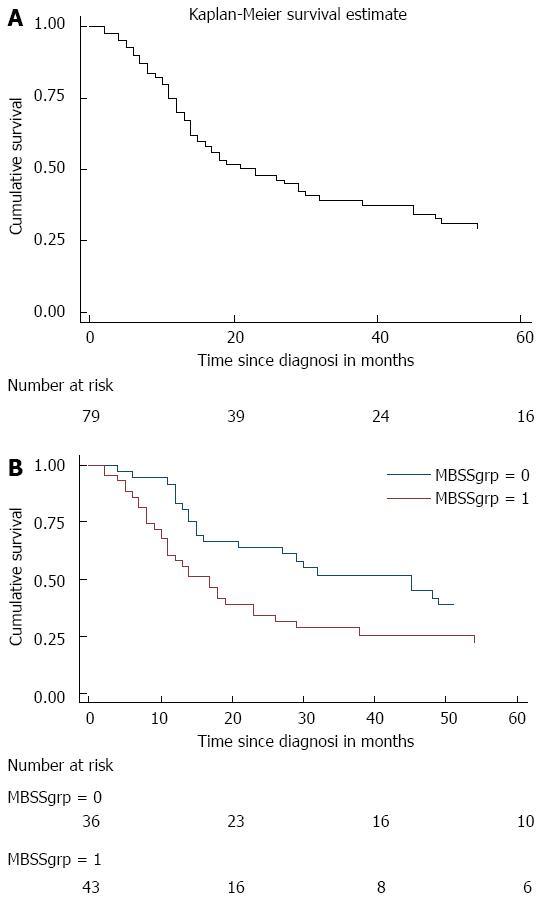
Survival data, using the Kaplan Meier survival method is displayed in Figure 1A. Median overall survival was 23 mo, and 5-year survival was 29.4% (95%CI: 19.2%-40.3%). Figure 1B shows the Kaplan Meier survival estimate stratified by MBSS category. For low and high MBSS the median survival was 45 and 17 mo respectively (P = 0.005; logrank test). At twelve months, survival was 83.3% (95%CI: 66.6-92.1) vs 58.1% (95%CI: 42.1-71.2) in those with low vs high MBSS, and 24 mo survival was 63.8% (95%CI: 45.9-77.1) and 34.0% (95%CI: 20.2-48.2) respectively.
The strongest univariate predictor of survival was the MBSS (HR = 2.13; P = 0.007) and additionally age and margin status were significant. Other co-variates not significantly associated with survival in univariate analysis included tumor size, sex and adjuvant chemotherapy. The MBSS grouping exhibited an interaction with the use of chemotherapy: survival probabilities according to MBSS and the use of adjuvant/neoadjuvant chemotherapy at 12 mo or at 24 mo are given in Table 3. The low MBSS group had a survival of 83% and 64% at 12 and 24 mo respectively irrespective of the use of adjuvant chemotherapy. However, in the high MBSS group, survival without chemotherapy was almost half that of the group receiving chemotherapy at 12 mo: 41% vs 69% (non statistically significant trend) although survival at 24 mo was similar (35% vs 33%) suggesting that chemotherapy may decrease first year mortality, but only within the high MBSS group.
| Time (mo) | Probability if high MBSS (95%CI) | Probability if low MBSS (95%CI) |
| Chemotherapy | ||
| 12 | 0.69 (0.48-0.83) | 0.80 (0.58-0.91) |
| 24 | 0.33 (0.16-0.52) | 0.64 (0.42-0.79) |
| No chemotherapy | ||
| 12 | 0.41 (0.19-0.63) | 0.90 (0.47-0.99) |
| 24 | 0.35 (0.14-0.57) | 0.60 (0.25-0.83) |
In the multivariate Cox model (stratified by chemotherapy), after addition of clinically meaningful covariates, MBSS was the variable with the strongest association (HR = 2.63; P = 0.001); only age (HR = 1.03; P = 0.027) and size of tumor (HR = 1.21; P = 0.101) improved the model further while gender and margin status did not and were excluded (Table 4). Table 4 also explores the above-mentioned possible interaction of MBSS and adjuvant chemotherapy in a multivariable model; chemotherapy was found to have an important interaction with MBSS as supported by a strong trend though this result was not significant. This confirmed the initial univariate results suggesting a lower mortality in high MBSS, but not low MBSS, patients receiving adjuvant chemotherapy. This interaction variable, on Cox model (Table 4), resulted in an adjusted mortality HR for the high MBSS (compared to low MBSS) of 4.14 (95%CI: 1.48-11.64) without chemotherapy and 2.11 (95%CI: 1.06-4.17) with chemotherapy.
| Variable | HR | Coef. | P value | 95%CI for HR | ||
| Model 1 (n = 83) Stratified by chemotherapy status | ||||||
| High MBSS | 2.63 | 0.969 | 0.001 | 1.49 | 4.67 | |
| Age | 1.03 | 0.030 | 0.027 | 1.00 | 1.06 | |
| Tumor size (cm) | 1.21 | 0.189 | 0.101 | 0.96 | 1.51 | |
| Model 2 (n = 74) Interaction between MBSS group and chemotherapy status | ||||||
| Low MBSS | Chemotherapy | 1.29 | 0.256 | 0.625 | 0.46 | 3.61 |
| Age | 1.03 | 0.032 | 0.024 | 1.00 | 1.06 | |
| Tumor size (cm) | 1.16 | 0.149 | 0.190 | 0.93 | 1.45 | |
| No Chemotherapy | High MBSS | 4.14 | 1.421 | 0.007 | 1.48 | 11.64 |
| Chemotherapy | High MBSS | 2.11 | 0.740 | 0.033 | 1.06 | 4.17 |
This report illustrates the survival of a modern cohort of patients with localized and resectable PHA: the overall median survival was 23 mo, and 5-year survival was 29%.Although all patients appeared similar in that they had resectable disease and underwent a pancreaticoduodenectomy with curative intent, the results demonstrate that they in fact represent a heterogeneous group with respect to ultimate survival. An important part of this heterogeneity can be discriminated on the basis of presenting symptoms alone as measured by the MBSS prior to pancreaticoduodenectomy. Overall survival in the cohort was 45 mo for patients with low MBSS, whereas it was 17 mo for high MBSS patients (P = 0.005). The ability of the preoperative MBSS to predict postoperative survival was significant (HR = 2.63; 95%CI: 1.49-4.67), and it outperformed conventional predictors such as tumor size, pathological margin status, and adjuvant administration of chemotherapy.
Although a majority of clinicians are sensitive to the local and systemic symptoms of a potentially resectable PHA patient, decision-making is usually based primarily on radiological staging[12] unless there is a strong suspicion of extra pancreatic disease[19]. The literature reports that the most common symptoms of pancreatic head cancer are jaundice, general fatigue, nausea, pain, and weight loss, and the most frequent of these at onset seem to be abdominal pain followed by back pain and jaundice[20,21]. In spite of this, there is a paucity of literature on the relationship between symptoms at diagnosis and prognosis from this disease. Earlier studies have demonstrated that patients with back pain were more likely to have a poorer prognosis[22-24], while more recent studies have looked at the timing of the onset of symptoms in relation to prognosis[21]. The latter authors concluded that jaundice was associated with significantly better survival than other symptoms, whereas back pain was associated with significantly worse prognosis[21]. Although this does not concur with our findings, one possible explanation is the exclusion of pancreatic tail tumors in our report. We chose to exclude patients undergoing resection for adenocarcinomas of the body and tail of the pancreas to address prognosis in a more homogeneous fashion. Pancreatic tail adenocarcinomas rarely exhibit jaundice, and these lesions may have a poorer prognosis because they tend to present clinically very late in the clinical course[25]. Although smoking is not a symptom per se, cigarettes are estimated to cause both a 75% increase in the risk of pancreatic cancer[25] (this risk persists up to 10-15 years after cessation[26]) and a worse pancreatic cancer survival[25,27]. This is why it was originally decided to model “smoking within the past 5 years” into the MBSS.
Few other clinical scores exist in the literature to predict survival after PHA. A nomogram was published by Brennan et al[28] in 2004 and was found to discriminate disease specific survival. The components of the nomogram were age, sex, need for portal vein resection, splenectomy, margin of resection, location of tumor in the pancreas, differentiation, number of positive and negative nodes, back pain, T stage, weight loss, and pathological features. Some patients in that study received preoperative chemotherapy and radiotherapy. Though this nomogram has been validated[28], disadvantages include that it cannot be utilized preoperatively for possible neoadjuvant decision-making, and that it is somewhat complex to apply in the absence of known regression coefficients. In comparison, the assignation of a high or low MBSS is strictly based on bedside preoperative clinical data. The MBSS can moreover be used in a dichotomized form (cut-off > 9) which makes it very useful for clinicians.
To originally create and validate the MBSS, we had previously analyzed a cohort of 126 unresectable PHA patients for symptoms and baseline factors which are widely documented upon initial clinical presentation[13]. We had found only four factors (weight loss, jaundice, abdominal pain, and smoking) to be significantly associated with survival. The current data mirrors in resectable PHA patients the strong association between the MBSS and survival previously observed in unresectable patients (HR = 2.7; P < 0.001)[13] and this hazard ratio is even greater in the absence of adjuvant chemotherapy (HR = 4.14; P = 0.007). The MBSS has now been validated in two different contexts: in unresectable pancreatic cancer patients, and in PHA patients undergoing pancreaticoduodenectomy, with or without adjuvant chemotherapy. The consistently similar hazard ratios hint to a reproducible biological classification of PHA patients at initial presentation. On multivariate analysis, the MBSS was moreover found to be a better indicator of survival than previous reported markers, including size of tumor and resection margin status (Table 4). Although we were not able to evaluate the usefulness of CA 19-9, it has been found by others[18] to be significantly associated with survival after resection for pancreatic cancer. Jaundice is known to up-regulate CA-19-9, and the lack of a standardized timing for its measurement after preoperative biliary decompression may have confounded our already small sample size.
A finding of particular importance is the possible interactive effect of chemotherapy in the high MBSS group. Table 4 demonstrates that this effect is not simply an effect of low MBSS patients being selected to receive chemotherapy and vice versa. We found that the use of chemotherapy was associated with a very strong trend towards improved survival in the high MBSS group as evidenced by a decrease in the hazard ratio for mortality from 4.14 to 2.11. This could mean that a survival benefit does accrue for patients with high MBSS who are given chemotherapy, though the interaction effect displayed wide confidence intervals and was not statistically significant possibly because of the sample size. These results should be considered of possible clinical significance and warrant exploration. Conversely, we were not able to demonstrate a positive effect for chemotherapy in the low MBSS group, perhaps because the survival of this group of patients is already much greater than the high MBSS patients. The possibility that patients with low MBSS may not benefit from chemotherapy in terms of survival, with its attendant possible side effects, may also warrant further evaluation in optimizing an individualized approach to treatment which balances survival with quality of life. The MBSS may be perceived as a clinical counterpart to the utility and application of molecular markers in a personalized medicine context, which is expected to increase in the future[29].
Perhaps the greatest immediate utility of the MBSS does not relate to possible decision-making but rather to a way of meaningfully stratifying PHA patients in an objective way similar to that already used subjectively by astute clinicians. Preoperative MBSS stratification of patients may allow investigators to detect a possible therapeutic effect more effectively and with fewer patients by controlling for what has up until now been inherent confounding. This ability may prove especially useful to better evaluate the results of newer prospective treatment options in the neoadjuvant context.
In conclusion, the MBSS is a simple yet powerful prognostic stratification tool for both resectable and unresectable PHA patients. The MBSS has now been validated in multiple different cohorts with PHA. The main advantage of the MBSS lies in its simplicity and its preoperative application to allow effective stratification of patients upon inclusion in trials in order to detect beneficial effects more efficiently. Also, the suggested effect of chemotherapy in our cohort possibly supports targeting patients with high MBSS more specifically for inclusion in newer oncology trials to optimize the potential of demonstrating a therapeutic difference.
Pancreatic adenocarcinoma is a lethal disease with low survival. However studies vary in the rates of their five years survival. This could be due to the heterogeneity of the disease. The McGill Brisbane Symptom Score (MBSS) is a score that was created and validated in a cohort of unresectable pancreatic adenocarcinoma. In this study, the authors validated it in a cohort of resectable pancreatic head adenocarcinoma (PHA) and showed its significance in this patient group.
The use of prognostic scores can help eliminate heterogeneity in research studies and permit stratification of patients according to their disease. This will result in more accurate studies with valid results.
The MBSS is an innovative score that is powerful and simple for use in patients with pancreatic adenocarcinoma. The major advantage of the MBSS is its preoperative, pre interventional application and use.
The MBSS can be used to predict survival of patients with pancreatic adenocarcinoma preoperatively, therefore treatment could be tailored based on scoring. Further research and further multicentre trials should be carried out to further support the wide use of MBSS.
There is a debate in the use of neoadjuvant or adjuvant chemotherapy in patients with pancreatic adenocarcinoma. Use of scoring methods such as the MBSS in resectable pancreatic adenocarcinoma patient could help stratify patients for different treatment modalities.
The article The MBSS for patients with resectable PHA is an useful and original research paper which highlights the utility of the MBSS to predict survival in patients with resectable PHA undergoing pancreaticoduodenectomy. The study is well structured, the subject is actual and interesting, providing a rationale for performing the research. The article has an adequate bibliography, the manuscript is correctly written and the conclusions are justified by the results found in the study.
P- Reviewer: Biondi A, Pedrazzoli S, Tanase CP S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9855] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 2. | Moon HJ, An JY, Heo JS, Choi SH, Joh JW, Kim YI. Predicting survival after surgical resection for pancreatic ductal adenocarcinoma. Pancreas. 2006;32:37-43. [PubMed] |
| 3. | Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199-210; discussion 1210-1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1122] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 4. | Shimada K, Sakamoto Y, Sano T, Kosuge T. Prognostic factors after distal pancreatectomy with extended lymphadenectomy for invasive pancreatic adenocarcinoma of the body and tail. Surgery. 2006;139:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Brown KM, Domin C, Aranha GV, Yong S, Shoup M. Increased preoperative platelet count is associated with decreased survival after resection for adenocarcinoma of the pancreas. Am J Surg. 2005;189:278-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Büchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 716] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 7. | Kuhlmann KF, de Castro SM, Wesseling JG, ten Kate FJ, Offerhaus GJ, Busch OR, van Gulik TM, Obertop H, Gouma DJ. Surgical treatment of pancreatic adenocarcinoma; actual survival and prognostic factors in 343 patients. Eur J Cancer. 2004;40:549-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 204] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 8. | Bauer J, Capra S, Battistutta D, Davidson W, Ash S. Compliance with nutrition prescription improves outcomes in patients with unresectable pancreatic cancer. Clin Nutr. 2005;24:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Srikureja W, Chang KJ. Endoscopic palliation of pancreatic adenocarcinoma. Curr Opin Gastroenterol. 2005;21:601-605. [PubMed] |
| 10. | Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1243] [Cited by in RCA: 1167] [Article Influence: 77.8] [Reference Citation Analysis (1)] |
| 11. | Preis M, Gardner TB, Gordon SR, Pipas JM, Mackenzie TA, Klein EE, Longnecker DS, Gutmann EJ, Sempere LF, Korc M. MicroRNA-10b expression correlates with response to neoadjuvant therapy and survival in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2011;17:5812-5821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 617] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 13. | Jamal MH, Doi SA, Simoneau E, Abou Khalil J, Hassanain M, Chaudhury P, Tchervenkov J, Metrakos P, Barkun JS. Unresectable pancreatic adenocarcinoma: do we know who survives? HPB (Oxford). 2010;12:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Tran KT, Smeenk HG, van Eijck CH, Kazemier G, Hop WC, Greve JW, Terpstra OT, Zijlstra JA, Klinkert P, Jeekel H. Pylorus preserving pancreaticoduodenectomy versus standard Whipple procedure: a prospective, randomized, multicenter analysis of 170 patients with pancreatic and periampullary tumors. Ann Surg. 2004;240:738-745. [PubMed] |
| 15. | Bassi C, Molinari E, Malleo G, Crippa S, Butturini G, Salvia R, Talamini G, Pederzoli P. Early versus late drain removal after standard pancreatic resections: results of a prospective randomized trial. Ann Surg. 2010;252:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 385] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 16. | Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2006;93:1232-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 444] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 17. | Verbeke CS. Resection margins and R1 rates in pancreatic cancer--are we there yet? Histopathology. 2008;52:787-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | Hartwig W, Hackert T, Hinz U, Gluth A, Bergmann F, Strobel O, Büchler MW, Werner J. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg. 2011;254:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 328] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 19. | Mayo SC, Austin DF, Sheppard BC, Mori M, Shipley DK, Billingsley KG. Evolving preoperative evaluation of patients with pancreatic cancer: does laparoscopy have a role in the current era? J Am Coll Surg. 2009;208:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Appelqvist P, Virén M, Minkkinen J, Kajanti M, Kostiainen S, Rissanen P. Operative finding, treatment, and prognosis of carcinoma of the pancreas: an analysis of 267 cases. J Surg Oncol. 1983;23:143-150. [PubMed] |
| 21. | Watanabe I, Sasaki S, Konishi M, Nakagohri T, Inoue K, Oda T, Kinoshita T. Onset symptoms and tumor locations as prognostic factors of pancreatic cancer. Pancreas. 2004;28:160-165. [PubMed] |
| 22. | Kalser MH, Barkin J, MacIntyre JM. Pancreatic cancer. Assessment of prognosis by clinical presentation. Cancer. 1985;56:397-402. [PubMed] |
| 23. | Ridder GJ, Klempnauer J. Back pain in patients with ductal pancreatic cancer. Its impact on resectability and prognosis after resection. Scand J Gastroenterol. 1995;30:1216-1220. [PubMed] |
| 24. | Kelsen DP, Portenoy R, Thaler H, Tao Y, Brennan M. Pain as a predictor of outcome in patients with operable pancreatic carcinoma. Surgery. 1997;122:53-59. [PubMed] |
| 25. | Iodice S, Gandini S, Maisonneuve P, Lowenfels AB. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg. 2008;393:535-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 368] [Article Influence: 21.6] [Reference Citation Analysis (1)] |
| 26. | Lynch SM, Vrieling A, Lubin JH, Kraft P, Mendelsohn JB, Hartge P, Canzian F, Steplowski E, Arslan AA, Gross M. Cigarette smoking and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Am J Epidemiol. 2009;170:403-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 244] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 27. | Yu GP, Ostroff JS, Zhang ZF, Tang J, Schantz SP. Smoking history and cancer patient survival: a hospital cancer registry study. Cancer Detect Prev. 1997;21:497-509. [PubMed] |
| 28. | Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293-298. [PubMed] |
| 29. | Smith RA, Tang J, Tudur-Smith C, Neoptolemos JP, Ghaneh P. Meta-analysis of immunohistochemical prognostic markers in resected pancreatic cancer. Br J Cancer. 2011;104:1440-1451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |