Published online Sep 14, 2014. doi: 10.3748/wjg.v20.i34.12182
Revised: March 27, 2014
Accepted: April 27, 2014
Published online: September 14, 2014
Processing time: 263 Days and 13.4 Hours
AIM: To build a consensus among Chilean specialists on the appropriate management of patients with nonalcoholic fatty liver disease (NAFLD) in clinical practice.
METHODS: NAFLD has now reached epidemic proportions worldwide. The optimal treatment for NAFLD has not been established due to a lack of evidence-based recommendations. An expert panel of members of the Chilean Gastroenterological Society and the Chilean Hepatology Association conducted a structured analysis of the current literature on NAFLD therapy. The quality of the evidence and the level of recommendations supporting each statement were assessed according to the recommendations of the United States Preventive Services Task Force. A modified three-round Delphi technique was used to reach a consensus among the experts.
RESULTS: A group of thirteen experts was established. The survey included 17 open-ended questions that were distributed among the experts, who assessed the articles associated with each question. The levels of agreement achieved by the panel were 93.8% in the first round and 100% in the second and third rounds. The final recommendations support the indication of lifestyle changes, including diet and exercise, for all patients with NAFLD. Proven pharmacological therapies include only vitamin E and pioglitazone, which can be used in nondiabetic patients with biopsy-proven nonalcoholic steatohepatitis (the progressive form of NAFLD), although the long-term safety and efficacy of these therapies have not yet been established.
CONCLUSION: Current NAFLD management is rapidly evolving, and new pathophysiology-based therapies are expected to be introduced in the near future. All NAFLD patients should be evaluated using a three-focused approach that considers the risks of liver disease, diabetes and cardiovascular events.
Core tip: Current nonalcoholic fatty liver disease (NAFLD) management is rapidly evolving, and new pathophysiology-based therapies are expected to be introduced in the near future. All NAFLD patients should be evaluated using a three-focused approach that considers risks of liver disease, diabetes and cardiovascular events. The final recommendations of this consensus support the indication of lifestyle changes, including diet and exercise, for all patients with NAFLD. Proven pharmacological therapies only consider vitamin E and pioglitazone, which can be used in nondiabetic patients with biopsy-proven nonalcoholic steatohepatitis (the progressive form of NAFLD), although the long-term safety and efficacy of these therapies have not been established.
- Citation: Arab JP, Candia R, Zapata R, Muñoz C, Arancibia JP, Poniachik J, Soza A, Fuster F, Brahm J, Sanhueza E, Contreras J, Cuellar MC, Arrese M, Riquelme A. Management of nonalcoholic fatty liver disease: An evidence-based clinical practice review. World J Gastroenterol 2014; 20(34): 12182-12201
- URL: https://www.wjgnet.com/1007-9327/full/v20/i34/12182.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i34.12182
Nonalcoholic fatty liver disease (NAFLD), a nosological entity of increasing importance in the medical community, is now the leading cause of chronic liver disease worldwide[1]. The prevalence of NAFLD has increased steadily due to its association with obesity and diabetes. The NAFLD epidemic has prompted a large body of research on the pathogenesis, epidemiology, clinical features, progression and treatment of this condition[2-4].
NAFLD is characterized by lipid accumulation in the liver (defined as the presence of lipids in > 5% of hepatocytes or a lipid content > 5% of liver weight), which is similar to what is observed in chronic alcohol users. The definition of NAFLD also includes the absence of significant alcohol intake (> 20 g of alcohol/d), hepatic viral infections and the use of potentially hepatotoxic medications. NAFLD, which ranges in prevalence from 5.7% to 30% in the general population, is currently considered the most common liver disease in Western countries[5,6]. In Chile, a clinical study using abdominal ultrasonography revealed a prevalence of fatty liver in 23% in the studied population[7]. From a histological viewpoint, NAFLD presents with different degrees of steatosis, inflammation and fibrosis; the spectrum of lesions ranges from simple steatosis to nonalcoholic steatohepatitis (NASH), the potentially progressive form of the disease[8]. The prevalence of NASH in the general population is estimated to range from 5% to 6%, and 3% to 20% of individuals with NASH will develop cirrhosis and associated complications in 10 or more years[9,10]. An unknown percentage of patients with NASH will develop hepatocellular carcinoma (HCC)[11]. Because patients with NASH have the potential to progress to cirrhosis, an adequate assessment of the risk factors involved in the progression of NASH, the establishment of therapeutic measures and, eventually, the use of pharmacotherapy are essential for the clinical management of these patients to prevent progressive liver damage[12,13].
Multiple risk factors are associated with the presence of NAFLD. Obesity, particularly the central obesity phenotype, type 2 diabetes mellitus (T2DM) - which is associated with a high risk of advanced liver disease, even in recently diagnosed patients[14,15] - and components of the metabolic syndrome, particularly insulin resistance (IR), are strongly associated with NAFLD in both Western and non-Western populations[16-19]. Currently, NAFLD is considered the hepatic manifestation of the metabolic syndrome. An increasing familial aggregation of NAFLD has been observed, which is explained by genetic and environmental factors, such as a sedentary lifestyle[20]. Additionally, recent studies have revealed that patients with NAFLD exhibit higher mortality rates than the general population, with an overrepresentation of liver-related deaths[10,21,22]. Moreover, NAFLD is recognized as an independent cardiovascular risk factor[23] and a clinical predictor of incident T2DM[24].
Although multiple interventions have been assessed in clinical studies of NAFLD management, no consensus exists on the evaluation of these patients or on the optimal pharmacological therapy[6]. Thus, the purpose of this work was to establish a national consensus on NAFLD management by combining an in-depth review of the current literature and responses to relevant questions by an expert panel, followed by a modified three-round Delphi technique aimed at reaching a consensus among the participating experts.
In January 2011, a group of 13 experts was established. The group received a survey that included 17 specific, open-ended clinical questions pre-established by the Consensus Organizing Committee concerning the different scenarios faced by clinicians when treating patients with NAFLD. The methodology of the consensus with regard to the different levels of evidence and the degree of recommendation for each statement were electronically distributed to the expert panel. To respond to the clinical questions, each expert evaluated the current level of evidence and established a recommendation according to the methodology described below. Each clinical question was discussed in detail, and members of the expert panel presented general outlines of epidemiology, clinical issues and the natural history of the disease a special meeting.
The quality of the evidence supporting each statement was assessed in a descriptive manner, based on the design of the studies, according to the recommendations of the United States Preventive Services Task Force[25,26].
The stratification of the evidence was conducted according to the study design, without methodological evaluation, as shown in Table 1, where each layer represents a quality level[27]. Each level is ranked decreasingly; thus, controlled clinical studies correspond to the highest quality level; observational studies correspond to an intermediate quality level; and pathophysiological studies and expert opinion correspond to the lowest quality level. The design of every level is variable, with randomized clinical studies with a large sample size and systematic reviews representing the highest level of evidence[28,29].
| Level of evidence | Description |
| Type I | Evidence obtained from at least one well-designed, randomized, controlled1 trial or from a systematic review of randomized clinical studies |
| Type II | II-1 Evidence obtained from nonrandomized, prospective, controlled1 studies |
| II-2 Evidence obtained from cohort observational studies2 or case-control studies, preferably multicenter | |
| II-3 Evidence obtained from case series | |
| Type III | Opinions of authorities on the subject matter based on expertise, expert committees, case reports, pathophysiological studies or basic science studies |
The present report does not include a methodological analysis of the internal validity of each study; this article only describes the design and results (with the corresponding 95%CIs and/or P values). A methodological analysis was performed for studies that assessed the same question and were ranked in the same level of evidence but produced discordant results[30].
Levels of recommendation were established (Table 2) based on a modification of the ratings of the United States Preventive Services Task Force[25,26].
| Recommendation | Available evidence |
| A | The Consensus strongly recommends the mentioned intervention or service. This recommendation is based on high-quality evidence, with benefits that significantly exceed the risks |
| B | The Consensus recommends the regular clinical use of the mentioned intervention or service. This recommendation is based on moderate-quality evidence of benefits that exceed the risks |
| C | The Consensus does not make any positive or negative recommendations regarding the mentioned intervention or service. A categorical recommendation is not provided because the evidence (of at least moderate quality) does not show a satisfactory risk/benefit relationship. Decisions must be made on a case-by-case basis |
| D | The Consensus makes a negative recommendation against the mentioned intervention or service. The recommendation is based on at least moderate-quality evidence that does not show any benefit or where the risk or damage exceeds the benefits of the intervention |
| I | The Consensus concludes that the evidence is insufficient due to low-quality studies or heterogeneous results or because the risk/benefit balance cannot be determined |
One to three questions were assigned to each expert to analyze and answer; the answers were to be supported through a review of the literature. Using the above-mentioned tools, a “level of evidence” and a “level of recommendation” were established for every subject analyzed; a final statement was created for every question and was presented as a “conclusion or recommendation”.
Delphi methodology: After each expert established a level of evidence, a level of recommendation and a conclusion or recommendation for each question, the consensus process began, using the Delphi methodology[31]. In the first stage, an initial draft (Spanish) of the text was sent (via e-mail) to the experts, who then submitted their opinions on all the questions to establish the “level of agreement” for each recommendation. The levels of agreement were standardized using a Likert-type scale with scores ranging from 1 to 5 (1: totally disagree; 2: disagree; 3: uncertain or with objections; 4: agree; and 5: totally agree). In cases in which the expert’s opinion was different from the statement that was written in the text, there was the option of inserting a comment to explain the reasons for this disagreement. If the agreement ranged from 4 to 5, the insertion of a comment was optional; however, if the agreement ranged from 1 to 3, a comment explaining the reasons or proposing a new final statement for the question was mandatory.
This first round involved testing. The organizing committee prepared a feedback document using the information that had been collected. The Spanish version of the survey was resubmitted to the experts to obtain their input for the reassessment of each question in the second round of the Delphi technique. The requested changes were subsequently incorporated into the feedback document, which was presented during the Annual Gastroenterology Postgraduate Course.
The third round of the Delphi technique involved an evaluation of all of the information provided by the expert panel and the updated information, which was derived from the literature review. A professional translator translated the document from Spanish to English, and all of the experts participated in the final phase of the consensus.
Literature search: Separate literature searches were conducted by one of the authors (Candia R), according to an established format: the specific wording of the question was determined, and PubMed was searched using the filters “systematic reviews” and “clinical studies”. At the time of manuscript preparation (May 2013), a new literature search was performed, and the information was made available to the experts during the third round of the Delphi technique to ensure an up-to-date data analysis. Moreover, relevant data published thereafter was retrieved an analyzed by the writers of the final report and commented in the discussion section.
The level of agreement obtained in the first round was > 4 (1-5 Likert scale) for 15 of 17 (93.8%) open-ended questions and their corresponding statements and recommendations. Each expert received feedback, including specific comments, on their responses to each question; the experts were asked to modify their answers and recommendations based on this feedback. The reviewed document (in Spanish) was distributed to the expert panel in the second round, and 100% agreement was obtained.
The public presentation phase of the Chilean NAFLD consensus was conducted during the Annual Gastroenterology Postgraduate Course, which was convened in June 2011 in Santiago, Chile and was attended by more than 400 Chilean gastroenterologists. Each expert presented the panel’s consensus position on one or several of the survey questions, with a brief description of the literature, the final statement, the level of evidence and the level of recommendation, and the audience voted in real-time on whether to approve the position. The third round of the Delphi consensus included all of the feedback provided by the audience. The literature review was updated, and the definitive consensus was translated into English, which had 100% agreement. The final version of the 17 open-ended questions and the responses to them, the level of evidence, the level of recommendation and the final recommendation statement are presented below.
The principal measure proposed relates to changes in lifestyle, i.e., weight loss and dietary modifications together with increased physical activity (Table 3)[32-41]. The pathophysiological basis of this statement is that a reduction in adipose tissue will reduce IR, which is considered the central mechanism of NAFLD[42]. The best results are obtained when this measure is associated with cognitive behavioral therapy, which must be considered and encouraged as a first-line therapy according to the severity of NAFLD or NASH[43-46].
| Ref. | Year | Study design1 | Intervention1 | Comparison | Duration (mo) | Histology | ALT |
| Scaglioni et al[218] | 2012 | OP-CS | D + E (n = 12) | 3 | N/A | + | |
| Thoma et al[67] | 2012 | SR | D + E2 (n = 338) | Control (n = 98) | 3-12 | N/A | + |
| Keating et al[79] | 2012 | SR | Exercise (n = 439) | Non-exercise control | 2-6 | + | - |
| Peng et al[66] | 2011 | SR | D + E (n = 78) | Control (n = 67) | 1-12 | N/A | N/A |
| Browning et al[55] | 2011 | OP-CS | Low-carbohydrate diet (n = 18) | Hypocaloric diet (n = 18) | 0.5 | N/A | + |
| Moscatiello et al[41] | 2011 | OP-CS | Cognitive behavioral therapy (n = 68) | D + E (n = 82) | 24 | N/A | + |
| Kistler et al[73] | 2011 | OR-CS | Intense exercise (n = 213) | Moderate exercise (n = 162) and inactive (n = 438) | + | - | |
| Elias et al[64] | 2010 | OP-CS | Diet 55% carbohydrates, 15% proteins and 30% fat (n = 17) | Control (n = 14) | 6 | N/A | + |
| Hayward et al[72] | 2010 | RCT | D + E (n = 28) | Control | 6 | + | + |
| 2Promrat et al[45] | 2010 | RCT | LSC (n = 21) | Control (n = 10) | 12 | + | + |
| 3Kantartzis et al[32] | 2009 | OP-CS | D + E (n = 50) | Control (n = 120) | 9 | N/A | + |
| St George et al[76] | 2009 | OP-CS | Exercise (n = 141) | Control (n = 34) | 3 | + | + |
| Chen et al[33] | 2008 | OP-CS | D + E (n = 16) | Exercise (n = 23) or control (n = 15) | 2.5 | N/A | + |
| Wang et al[34] | 2008 | OP-CS | LSC (n = 19) | Control (n = 38) | 1 | N/A | + |
| Krasnoff et al[69] | 2008 | OT-S | Exercise (n = 37) | + | N/A | ||
| Ryan et al[54] | 2007 | OP-CS | Diet 60% Carbohydrates/25% fat (n = 26) | Diet 40% Carbohydrates/45% fat (n = 26) | 4 | N/A | + |
| Tendler et al[219] | 2007 | OP-CS | Diet (n = 5) | 6 | + | - | |
| Zelber-Sagi et al[80] | 2006 | RCT | D + E (n = 44) | 6 | + | + | |
| Thomas et al[220] | 2006 | OP-CS | D + E (n = 10) | 6 | N/A | + | |
| Sreenivasa Baba et al[35] | 2006 | OP-CS | D + E (n = 65) | 6 | N/A | + | |
| 4Huang et al[49] | 2005 | OP-CS | D + E (n = 23) | 12 | - | + | |
| Suzuki et al[59] | 2005 | OP-CS | D + E (n = 348) | 12 | N/A | + | |
| Hickman et al[50] | 2004 | OP-CS | D + E (n = 31) | 15 | + | + | |
| Okita et al[221] | 2001 | OP-CS | Diet (n = 14) | 6 | N/A | + | |
| Knobler et al[222] | 1999 | OP-CS | Diet (n = 48) | 24 | N/A | + | |
| 5Ueno et al[36] | 1997 | OP-CS | D + E (n = 15) | Control (n = 10) | 3 | - | + |
| Park et al[46] | 1995 | OP-CS | D + E (n = 13) | Control (n = 12) | 12 | N/A | + |
| Palmer et al[47] | 1990 | OR-CS | D + E (n = 39) | 16 | N/A | + | |
| Eriksson et al[223] | 1986 | OT-S | Diet (n = 3) | 12 | N/A | + |
Are diet and weight loss effective for the treatment of NAFLD? The dietary recommendations for patients with NAFLD should include a reduction of 600-800 calories in the usual daily oral intake or caloric restriction set to 25-30 kcal/kg per day of the ideal body weight[36,47,48]. Protein intake should be 1-1.5 g/kg per day, and carbohydrates should be restricted to 40%-45% of the caloric intake[49]. Of the total daily fat in the diet, saturated fat should also be restricted to < 30% of the caloric intake; < 10% of the caloric intake should be saturated fat[50-52]. The patient should be instructed to preferentially consume fruits and vegetables instead of fructose-rich food[49]. Low-carbohydrate diets are associated with reductions in hepatic triglycerides and serum aminotransferases[53-55]. High-cholesterol/high-fat diets, with low levels of polyunsaturated fat, fiber and antioxidants (vitamins C and E), are associated with NASH[48,51,56]. Weight loss should be gradual and controlled (0.5 kg/wk) because dramatic changes (> 1.6 kg/wk) can be associated with portal inflammation and progressive fibrosis[57]; therefore, this recommendation should be emphasized to prevent liver damage.
Weight loss, preferably through diet, must be at least 5%-10% to observe a significant clinical benefit[50,58-65]. Two recently published systematic reviews assessed diet, weight loss and lifestyle changes related to NAFLD[66,67]. Both reviews analyzed 6 randomized studies and 17 observational studies; these studies consistently showed reductions in serum aminotransferases, and 5 of these studies showed histological improvements in hepatic steatosis and inflammation (but not in fibrosis). However, the methodological quality of these studies was poor and heterogeneous; therefore, no meta-analysis of the data was performed. On this basis, the consensus statement regarding diet and weight loss in NAFLD was as follows: Lifestyle changes involving diet (caloric intake restriction set to 25-35 kcal/kg per day and a low-fat, low-carbohydrate diet) leading to gradual but significant weight loss (5%-10% of baseline weight), improve insulin resistance and liver test abnormalities and decrease histological inflammation; therefore, these measures should be indicated for all patients with NASH. Furthermore, due to the optimal cost-benefit ratio, the absence of contraindications or side effects and all the additional medical benefits, these measures should be recommended to all NAFLD patients[40] (Level of Evidence: I, Level of Recommendation: A).
Is exercise effective for the treatment of NAFLD? Physical exercise can improve IR and modify hepatic fat content[68]. Physical activity should be objectively assessed and recommended to all patients with NAFLD[36,69]. Thirty minutes of moderate-to-intense aerobic physical exercise (e.g., treadmill, elliptical trainer, bike or swimming) 3 to 5 times per week must be recommended, applying caution for individuals at high cardiovascular risk[12]. An optimal exercise prescription has not been established. A recent study and a recent meta-analysis suggested that aerobic exercise is superior to resistance training[70,71]. If the patient has not followed a hypocaloric diet, they may need more intense exercise to obtain better results; moderate exercise is sufficient if the patient has adhered to a hypocaloric diet[35,72-74].
A recent, large, cross-sectional study of 72359 healthy Korean adults without diabetes demonstrated that regular exercise (at least 3 times per week, for at least 30 min each time) was associated with a significant reduction in the risk of NAFLD (for all body mass index categories over 19.6 kg/m2) with age- and sex-adjusted ORs of 0.53-0.72 and decreases in liver enzymes (reduced risk of elevated aminotransferases with multivariate adjusted ORs of 0.85 and 0.74, respectively) among individuals with recognized NAFLD independent of obesity[75]. This recent study is supported by other studies showing that exercise does not need to reduce body weight to have beneficial effects on improving lipid metabolism and reducing hepatic fat.
Physical activity is associated with improvements in cardiovascular condition and IR and the normalization of hepatic enzymes, regardless of weight loss[40,70,76-78]. However, a recent systematic review of 12 of the 16822 studies identified in an initial search, emphasized that individual reports of exercise interventions often have small sample sizes and insufficient power to detect clinically meaningful hepatic benefits; nevertheless, there is clear evidence that exercise therapy has a definite benefit on liver fat, but the benefit on alanine aminotransferase levels is unclear[79]. This benefit of exercise was apparent even if minimal or no weight loss was achieved and at exercise levels below the current lifestyle recommendations for obesity management. The consensus statement on exercise was as follows: In patients with NAFLD, physical exercise - at least of moderate intensity - improves insulin resistance and liver fat, with uncertain effects on liver test abnormalities; therefore, being a valid and low-cost therapy, it should be recommended to all NAFLD patients (Level of Evidence: II-2, Level of Recommendation: B).
Is orlistat effective as a weight loss medication for the treatment of NAFLD? Orlistat, an enteric lipase inhibitor that causes fat malabsorption, is commonly indicated to achieve weight loss. Few clinical studies have directly assessed orlistat in NAFLD, although a study by Zelber-Sagi et al[80] demonstrated an average weight loss of 10.3 kg and a significant reduction in serum aminotransferase levels in obese patients with NASH. Harrison et al[81] found improvements in liver function tests and steatosis using ultrasonography. A recent systematic review[66] found that 2 randomized studies of low methodological quality had findings that were consistent with the results of other studies on weight loss without the use of medications; therefore, a direct effect on NAFLD cannot be attributed to orlistat. The consensus statement on orlistat was as follows: Orlistat has not shown any significant benefit on the liver independent of weight loss; therefore, this drug must be used in combination with a guided nutritional and physical exercise program to achieve the desired weight loss[81,82] (Level of Evidence: II-1, Level of Recommendation: C).
Is sibutramine effective as a weight loss medication for the treatment of NAFLD? Sibutramine, a serotonin-norepinephrine reuptake inhibitor that reduces the postprandial satiety threshold and increases energy expenditure, has been found to be temporarily useful for weight loss. Only one study with a small number of patients has shown a reduction in IR and moderate improvements in hepatic parameters in laboratory tests and ultrasonography[83]. However, there is an absence of methodologically well-structured studies to support the effectiveness of sibutramine in the treatment of NAFLD[84-86] independent of weight loss. The results of the sibutramine cardiovascular outcomes trial[87] showed a significant risk of cardiovascular side effects, and other data have indicated a modest efficacy of this medication. In response to these findings, in 2010 international organizations such as the Committee of Medicinal Products for Human Use of the European Medicines Agency and the United States Food and Drug Administration (FDA) recommended against the continued use of sibutramine[88]. In our country, the Chilean National Institute of Public Health recently prohibited the use of this medication. The consensus statement on sibutramine was as follows: Sibutramine is not recommended for the treatment of NAFLD due to the potential for severe cardiovascular adverse events and the absence of a significant benefit on NAFLD (Level of Evidence: II-2, Level of Recommendation: D).
Is rimonabant effective as a weight loss medication for the treatment of NAFLD? Rimonabant, a cannabinoid 1 receptor antagonist, has been proposed for the treatment of NAFLD because of its effect on the reduction of caloric intake. However, it also has potential psychiatric adverse effects associated with anxiety and depression (RR = 2.35; 95%CI: 1.66-3.34)[89,90]; hence, all prospective studies on this medication were halted, and the product was recalled. The final statement from the panel was as follows: Rimonabant is not recommended for the treatment of NAFLD (Level of evidence: II-1, Level of recommendation: D).
For morbidly obese patients, bariatric surgery is a therapeutic option for weight loss[91]; moreover, surgery is the best alternative option for weight reduction if lifestyle modifications and pharmacological therapy have not yielded long-term success. In 2004, the Swedish Obese Subjects study suggested that bariatric surgery might be related to long-term improvements in cardiovascular risk factors, such as diabetes mellitus, hypertriglyceridemia and hypertension, compared with a control group with conventional weight loss[92]. However, the effects on the liver were not specifically studied. Another 2004 study showed that NASH was resolved in 82% of patients who underwent laparoscopic surgery for the placement of an adjustable gastric band, who experienced an average weight reduction of 34 ± 17 kg[93]. A systematic review conducted in 2008 reported that bariatric surgery led to the regression of inflammation and fatty infiltration of the liver; therefore, it could be considered a feasible alternative in selected cases[94]. This information suggests that in morbidly obese patients, bariatric surgery is associated with significant weight loss, leading to improvements in the metabolic syndrome and improved control of diabetes mellitus, steatosis, inflammation and even liver fibrosis in patients with established NASH.
Other recent studies have suggested potential benefits of bariatric surgery[95-98]. However, due to a lack of randomized studies assessing the effect of bariatric surgery on NASH, a recent review in the Cochrane Database concluded that the lack of studies hinders the recommendation of the use of bariatric surgery for NASH therapy[99]; its long-term effects have not been studied. On this basis, the consensus statement on bariatric surgery for NAFLD was as follows: Bariatric surgery may play a role in the treatment of morbidly obese patients with established NASH. However, bariatric surgery may have complications, which vary depending on the center where it is performed (average mortality of 0.3% and morbidity of 10%). The indication of this procedure must be individualized, and it should be performed in specialized medical centers with a multidisciplinary approach (Level of Evidence: II-1 Level of Recommendation: B).
Reasonable pharmacological approaches to NAFLD treatment include oral hypoglycemic and insulin-sensitizing medications[100-104]. Metformin and thiazolidinediones (pioglitazone and rosiglitazone) have been associated with the normalization of aminotransferases in 50% of cases, reductions in steatosis (assessed using ultrasonography and magnetic resonance spectroscopy), partial improvements in necrosis and inflammation and, although less evident, a partial improvement in fibrosis after one year of follow-up[105-107]. However, the regression of liver function to pretreatment levels after therapy is suspended has been reported[105,108]. The following questions regarding specific medications were addressed by the panel:
Is metformin effective for the treatment of NAFLD? Metformin, a biguanide, improves IR and hyperinsulinemia by reducing hepatic glucose production, increasing the peripheral uptake of glucose by muscles and reverting IR induced by tumor necrosis factor-α[109]. Clinical studies have shown an initial improvement in serum aminotransferases[110], although there was no improvement after one year of treatment[111]. Other studies of metformin have produced promising data; however, the lack of follow-up and histological end-points make it impossible to form solid conclusions[112,113]. A Cochrane Review highlighted the normalization of aminotransferases compared with diet (OR = 2.83; 95%CI: 1.27-6.31) and improvements in ultrasound findings (OR = 5.25; 95%CI: 1.09-25.21)[114]. However, a small number of studies were included due to methodological problems. A study of obese Brazilian adolescents showed that metformin, together with lifestyle changes, was more effective than placebo for improving clinical parameters related to obesity and steatosis. The study did not include liver biopsy[115].
Recently, the US by the Nonalcoholic Steatohepatitis Clinical Research Network Research Group conducted a multicenter study, named treatment of NAFLD in children (TONIC). The study compared metformin and vitamin E in 173 pediatric patients with NAFLD who were followed for 96 wk and underwent post-treatment biopsy[116]. This study did not demonstrate any significant benefits of metformin on aminotransferase levels or liver histology[117]. A more recent study of 2153 patients did not show beneficial effects of metformin, regardless of weight loss[118]. Finally, a systematic review of 8 randomized controlled trials did not show any beneficial effects of metformin on histology[119]. The consensus statement on metformin for NAFLD was as follows: Metformin might have a temporary beneficial effect on serum aminotransferases. Histological benefits have not been demonstrated in children, but further studies with histological follow-up on adults are needed. The consensus does not recommend the regular use of metformin for the treatment of NAFLD; however, it can be used in insulin-resistant patients (without renal insufficiency or heart failure) (Level of evidence: I, Level of recommendation: C).
Are thiazolidinediones effective for the treatment of NAFLD? Thiazolidinediones improve insulin sensitivity in adipose tissue by activating the nuclear transcription factor PPAR-gamma[120,121]. IR improvements associated with the normalization of biochemical and histological parameters were observed in a recent study of 30 patients with NASH who received 4 mg of rosiglitazone twice per day for 48 wk[122]; this clear trend was also observed in another study, in which 18 patients with NASH received 30 mg of pioglitazone for 48 wk[107], and in a third study comparing pioglitazone 45 mg/d and a hypocaloric diet vs hypocaloric diet alone for 6 mo[123]. However, after discontinuing the medication, the patients gained weight and their biochemical and histological abnormalities reappeared. Other studies with good methodological quality have shown improvements in both biochemical parameters and histology[124,125]. The FLIRT study showed that rosiglitazone resulted in a significant improvement in steatosis without changes in other parameters of liver damage[106,126]. A recently published multicenter study (the PIVENS study)[127] compared pioglitazone to vitamin E in 247 patients with histological NAFLD who were followed up for 96 wk and underwent post-treatment biopsy. Compared with placebo, vitamin E therapy was associated with a significantly higher rate of improvement in NASH (43% vs 19%, P = 0.001); however, pioglitazone was not associated with a significant difference in the rate of improvement compared with placebo (34% and 19%, respectively, P = 0.04, with a prespecified significance level of 0.025). Serum aminotransferase levels were reduced with both vitamin E and pioglitazone treatment, compared with placebo (P < 0.001 for both comparisons), and both agents were associated with reductions in hepatic steatosis (P = 0.005 for vitamin E and P < 0.001 for pioglitazone) and in lobular inflammation (P = 0.02 for vitamin E and P = 0.004 for pioglitazone), but there were no improvements in fibrosis scores (P = 0.24 for vitamin E and P = 0.12 for pioglitazone). Subjects who received pioglitazone gained more weight than did those who received vitamin E or placebo; the rates of other side effects were similar among the three groups. Serum aminotransferase abnormalities reappeared after treatment discontinuation. Finally, two recent meta-analyses supported the beneficial histological effects of insulin sensitizers[119], particularly pioglitazone[128], in patients with NASH.
The long-term safety of thiazolidinediones has been questioned in several studies showing cardiovascular adverse effects, including congestive heart failure and increased rates of coronary events[129]. Additionally, increased rates of bladder cancer and bone loss have been reported in thiazolidinedione users. For these reasons, rosiglitazone is no longer marketed; pioglitazone is the only available agent in this class. The consensus statement on thiazolidinediones for NAFLD was as follows: Thiazolidinediones (pioglitazone and rosiglitazone) improve insulin sensitivity and may reduce steatosis and liver inflammation. The panel recommends the use of pioglitazone in nondiabetic patients with biopsy-proven steatohepatitis (Level of evidence: I, Level of recommendation: B). The long-term safety of pioglitazone has not been established.
Hypolipidemic medications, such as statins and fibrates, are suggested as a potential treatment options for NAFLD due to their effects on common abnormalities among metabolic syndrome patients such as hypertriglyceridemia and low HDL-cholesterol levels[130,131].
Are fibrates effective for the treatment of NAFLD? A clinical study of gemfibrozil use by NAFLD patients showed a moderate improvement in biochemical parameters after the administration of 600 mg/d for 4 wk[132]. On the contrary, the use of clofibrate in another study did not result in any biochemical or histological improvements[133]. In another study, the use of fenofibrate reduced plasma triglyceride levels without changing the hepatic triglyceride content[134]. There are no data from large multicenter studies on which to base solid conclusions[130,135]. Thus, the consensus statement on the use of fibrates in NAFLD was as follows: Fibrates have been studied in NAFLD, mostly in small studies, with conflicting results. Hence, the regular use of fibrates is not recommended for patients outside the context of clinical studies (Level of evidence: II-1, Level of recommendation: C).
Are statins effective for the treatment of NAFLD? In small randomized studies, HMG-CoA reductase inhibitors (statins) have produced partial improvements in aminotransferases; however, the results were not consistent in all of the studies[13,105,136-141]. A small pilot study of 20 mg/d pravastatin for a 6-mo period showed the normalization of aminotransferases and improvements in liver inflammation in patients with NASH[142]. Another study of atorvastatin showed the normalization of serum aminotransferases and lipid levels[143]. A larger study demonstrated a beneficial effect of atorvastatin, but in association with vitamins C and E[144]. There is no current evidence of an increased risk of hepatotoxicity when statins are administered in standard doses to patients with NAFLD and other liver diseases[145-150]. The consensus statement on statins was as follows: Statins have shown discordant effects on NAFLD. Their regular use is not recommended for the treatment of NAFLD. Statins can be used to treat dyslipidemia in NAFLD patients (Level of evidence: II-1, Level of recommendation: C).
Is ezetimibe effective for the treatment of NAFLD? Ezetimibe is a selective inhibitor of cholesterol and phytosterol absorption in the intestine. The Niemann-Pick C1 like 1 protein, the target of ezetimibe, is present not only in the intestine[151] but also expressed in the human liver[152]. This protein appears to play an important role in the development of NAFLD because the loss of the expression of this protein prevents the occurrence of NAFLD in animal models[153,154]. An improvement in liver sensitivity to insulin and reductions in liver inflammation and lipid accumulation have been observed[155]. Additionally, ezetimibe has been suggested to function as an antioxidant, which could reduce inflammatory processes during its metabolism in the liver[156,157]. To date, only preliminary studies of ezetimibe with few patients have been conducted; some were of high quality and demonstrated positive evidence regarding the effect of this drug on patients with NAFLD[157-160]. Due to its antioxidant capacity and safety profile, ezetimibe is a good option for NAFLD patients with high cardiovascular risk factors[140,161-164]. A recent prospective cohort study of 45 patients with NAFLD revealed interesting beneficial biochemical, metabolic and histological effects after 96 wk of follow-up[165]. The consensus statement on ezetimibe use in NAFLD was as follows: Ezetimibe is a drug with an adequate safety profile and scarce but positive evidence regarding its beneficial effect on NAFLD patients. Studies with good methodological design are needed before its use can be widely recommended. However, ezetimibe could be used in patients affected by both NAFLD and dyslipidemia because it might have a clinical benefit (Level of Evidence: III, Level of Recommendation: I).
Recent experimental and clinical evidence strongly suggests that the renin-angiotensin-aldosterone axis may play a role in mediating hepatic inflammation and fibrosis in NAFLD. Thus, suppressing the renin-angiotensin system with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) has been studied in small trials, which demonstrated both biochemical and histological improvements[166]. It has been observed that telmisartan and irbesartan not only antagonize ARAII but also activate peroxisome proliferator-activated receptor-γ; therefore, these drugs may positively influence hepatic lipid metabolism. However, to date, the evidence supporting the use of either ACEIs or ARBs in NAFLD is scarce and limited to retrospective studies, prospective pilot studies and post hoc analyses of clinical trials[167-170]. Thus, additional studies are needed to recommend these agents as primary medications for patients with NAFLD. However, in hypertensive patients with NAFLD, the use of ARB may be promoted. The use of telmisartan and irbesartan may produce a protective effect in recently diagnosed patients with diabetes, cardiovascular disease and NAFLD (by reducing IR)[171-174]. The approved statement was as follows: The control of blood pressure is advisable for cardiovascular risk reduction. Angiotensin II receptor antagonists (losartan and telmisartan) are recommended for patients affected by NAFLD and hypertension until additional clinical evidence becomes available[175] (Level of evidence: II-1, Level of recommendation: B).
The presence of inflammation or oxidative stress in NAFLD supports the idea that protecting cellular structures from the damage caused by free radicals and reactive products of lipid peroxidation would be beneficial in affected individuals. However, the available evidence is contradictory, and studies have not shown significant advantages related to lifestyle modifications and improved biochemical parameters[13,48,51,176,177]. Moreover, a recent Cochrane Review assessed the available information regarding the use of antioxidants and concluded that although the use of these agents is associated with reductions in aminotransferase levels, there is insufficient evidence to either support or refute the utility of antioxidants in NAFLD[178]. Specific agents are reviewed below.
Is vitamin C effective for the treatment of NAFLD? Vitamin C has been studied primarily in the pediatric population. These studies have not shown clear beneficial effects and did not assess the benefit of vitamin C separately (in several studies, the intervention group received both vitamins C and E)[13,58,102,105,179,180]. The statement of the consensus was as follows: Vitamin C is not recommended for patients with NAFLD outside the context of research protocols (Level of evidence: II-3, Level of recommendation: I).
Is vitamin E effective for the treatment of NAFLD? Several studies have assessed the use of vitamin E in patients with NAFLD[58,112,177,179-184]. Data from the PIVENS study, published in 2010 and described in question 4b, showed that the use of vitamin E resulted in histological improvement in 43% of the patients vs 19% of the placebo group (P < 0.001), primarily in the form of decreases in steatosis and lobular inflammation, with no changes in fibrosis. Additionally, a normalization of serum levels of aminotransferases was observed; the values returned to pretreatment levels after the therapy was discontinued. No significant adverse effects have been shown for vitamin E after two-years of treatment[127]. The use of vitamin E has also been combined with ursodeoxycholic acid (UDCA), with promising results[185].
Recently, the Nonalcoholic Steatohepatitis Clinical Research Network conducted a multicenter study comparing metformin and vitamin E in 173 pediatric patients with NAFLD, who were followed up for 96 wk and underwent a post-treatment biopsy (the TONIC study)[116]. This study did not show significant benefits of vitamin E for aminotransferase levels; however, it did show differences in the histological characteristics (ballooning and NAFLD activity score) of the liver biopsy performed at 96 wk[117].
Several concerns have been raised regarding an increase in all-cause mortality with the long-term use of vitamin E[186]. However, this issue is controversial[187]. Thus, the statement of the consensus was as follows: The use of vitamin E is well supported for nondiabetic adults with biopsy-proven NASH by recent studies showing improvements in histological and laboratory findings. The reversal of these beneficial effects after discontinuation of therapy suggests that long-term treatment is required (Level of evidence: I, Level of recommendation: A).
Is omega-3 polyunsaturated fatty acid supplementation effective for the treatment of NAFLD? Omega-3 polyunsaturated fatty acids (PUFAs) are widely used as an antioxidant. Several studies have assessed their efficacy in patients with NAFLD. A recent systematic review and meta-analysis[188] found significant heterogeneity between these studies and concluded that although omega-3 PUFA supplementation may decrease liver fat (with no effects on aminotransferase levels), the optimal dose has not been established. Additional trials are needed to support the routine use of omega-3 PUFA in patients with NAFLD. The statement of the consensus was as follows: To date, there is insufficient evidence to support the routine use of omega-3 PUFA supplementation in patients with NAFLD (Level of evidence: I, Level of recommendation: C).
Is pentoxifylline effective for the treatment of NAFLD? Another approach to NAF-LD treatment involves the use of anti-TNFα drugs because this cytokine induces both necroinflammation and IR[121]. Pentoxifylline, a TNFα inhibitor, has been used in animal models[189] and in patients with NASH[141,190-193]. Two pilot studies reported improvements in aminotransferase levels and IR[194,195]. A recently published systematic review[196] found 2 randomized studies[191,197] and 6 prospective cohort studies that showed improvements in aminotransferase levels only. A recent randomized study, which was not included in the mentioned systematic review, reported histological improvements in 50% of patients who received pentoxifylline compared with 16% of patients who received placebo[198,199]. Another small, randomized trial, which showed a benefit of pentoxifylline, was recently published[200]. More controlled studies with adequate sample sizes are required before its use can be recommended. The approved statement by the consensus panel was as follows: The use of pentoxifylline is not recommended on a regular basis for the treatment of NAFLD. Its indication must be evaluated on a case-by-case basis because although the current evidence is favorable, it is weak and scarce. A recent randomized, controlled trial showed clear benefits. More studies are required before recommending its routine use (Level of evidence: I, Level of recommendation: C).
UDCA, a hydrophilic cytoprotective bile acid, has been studied in patients with NAFLD in several trials, with contradictory results. Initial studies held promise[133]. However, a systematic Cochrane Review published in 2007 found 4 studies on UDCA in NAFLD (only one with adequate methodological quality) and concluded that UDCA did not show any significant benefit in patients with NAFLD[201]. The sole included study with a relatively large, well-designed, double-blinded, randomized, placebo-controlled design assessed a group of 166 patients with NASH who were randomized to receive 13-15 mg/kg/d of UDCA or placebo for one year; however, the study did not show clear benefits[202]. Subsequently, another randomized controlled study, in which high doses of UDCA were administered to 185 patients, also failed to indicate any benefit for patients with NAFLD[203]. Currently, there is no robust evidence recommending the use of UDCA[133,181,201-203]. The approved statement by the consensus panel was as follows: There is a lack of evidence of benefit of UDCA for the treatment of NAFLD. Thus, its routine use is not currently recommended (Level of evidence: I, Level of recommendation: D).
Our evidence-based analysis has several strengths. One is the use of the Delphi method to develop this consensus. The Delphi method is used for analyzing facts and reaching agreements on topics with insufficient evidence to support a given medical intervention. This method is a useful tool for creating a consensus without all of the panel members physically present in the same room to discuss issues. The main objective of the Delphi method is to reach a reliable agreement among the different members of an expert panel by using several questionnaires, which are completed anonymously[204]. The experts must be asked about one issue at least twice so that they can reconsider their answers after receiving information from the other members. The literature recommends a number of experts between 10 and 18. Our panel consisted of 13 members; however, none of the members were from countries outside of Chile, which is one of the limitations of our consensus.
While this report was under preparation, guidelines for the diagnosis and management of NAFLD, which were jointly endorsed by the American Gastroenterological Association (AGA), the American Association for the Study of Liver Diseases (AASLD) and the American College of Gastroenterology (ACG), were published. Thus, the conclusions of our national expert panel are discussed in the context of these guidelines in the following paragraphs.
In line with the recently published guidelines, our consensus stated that the most effective current therapeutic strategies for NAFLD are diet and lifestyle modifications, such as regular exercise, and suggested that both strategies must be advocated in every patient. The target weight loss must be approximately 5%-10% of the original body weight to achieve clinical and histopathological benefits. The AGA, AASLD and ACG guideline advise a weight loss of at least 3%-5% to recover from steatosis, but a weight loss greater than 10% is recommended to observe changes in necrosis and inflammation[205]. Thus, dietary changes and regular exercise should be recommended for all patients with NAFLD, although the optimal diet composition has not been determined.
When asked about weight loss medications used for the treatment of NAFLD in Chile such as orlistat, sibutramine and rimonabant, our panel concluded that orlistat did not provide any benefit in liver disease despite the weight loss achieved. Therefore, this agent should be used in combination with dietary modifications and exercise. Moreover, an FDA warning was recently issued regarding potential orlistat-related hepatotoxicity[206]. The panel argued against the use of sibutramine and rimonabant due to reports of serious adverse reactions from these agents. The AGA/AASLD/ACG guidelines contain no statements concerning this specific topic.
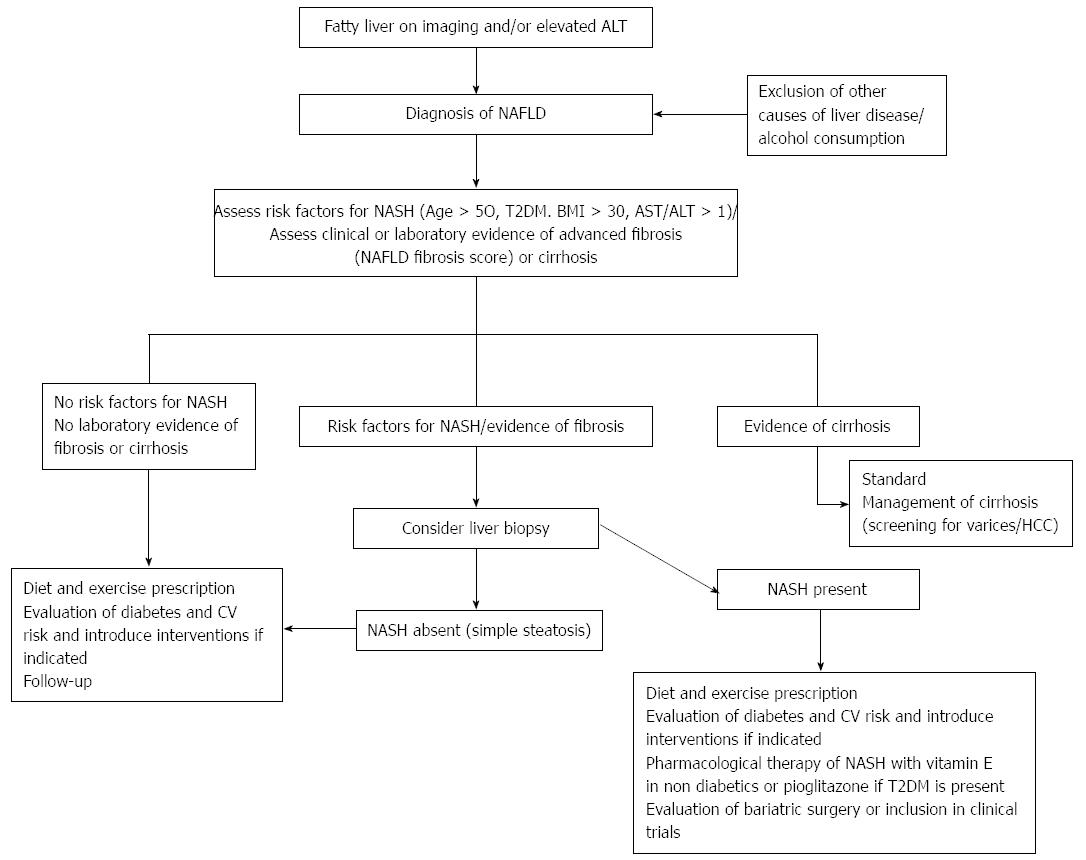
Although the consensus was largely focused on therapy, the ongoing debates about the necessity of liver biopsy in the setting of NAFLD and about the correct use of noninvasive methods for risk stratification indicate that these topics are highly relevant for the management of patients with NAFLD. The majority of patients have simple steatosis, which carries a relatively benign prognosis. However, patients with NASH exhibit increased liver-related and cardiovascular mortality; therefore, it is crucial to identify individuals at risk of developing this progressive disease. Although liver biopsy remains the gold standard for the precise diagnosis and staging of the disease, its invasive nature and cost preclude its widespread use in patients with NAFLD and other etiologies[207,208]. The use of noninvasive tools to identify patients with advanced disease is becoming more common, although firm evidence-based recommendations have not been established[209]. The statement of the recent AGA/AASLD/ACG guidelines on this topic indicates the relevance of the metabolic syndrome as a predictor of NASH and the usefulness of the NAFLD Fibrosis Score in patients with NAFLD for guiding clinicians in identifying patients who are at risk of NASH and advanced fibrosis. Additionally, a liver biopsy may be indicated in patients with NAFLD who have coexisting chronic liver diseases or competing etiologies for hepatic steatosis.
With regard to the use of bariatric surgery for NAFLD management, the panel agreed that these surgical procedures could play a role in the scenario of an obese patient with established NASH, with the acknowledgment that these procedures carry a risk of morbidity and that their indication should be individualized. The AGA/AASLD/ACG guidelines note that surgery is not contraindicated in patients with NAFLD or NASH without established cirrhosis, but they also state that it is premature to consider bariatric surgery as a standard therapeutic measure to treat patients with NASH. Staging of the disease is particularly important in this setting since surgery should be avoided in patients with cirrhosis particularly if portal hypertension is present[210].
The use of insulin sensitizers has been widely explored in the field of NAFLD. Based on their review of the data on different medications and the discussions during the Delphi rounds, our panel concluded that although metformin does not have a significant effect on liver histopathology in adult patients with NASH, thiazolidinediones, such as pioglitazone and rosiglitazone, could reduce liver steatosis and inflammation because they improve insulin sensitivity. The AGA/AASLD/ACG guidelines mention that pioglitazone can be used to treat patients with biopsy-proven NASH. However, it should be noted that the majority of patients who participated in clinical trials investigating pioglitazone for NASH were nondiabetic, and the long-term safety of pioglitazone in patients with NASH has not been established. In fact, due to the increased risk of coronary events, rosiglitazone is no longer marketed in Europe, and its use is highly restricted in the United States. Additionally, recent evidence concerning an increased risk of bladder cancer among pioglitazone users[211] and the associated undesirable weight gain will likely limit the use of this medication in patients with NAFLD in the near future.
Regarding the use of lipid-lowering agents to treat NAFLD, the panel agreed that fibrates are no longer recommended in NAFLD. With respect to omega-3 fatty acids, the panel agreed that current evidence does not support their use, but they could be indicated to treat hypertriglyceridemia. Of note, a recently published report of a small double-blind randomized placebo-controlled clinical trial showed that omega 3 fatty acids provided no benefit over placebo in NASH patients with diabetes[212]. With regard to other hypolipidemic drugs, the panel concluded that statins could be used to treat dyslipidemia in patients with NAFLD and NASH without safety concerns. In the case of ezetimibe administration in patients with NAFLD current data are limited but encouraging. The panel advised waiting for larger, more reliable studies of this medication, which is not addressed in the AGA/AASLD/ACG guidelines.
The panel advised the prolonged use of vitamin E by adult patients with NAFLD, based on recent studies showing histological improvement from treatment with this compound. The American guidelines recommended its use in nondiabetic patients with biopsy-confirmed NASH and stated that vitamin E should be considered as a first-line pharmacotherapy for this patient population. Similarly, AGA/AASLD/ACG guidelines emphasized that until further data supporting its effectiveness become available, vitamin E is not recommended to treat NASH in diabetic patients, NAFLD without liver biopsy, NASH cirrhosis or cryptogenic cirrhosis. Several concerns have been raised regarding the safety of long-term vitamin E administration, based on meta-analytic data showing that vitamin E supplementation increases all-cause mortality. However, this issue remains unresolved because this observation has not been confirmed by other trials and meta-analyses[213].
With regard to other agents, after reviewing the evidence regarding pentoxifylline the panel concluded, in agreement with a recent metanalysis[214], that this drug may be a useful treatment option for NAFLD but more evidence is needed to make firm recommendations. Lastly, the use of ursodeoxycholic acid in the treatment of patients with NASH, the panel agreed that this agent could not be recommended due to a lack of efficacy, which is in agreement with the statement of the American guidelines.
Finally, during the presentations and discussions, the participating panel agreed that patients with NAFLD should undertake active measures to control hypertension as part of integrated CV risk factor management in this patient population. To that end, the use of angiotensin receptor blockers, such as losartan or telmisartan, is recommended on the basis of their potential beneficial hepatic effects, which may halt fibrogenesis in patients with NASH. In fact, considering the strong association of NAFLD with components of the metabolic syndrome and the recently recognized role of NAFLD as an independent risk factor for CVD and the future development of T2DM[215], we strongly recommend an individual approach based on the following 3 points: (1) Assessment of T2DM risk: Measure fasting glycemia and serum insulin levels to estimate IR, measure HbA1c levels and, in selected patients, conduct a 75-g glucose load tolerance test. NAFLD patients should be advised that NAFLD conveys an increased risk of T2DM and that the disease can be prevented or delayed by introducing consistent lifestyle changes[204]; (2) Assessment of CV risk: Determine risk factors and calculate the patient’s individual CV risk using an objective score (e.g., Framingham) and treat risk factors accordingly. In this regard, the American College of Cardiology and the American Heart Association, in collaboration with the National Heart, Lung, and Blood Institute and other medical governing bodies, released the revised United States guidelines on CV risk assessment[216], lifestyle modification[217] and high blood cholesterol treatment[211] which provided guidelines for current management; and (3) Assessment of liver-related risk: In addition to common variables, such as a physical examination and measurements of serum aminotransferases and platelets, the use of validated scores, such as the NAFLD fibrosis score for advanced fibrosis detection, is recommended. NASH could be suspected combining routine assessed clinical variables with laboratory tests and taken into consideration the presence of metabolic syndrome. Referring the patient to a gastroenterologist for a liver biopsy is advisable if NASH, advanced fibrosis or cirrhosis is suspected. A proposed algorithm based in the presence of risk factor for NASH or fibrosis is shown in Figure 1[213].
In conclusion, the management of NAFLD is evolving; however, the current therapeutic options are limited to lifestyle changes and to the use of vitamin E and pioglitazone in selected patients with NASH. All patients with NAFLD need to be evaluated using a three-focused approach that considers the risks of developing advanced liver, T2DM and/or CV events. The Delphi technique, which is recommended for defining behaviors, is a useful tool for creating a consensus on the management of multifaceted entities, such as NAFLD.
Nonalcoholic fatty liver disease (NAFLD) is now the most common type of liver disease worldwide. The optimal treatment for NAFLD is evolving, and the current evidence-based recommendations are insufficient. To generate practical recommendations on the appropriate management of patients with NAFLD, an expert panel of members of the Chilean Gastroenterological Society and the Chilean Hepatological Association conducted a structured analysis of the current literature on NAFLD therapy.
NAFLD management is evolving, and large, well-designed trials assessing new agents with clear endpoints are being conducted. Their results are eagerly awaited.
Using the Delphi technique to reach a consensus among experts after assessing the quality of the available evidence is a reasonable approach for generating up-to-date practical recommendations on NAFLD management. A three-pronged approach that considers the liver, diabetes and cardiovascular risks in patients with NAFLD is well supported by evidence but is not emphasized in current guidelines.
The statements of the current evidence-based clinical practice review provide a rationale for current NAFLD management in clinical practice.
The Delphi technique is a method for gathering data from specialists within their domain of expertise to achieve a convergence of opinion on a specific real-world issue. The Delphi technique is well suited as a method of consensus building in scenarios where evidence-based recommendations are insufficient.
This report represents a well-constructed, evidence-based literature review and practice guidelines for Chilean hepatologists. The expert panel analyzed published clinical trials and evaluated current therapies for patients with NAFLD, especially patients with nonalcoholic steatohepatitis. The recommendations are well supported and a final consensus was reached. The consensus is well prepared with few linguistic errors. The recommendations regarding interventional options are evidence based and well balanced in terms of what should be prescribed for patients based on individual conditions. Therefore, these guidelines are instructive not only for Chilean physicians but also for those who manage patients with NAFLD in other nations.
P- Reviewer: Federico A, Teschke R, Wu J S- Editor: Gou SX L- Editor: A E- Editor: Ma S
| 1. | Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2002;17 Suppl:S186-S190. [PubMed] |
| 2. | Cheung O, Sanyal AJ. Recent advances in nonalcoholic fatty liver disease. Curr Opin Gastroenterol. 2009;25:230-237. [PubMed] |
| 3. | Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci. 2010;55:560-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 237] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 4. | Méndez-Sánchez N, Arrese M, Zamora-Valdés D, Uribe M. Current concepts in the pathogenesis of nonalcoholic fatty liver disease. Liver Int. 2007;27:423-433. [PubMed] |
| 5. | Oh MK, Winn J, Poordad F. Review article: diagnosis and treatment of non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;28:503-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Younossi ZM. Review article: current management of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2008;28:2-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Riquelme A, Arrese M, Soza A, Morales A, Baudrand R, Pérez-Ayuso RM, González R, Alvarez M, Hernández V, García-Zattera MJ. Non-alcoholic fatty liver disease and its association with obesity, insulin resistance and increased serum levels of C-reactive protein in Hispanics. Liver Int. 2009;29:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51:371-379. [PubMed] |
| 9. | Bellentani S, Marino M. Epidemiology and natural history of non-alcoholic fatty liver disease (NAFLD). Ann Hepatol. 2009;8 Suppl 1:S4-S8. [PubMed] |
| 10. | Caldwell S, Argo C. The natural history of non-alcoholic fatty liver disease. Dig Dis. 2010;28:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1012] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 12. | Méndez-Sánchez N, Arrese M, Zamora-Valdés D, Uribe M. Treating nonalcoholic fatty liver disease. Liver Int. 2007;27:1157-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 2009;49:306-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 428] [Cited by in RCA: 417] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 14. | Arab JP, Ramírez C, Arrese M. Early warning of liver disease in diabetics. Ann Hepatol. 2010;9:307-309. [PubMed] |
| 15. | Porepa L, Ray JG, Sanchez-Romeu P, Booth GL. Newly diagnosed diabetes mellitus as a risk factor for serious liver disease. CMAJ. 2010;182:E526-E531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Adams LA, Angulo P. Recent concepts in non-alcoholic fatty liver disease. Diabet Med. 2005;22:1129-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 207] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 17. | Fan JG, Farrell GC. Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol. 2009;50:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 437] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 18. | Rector RS, Thyfault JP, Wei Y, Ibdah JA. Non-alcoholic fatty liver disease and the metabolic syndrome: an update. World J Gastroenterol. 2008;14:185-192. [PubMed] |
| 19. | Trauner M, Arrese M, Wagner M. Fatty liver and lipotoxicity. Biochim Biophys Acta. 2010;1801:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 20. | Schwimmer JB, Celedon MA, Lavine JE, Salem R, Campbell N, Schork NJ, Shiehmorteza M, Yokoo T, Chavez A, Middleton MS. Heritability of nonalcoholic fatty liver disease. Gastroenterology. 2009;136:1585-1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 358] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 21. | Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113-121. [PubMed] |
| 22. | Angulo P. Long-term mortality in nonalcoholic fatty liver disease: is liver histology of any prognostic significance? Hepatology. 2010;51:373-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 23. | Edens MA, Kuipers F, Stolk RP. Non-alcoholic fatty liver disease is associated with cardiovascular disease risk markers. Obes Rev. 2009;10:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Zelber-Sagi S, Lotan R, Shibolet O, Webb M, Buch A, Nitzan-Kaluski D, Halpern Z, Santo E, Oren R. Non-alcoholic fatty liver disease independently predicts prediabetes during a 7-year prospective follow-up. Liver Int. 2013;33:1406-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, Atkins D. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20:21-35. [PubMed] |
| 26. | Sawaya GF, Guirguis-Blake J, LeFevre M, Harris R, Petitti D. Update on the methods of the U.S. Preventive Services Task Force: estimating certainty and magnitude of net benefit. Ann Intern Med. 2007;147:871-875. [PubMed] |
| 27. | American Gastroenterological Association Medical Position Statement: guidelines for the use of enteral nutrition. Gastroenterology. 1995;108:1280-1281. [PubMed] |
| 28. | Hayward RS, Wilson MC, Tunis SR, Bass EB, Guyatt G. Users’ guides to the medical literature. VIII. How to use clinical practice guidelines. A. Are the recommendations valid? The Evidence-Based Medicine Working Group. JAMA. 1995;274:570-574. [PubMed] |
| 29. | Letelier LM, Moore P. [Evidence based medicine: a view after a decade]. Rev Med Chil. 2003;131:939-946. [PubMed] |
| 30. | Guyatt GH, Sackett DL, Cook DJ. Users’ guides to the medical literature. II. How to use an article about therapy or prevention. A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA. 1993;270:2598-2601. [PubMed] |
| 31. | Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32:1008-1015. [PubMed] |
| 32. | Kantartzis K, Thamer C, Peter A, Machann J, Schick F, Schraml C, Königsrainer A, Königsrainer I, Kröber S, Niess A. High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut. 2009;58:1281-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 33. | Chen SM, Liu CY, Li SR, Huang HT, Tsai CY, Jou HJ. Effects of therapeutic lifestyle program on ultrasound-diagnosed nonalcoholic fatty liver disease. J Chin Med Assoc. 2008;71:551-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Wang CL, Liang L, Fu JF, Zou CC, Hong F, Xue JZ, Lu JR, Wu XM. Effect of lifestyle intervention on non-alcoholic fatty liver disease in Chinese obese children. World J Gastroenterol. 2008;14:1598-1602. [PubMed] |
| 35. | Sreenivasa Baba C, Alexander G, Kalyani B, Pandey R, Rastogi S, Pandey A, Choudhuri G. Effect of exercise and dietary modification on serum aminotransferase levels in patients with nonalcoholic steatohepatitis. J Gastroenterol Hepatol. 2006;21:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 36. | Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, Torimura T, Inuzuka S, Sata M, Tanikawa K. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27:103-107. [PubMed] |
| 37. | Satapathy SK, Sanyal AJ. Novel treatment modalities for nonalcoholic steatohepatitis. Trends Endocrinol Metab. 2010;21:668-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, Fowler S. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142:611-619. [PubMed] |
| 39. | Vilar Gomez E, Rodriguez De Miranda A, Gra Oramas B, Arus Soler E, Llanio Navarro R, Calzadilla Bertot L, Yasells Garcia A, Del Rosario Abreu Vazquez M. Clinical trial: a nutritional supplement Viusid, in combination with diet and exercise, in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2009;30:999-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Wang RT, Koretz RL, Yee HF. Is weight reduction an effective therapy for nonalcoholic fatty liver? A systematic review. Am J Med. 2003;115:554-559. [PubMed] |
| 41. | Moscatiello S, Di Luzio R, Bugianesi E, Suppini A, Hickman IJ, Di Domizio S, Dalle Grave R, Marchesini G. Cognitive-behavioral treatment of nonalcoholic Fatty liver disease: a propensity score-adjusted observational study. Obesity (Silver Spring). 2011;19:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Moschen AR, Tilg H. Nutrition in pathophysiology and treatment of nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care. 2008;11:620-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Bellentani S, Dalle Grave R, Suppini A, Marchesini G. Behavior therapy for nonalcoholic fatty liver disease: The need for a multidisciplinary approach. Hepatology. 2008;47:746-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 44. | Neuschwander-Tetri BA. Lifestyle modification as the primary treatment of NASH. Clin Liver Dis. 2009;13:649-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 968] [Article Influence: 64.5] [Reference Citation Analysis (1)] |
| 46. | Park HS, Kim MW, Shin ES. Effect of weight control on hepatic abnormalities in obese patients with fatty liver. J Korean Med Sci. 1995;10:414-421. [PubMed] |
| 47. | Palmer M, Schaffner F. Effect of weight reduction on hepatic abnormalities in overweight patients. Gastroenterology. 1990;99:1408-1413. [PubMed] |
| 48. | Vajro P, Mandato C, Franzese A, Ciccimarra E, Lucariello S, Savoia M, Capuano G, Migliaro F. Vitamin E treatment in pediatric obesity-related liver disease: a randomized study. J Pediatr Gastroenterol Nutr. 2004;38:48-55. [PubMed] |
| 49. | Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, Emick D, Lok AS, Conjeevaram HS. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100:1072-1081. [PubMed] |
| 50. | Hickman IJ, Jonsson JR, Prins JB, Ash S, Purdie DM, Clouston AD, Powell EE. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut. 2004;53:413-419. [PubMed] |
| 51. | Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 362] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 52. | Schäfer S, Kantartzis K, Machann J, Venter C, Niess A, Schick F, Machicao F, Häring HU, Fritsche A, Stefan N. Lifestyle intervention in individuals with normal versus impaired glucose tolerance. Eur J Clin Invest. 2007;37:535-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 53. | Gasteyger C, Larsen TM, Vercruysse F, Astrup A. Effect of a dietary-induced weight loss on liver enzymes in obese subjects. Am J Clin Nutr. 2008;87:1141-1147. [PubMed] |
| 54. | Ryan MC, Abbasi F, Lamendola C, Carter S, McLaughlin TL. Serum alanine aminotransferase levels decrease further with carbohydrate than fat restriction in insulin-resistant adults. Diabetes Care. 2007;30:1075-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 55. | Browning JD, Baker JA, Rogers T, Davis J, Satapati S, Burgess SC. Short-term weight loss and hepatic triglyceride reduction: evidence of a metabolic advantage with dietary carbohydrate restriction. Am J Clin Nutr. 2011;93:1048-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 56. | Musso G, Gambino R, De Michieli F, Cassader M, Rizzetto M, Durazzo M, Fagà E, Silli B, Pagano G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003;37:909-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 534] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 57. | Andersen T, Gluud C, Franzmann MB, Christoffersen P. Hepatic effects of dietary weight loss in morbidly obese subjects. J Hepatol. 1991;12:224-229. [PubMed] |
| 58. | Nobili V, Manco M, Devito R, Ciampalini P, Piemonte F, Marcellini M. Effect of vitamin E on aminotransferase levels and insulin resistance in children with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2006;24:1553-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 59. | Suzuki A, Lindor K, St Saver J, Lymp J, Mendes F, Muto A, Okada T, Angulo P. Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol. 2005;43:1060-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 212] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 60. | Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13206] [Cited by in RCA: 12387] [Article Influence: 538.6] [Reference Citation Analysis (1)] |
| 61. | Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1999] [Cited by in RCA: 2068] [Article Influence: 129.3] [Reference Citation Analysis (1)] |
| 62. | Rafiq N, Younossi ZM. Effects of weight loss on nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 63. | Clark JM. Weight loss as a treatment for nonalcoholic fatty liver disease. J Clin Gastroenterol. 2006;40 Suppl 1:S39-S43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 64. | Elias MC, Parise ER, de Carvalho L, Szejnfeld D, Netto JP. Effect of 6-month nutritional intervention on non-alcoholic fatty liver disease. Nutrition. 2010;26:1094-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 65. | Lazo M, Solga SF, Horska A, Bonekamp S, Diehl AM, Brancati FL, Wagenknecht LE, Pi-Sunyer FX, Kahn SE, Clark JM. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care. 2010;33:2156-2163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 258] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 66. | Peng L, Wang J, Li F. Weight reduction for non-alcoholic fatty liver disease. Cochrane Database Syst Rev. 2011;CD003619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 67. | Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 390] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 68. | Mager DR, Patterson C, So S, Rogenstein CD, Wykes LJ, Roberts EA. Dietary and physical activity patterns in children with fatty liver. Eur J Clin Nutr. 2010;64:628-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 69. | Krasnoff JB, Painter PL, Wallace JP, Bass NM, Merriman RB. Health-related fitness and physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2008;47:1158-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 70. | Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, George J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50:1105-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 440] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 71. | Ismail I, Keating SE, Baker MK, Johnson NA. A systematic review and meta-analysis of the effect of aerobic vs. resistance exercise training on visceral fat. Obes Rev. 2012;13:68-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 72. | Hayward C, Lockwood J, Williams C, Cole R, Torres D, Harrison S. Lifestyle Modification and NAFLD: A Prospective, Randomized Trial. Hepatology. 2010;52:622A. |
| 73. | Kistler KD, Brunt EM, Clark JM, Diehl AM, Sallis JF, Schwimmer JB. Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am J Gastroenterol. 2011;106:460-48; quiz 469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 334] [Cited by in RCA: 317] [Article Influence: 22.6] [Reference Citation Analysis (1)] |
| 74. | Conjeevaram HS, Tiniakos DG. Editorial: Exercise for NAFLD: does intensity matter? Am J Gastroenterol. 2011;106:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 75. | Bae JC, Suh S, Park SE, Rhee EJ, Park CY, Oh KW, Park SW, Kim SW, Hur KY, Kim JH. Regular exercise is associated with a reduction in the risk of NAFLD and decreased liver enzymes in individuals with NAFLD independent of obesity in Korean adults. PLoS One. 2012;7:e46819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 76. | St George A, Bauman A, Johnston A, Farrell G, Chey T, George J. Independent effects of physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2009;50:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 221] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 77. | Chan HL, de Silva HJ, Leung NW, Lim SG, Farrell GC. How should we manage patients with non-alcoholic fatty liver disease in 2007? J Gastroenterol Hepatol. 2007;22:801-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 78. | Devries MC, Samjoo IA, Hamadeh MJ, Tarnopolsky MA. Effect of endurance exercise on hepatic lipid content, enzymes, and adiposity in men and women. Obesity (Silver Spring). 2008;16:2281-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 79. | Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 371] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 80. | Zelber-Sagi S, Kessler A, Brazowsky E, Webb M, Lurie Y, Santo M, Leshno M, Blendis L, Halpern Z, Oren R. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006;4:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 212] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 81. | Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009;49:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 334] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 82. | Neff LM, Aronne LJ. Pharmacotherapy for obesity. Curr Atheroscler Rep. 2007;9:454-462. [PubMed] |
| 83. | Sabuncu T, Nazligul Y, Karaoglanoglu M, Ucar E, Kilic FB. The effects of sibutramine and orlistat on the ultrasonographic findings, insulin resistance and liver enzyme levels in obese patients with non-alcoholic steatohepatitis. Rom J Gastroenterol. 2003;12:189-192. [PubMed] |
| 84. | Harrison SA, Neuschwander-Tetri BA. Pharmacologic management of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:715-28, xii. [PubMed] |
| 85. | Sabuncu T, Harma M, Harma M, Nazligul Y, Kilic F. Sibutramine has a positive effect on clinical and metabolic parameters in obese patients with polycystic ovary syndrome. Fertil Steril. 2003;80:1199-1204. [PubMed] |
| 86. | Filippatos TD, Elisaf MS. Combination drug treatment in patients with non-alcoholic fatty liver disease. World J Hepatol. 2010;2:139-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 87. | James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, Torp-Pedersen C, Sharma AM, Shepherd GM, Rode RA. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363:905-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 615] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 88. | Scheen AJ. Cardiovascular risk-benefit profile of sibutramine. Am J Cardiovasc Drugs. 2010;10:321-334. [PubMed] |
| 89. | Chavez-Tapia NC, Tellez-Avila FI, Bedogni G, Crocè LS, Masutti F, Tiribelli C. Systematic review and meta-analysis on the adverse events of rimonabant treatment: considerations for its potential use in hepatology. BMC Gastroenterol. 2009;9:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Lee HK, Choi EB, Pak CS. The current status and future perspectives of studies of cannabinoid receptor 1 antagonists as anti-obesity agents. Curr Top Med Chem. 2009;9:482-503. [PubMed] |
| 91. | Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288:2793-2796. [PubMed] |
| 92. | Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683-2693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3301] [Cited by in RCA: 3026] [Article Influence: 144.1] [Reference Citation Analysis (0)] |
| 93. | Dixon JB, Bhathal PS, Hughes NR, O’Brien PE. Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 524] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 94. | Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008;6:1396-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 351] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 95. | Stephen S, Baranova A, Younossi ZM. Nonalcoholic fatty liver disease and bariatric surgery. Expert Rev Gastroenterol Hepatol. 2012;6:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 96. | De Ridder RJ, Schoon EJ, Smulders JF, van Hout GC, Stockbrügger RW, Koek GH. Review article: Non-alcoholic fatty liver disease in morbidly obese patients and the effect of bariatric surgery. Aliment Pharmacol Ther. 2007;26 Suppl 2:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 97. | Hafeez S, Ahmed MH. Bariatric surgery as potential treatment for nonalcoholic fatty liver disease: a future treatment by choice or by chance? J Obes. 2013;2013:839275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 98. | Lee YM, Low HC, Lim LG, Dan YY, Aung MO, Cheng CL, Wee A, Lim SG, Ho KY. Intragastric balloon significantly improves nonalcoholic fatty liver disease activity score in obese patients with nonalcoholic steatohepatitis: a pilot study. Gastrointest Endosc. 2012;76:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 99. | Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, Mendez-Sanchez N, Lizardi-Cervera J, Uribe M. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010;CD007340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 100. | Ratziu V, Caldwell S, Neuschwander-Tetri BA. Therapeutic trials in nonalcoholic steatohepatitis: insulin sensitizers and related methodological issues. Hepatology. 2010;52:2206-2215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 101. | Rakoski MO, Singal AG, Rogers MA, Conjeevaram H. Meta-analysis: insulin sensitizers for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2010;32:1211-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 102. | Socha P, Horvath A, Vajro P, Dziechciarz P, Dhawan A, Szajewska H. Pharmacological interventions for nonalcoholic fatty liver disease in adults and in children: a systematic review. J Pediatr Gastroenterol Nutr. 2009;48:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 103. | Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila FI, Sanchez-Avila F, Montano-Reyes MA, Uribe M. Insulin sensitizers in treatment of nonalcoholic fatty liver disease: Systematic review. World J Gastroenterol. 2006;12:7826-7831. [PubMed] |
| 104. | Utzschneider KM, Kahn SE. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753-4761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 640] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 105. | Moscatiello S, Marzocchi R, Villanova N, Bugianesi E, Marchesini G. Which treatment for nonalcoholic fatty liver disease? Mini Rev Med Chem. 2008;8:767-775. [PubMed] |
| 106. | Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, Podevin P, Lacorte JM, Bernhardt C, Bruckert E. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135:100-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 479] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 107. | Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, Doo E, Ghany M, Premkumar A, Park Y. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188-196. [PubMed] |
| 108. | Lutchman G, Modi A, Kleiner DE, Promrat K, Heller T, Ghany M, Borg B, Loomba R, Liang TJ, Premkumar A. The effects of discontinuing pioglitazone in patients with nonalcoholic steatohepatitis. Hepatology. 2007;46:424-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 173] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 109. | Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893-894. [PubMed] |
| 110. | Schwimmer JB, Middleton MS, Deutsch R, Lavine JE. A phase 2 clinical trial of metformin as a treatment for non-diabetic paediatric non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2005;21:871-879. [PubMed] |
| 111. | Nair S, Diehl AM, Wiseman M, Farr GH, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 249] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 112. | Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, David E, Rizzetto M, Marchesini G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082-1090. [PubMed] |
| 113. | Garinis GA, Fruci B, Mazza A, De Siena M, Abenavoli S, Gulletta E, Ventura V, Greco M, Abenavoli L, Belfiore A. Metformin versus dietary treatment in nonalcoholic hepatic steatosis: a randomized study. Int J Obes (Lond). 2010;34:1255-1264. [PubMed] [DOI] [Full Text] |
| 114. | Angelico F, Burattin M, Alessandri C, Del Ben M, Lirussi F. Drugs improving insulin resistance for non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2007;CD005166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 115. | Tock L, Dâmaso AR, de Piano A, Carnier J, Sanches PL, Lederman HM, Ernandes RM, de Mello MT, Tufik S. Long-term effects of metformin and lifestyle modification on nonalcoholic Fatty liver disease obese adolescents. J Obes. 2010;2010:pii 831901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 116. | Lavine JE, Schwimmer JB, Molleston JP, Scheimann AO, Murray KF, Abrams SH, Rosenthal P, Sanyal AJ, Robuck PR, Brunt EM. Treatment of nonalcoholic fatty liver disease in children: TONIC trial design. Contemp Clin Trials. 2010;31:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 117. | Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 844] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 118. | Krakoff J, Clark JM, Crandall JP, Wilson C, Molitch ME, Brancati FL, Edelstein SL, Knowler WC. Effects of metformin and weight loss on serum alanine aminotransferase activity in the diabetes prevention program. Obesity (Silver Spring). 2010;18:1762-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 119. | Shyangdan D, Clar C, Ghouri N, Henderson R, Gurung T, Preiss D, Sattar N, Fraser A, Waugh N. Insulin sensitisers in the treatment of non-alcoholic fatty liver disease: a systematic review. Health Technol Assess. 2011;15:1-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 120. | Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1612] [Cited by in RCA: 1539] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 121. | Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 437] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 122. | Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38:1008-1017. [PubMed] |
| 123. | Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307. [PubMed] |
| 124. | Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, Austin AS, Freeman JG, Morgan L, Webber J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 554] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 125. | Gupta AK, Bray GA, Greenway FL, Martin CK, Johnson WD, Smith SR. Pioglitazone, but not metformin, reduces liver fat in Type-2 diabetes mellitus independent of weight changes. J Diabetes Complications. 2010;24:289-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 126. | Ratziu V, Charlotte F, Bernhardt C, Giral P, Halbron M, Lenaour G, Hartmann-Heurtier A, Bruckert E, Poynard T. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010;51:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 292] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 127. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2458] [Article Influence: 163.9] [Reference Citation Analysis (2)] |
| 128. | Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 129. | Taylor C, Hobbs FD. Type 2 diabetes, thiazolidinediones, and cardiovascular risk. Br J Gen Pract. 2009;59:520-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 130. | Zambon A, Cusi K. The role of fenofibrate in clinical practice. Diab Vasc Dis Res. 2007;4 Suppl 3:S15-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 131. | Liberopoulos EN, Athyros VG, Elisaf MS, Mikhailidis DP. Statins for non-alcoholic fatty liver disease: a new indication? Aliment Pharmacol Ther. 2006;24:698-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 132. | Basaranoglu M, Acbay O, Sonsuz A. A controlled trial of gemfibrozil in the treatment of patients with nonalcoholic steatohepatitis. J Hepatol. 1999;31:384. [PubMed] |
| 133. | Laurin J, Lindor KD, Crippin JS, Gossard A, Gores GJ, Ludwig J, Rakela J, McGill DB. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology. 1996;23:1464-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 360] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 134. | Fabbrini E, Mohammed BS, Korenblat KM, Magkos F, McCrea J, Patterson BW, Klein S. Effect of fenofibrate and niacin on intrahepatic triglyceride content, very low-density lipoprotein kinetics, and insulin action in obese subjects with nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2010;95:2727-2735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 135. | Athyros VG, Mikhailidis DP, Didangelos TP, Giouleme OI, Liberopoulos EN, Karagiannis A, Kakafika AI, Tziomalos K, Burroughs AK, Elisaf MS. Effect of multifactorial treatment on non-alcoholic fatty liver disease in metabolic syndrome: a randomised study. Curr Med Res Opin. 2006;22:873-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 192] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 136. | Hyogo H, Tazuma S, Arihiro K, Iwamoto K, Nabeshima Y, Inoue M, Ishitobi T, Nonaka M, Chayama K. Efficacy of atorvastatin for the treatment of nonalcoholic steatohepatitis with dyslipidemia. Metabolism. 2008;57:1711-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 137. | Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized placebo-controlled trial. J Clin Gastroenterol. 2009;43:990-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 206] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 138. | Hatzitolios A, Savopoulos C, Lazaraki G, Sidiropoulos I, Haritanti P, Lefkopoulos A, Karagiannopoulou G, Tzioufa V, Dimitrios K. Efficacy of omega-3 fatty acids, atorvastatin and orlistat in non-alcoholic fatty liver disease with dyslipidemia. Indian J Gastroenterol. 2004;23:131-134. [PubMed] |
| 139. | Kiyici M, Gulten M, Gurel S, Nak SG, Dolar E, Savci G, Adim SB, Yerci O, Memik F. Ursodeoxycholic acid and atorvastatin in the treatment of nonalcoholic steatohepatitis. Can J Gastroenterol. 2003;17:713-718. [PubMed] |
| 140. | Abel T, Fehér J, Dinya E, Eldin MG, Kovács A. Safety and efficacy of combined ezetimibe/simvastatin treatment and simvastatin monotherapy in patients with non-alcoholic fatty liver disease. Med Sci Monit. 2009;15:MS6-M11. [PubMed] |
| 141. | Mihaila RG, Nedelcu L, Fratila O, Rezi EC, Domnariu C, Deac M. Effects of lovastatin and pentoxyphyllin in nonalcoholic steatohepatitis. Hepatogastroenterology. 2009;56:1117-1121. [PubMed] |
| 142. | Rallidis LS, Drakoulis CK, Parasi AS. Pravastatin in patients with nonalcoholic steatohepatitis: results of a pilot study. Atherosclerosis. 2004;174:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 143. | Gómez-Domínguez E, Gisbert JP, Moreno-Monteagudo JA, García-Buey L, Moreno-Otero R. A pilot study of atorvastatin treatment in dyslipemid, non-alcoholic fatty liver patients. Aliment Pharmacol Ther. 2006;23:1643-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 144. | Foster T, Budoff MJ, Saab S, Ahmadi N, Gordon C, Guerci AD. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the St Francis Heart Study randomized clinical trial. Am J Gastroenterol. 2011;106:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 145. | Chalasani N. Statins and hepatotoxicity: focus on patients with fatty liver. Hepatology. 2005;41:690-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 181] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 146. | Vuppalanchi R, Teal E, Chalasani N. Patients with elevated baseline liver enzymes do not have higher frequency of hepatotoxicity from lovastatin than those with normal baseline liver enzymes. Am J Med Sci. 2005;329:62-65. [PubMed] |
| 147. | Ekstedt M, Franzén LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: a histopathological follow-up study. J Hepatol. 2007;47:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 195] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 148. | Riley P, Al Bakir M, O’Donohue J, Crook M. Prescribing statins to patients with nonalcoholic fatty liver disease: real cardiovascular benefits outweigh theoretical hepatotoxic risk. Cardiovasc Ther. 2009;27:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 149. | Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, Pagourelias ED, Theocharidou E, Karagiannis A, Mikhailidis DP. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376:1916-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 500] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 150. | Tzefos M, Olin JL. 3-hydroxyl-3-methylglutaryl coenzyme A reductase inhibitor use in chronic liver disease: a therapeutic controversy. J Clin Lipidol. 2011;5:450-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 151. | Rudovich NN, Weickert MO, Machann J, Pfeiffer AF. Combination of acarbose and ezetimibe prevents non-alcoholic fatty liver disease: a break of intestinal insulin resistance? J Hepatol. 2010;52:952-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 152. | Enjoji M, Nakamuta M. Is the control of dietary cholesterol intake sufficiently effective to ameliorate nonalcoholic fatty liver disease? World J Gastroenterol. 2010;16:800-803. [PubMed] |
| 153. | Zheng S, Hoos L, Cook J, Tetzloff G, Davis H, van Heek M, Hwa JJ. Ezetimibe improves high fat and cholesterol diet-induced non-alcoholic fatty liver disease in mice. Eur J Pharmacol. 2008;584:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 154. | Nomura M, Ishii H, Kawakami A, Yoshida M. Inhibition of hepatic Niemann-Pick C1-like 1 improves hepatic insulin resistance. Am J Physiol Endocrinol Metab. 2009;297:E1030-E1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 155. | Ahmed MH, Byrne CD. Ezetimibe as a potential treatment for non-alcoholic fatty liver disease: is the intestine a modulator of hepatic insulin sensitivity and hepatic fat accumulation? Drug Discov Today. 2010;15:590-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 156. | Yamagishi S, Nakamura K, Matsui T, Sato T, Takeuchi M. Inhibition of intestinal cholesterol absorption by ezetimibe is a novel therapeutic target for fatty liver. Med Hypotheses. 2006;66:844-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 157. | Hughes EA, Tracey I, Singhal S, Patel J. Unexpected beneficial effect in the use of ezetimibe in non-alcoholic fatty liver disease. Med Hypotheses. 2006;67:1463-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 158. | Patel JV, Hughes EA. Efficacy, safety and LDL-C goal attainment of ezetimibe 10 mg-simvastatin 20 mg vs. placebo-simvastatin 20 mg in UK-based adults with coronary heart disease and hypercholesterolaemia. Int J Clin Pract. 2006;60:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 159. | Chan DC, Watts GF, Gan SK, Ooi EM, Barrett PH. Effect of ezetimibe on hepatic fat, inflammatory markers, and apolipoprotein B-100 kinetics in insulin-resistant obese subjects on a weight loss diet. Diabetes Care. 2010;33:1134-1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 160. | Enjoji M, Machida K, Kohjima M, Kato M, Kotoh K, Matsunaga K, Nakashima M, Nakamuta M. NPC1L1 inhibitor ezetimibe is a reliable therapeutic agent for non-obese patients with nonalcoholic fatty liver disease. Lipids Health Dis. 2010;9:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 161. | Ahmed MH, Saad RA, Osman MM. Ezetimibe: effective and safe treatment for dyslipidaemia associated with nonalcoholic fatty liver disease. Response to: Toth PP, Davidson MH: simvastatin and ezetimibe: combination therapy for the management of dyslipidaemia. Expert Opin. Pharmacother. (2005) 6(1): 131-139. Expert Opin Drug Saf. 2006;5:487-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 162. | Toth PP, Davidson MH. Simvastatin plus ezetimibe: combination therapy for the management of dyslipidaemia. Expert Opin Pharmacother. 2005;6:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 163. | Yoneda M, Fujita K, Imajo K, Mawatari H, Kirikoshi H, Saito S, Nakajima A. Induction of microsomal triglyceride transfer protein expression is a candidate mechanism by which ezetimibe therapy might exert beneficial effects in patients with nonalcoholic steatohepatitis. J Gastroenterol. 2011;46:415-46; author reply 417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 164. | Yoneda M, Fujita K, Nozaki Y, Endo H, Takahashi H, Hosono K, Suzuki K, Mawatari H, Kirikoshi H, Inamori M. Efficacy of ezetimibe for the treatment of non-alcoholic steatohepatitis: An open-label, pilot study. Hepatol Res. 2010;40:566-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 165. | Park H, Shima T, Yamaguchi K, Mitsuyoshi H, Minami M, Yasui K, Itoh Y, Yoshikawa T, Fukui M, Hasegawa G. Efficacy of long-term ezetimibe therapy in patients with nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 166. | Yokohama S, Yoneda M, Haneda M, Okamoto S, Okada M, Aso K, Hasegawa T, Tokusashi Y, Miyokawa N, Nakamura K. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology. 2004;40:1222-1225. [PubMed] |
| 167. | Paschos P, Tziomalos K. Nonalcoholic fatty liver disease and the renin-angiotensin system: Implications for treatment. World J Hepatol. 2012;4:327-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 168. | Georgescu EF, Ionescu R, Niculescu M, Mogoanta L, Vancica L. Angiotensin-receptor blockers as therapy for mild-to-moderate hypertension-associated non-alcoholic steatohepatitis. World J Gastroenterol. 2009;15:942-954. [PubMed] |
| 169. | Eriksson JW, Jansson PA, Carlberg B, Hägg A, Kurland L, Svensson MK, Ahlström H, Ström C, Lönn L, Ojbrandt K. Hydrochlorothiazide, but not Candesartan, aggravates insulin resistance and causes visceral and hepatic fat accumulation: the mechanisms for the diabetes preventing effect of Candesartan (MEDICA) Study. Hypertension. 2008;52:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 170. | Fogari R, Maffioli P, Mugellini A, Zoppi A, Lazzari P, Derosa G. Effects of losartan and amlodipine alone or combined with simvastatin in hypertensive patients with nonalcoholic hepatic steatosis. Eur J Gastroenterol Hepatol. 2012;24:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 171. | Scheen AJ. Renin-angiotensin system inhibition prevents type 2 diabetes mellitus. Part 2. Overview of physiological and biochemical mechanisms. Diabetes Metab. 2004;30:498-505. [PubMed] |
| 172. | Scheen AJ. Renin-angiotensin system inhibition prevents type 2 diabetes mellitus. Part 1. A meta-analysis of randomised clinical trials. Diabetes Metab. 2004;30:487-496. [PubMed] |
| 173. | Vitale C, Mercuro G, Castiglioni C, Cornoldi A, Tulli A, Fini M, Volterrani M, Rosano GM. Metabolic effect of telmisartan and losartan in hypertensive patients with metabolic syndrome. Cardiovasc Diabetol. 2005;4:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 174. | Sloniger JA, Saengsirisuwan V, Diehl CJ, Kim JS, Henriksen EJ. Selective angiotensin II receptor antagonism enhances whole-body insulin sensitivity and muscle glucose transport in hypertensive TG(mREN2)27 rats. Metabolism. 2005;54:1659-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 175. | Loria P, Adinolfi LE, Bellentani S, Bugianesi E, Grieco A, Fargion S, Gasbarrini A, Loguercio C, Lonardo A, Marchesini G. Practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease. A decalogue from the Italian Association for the Study of the Liver (AISF) Expert Committee. Dig Liver Dis. 2010;42:272-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 176. | Hasegawa T, Yoneda M, Nakamura K, Makino I, Terano A. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: a pilot study. Aliment Pharmacol Ther. 2001;15:1667-1672. [PubMed] |
| 177. | Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr. 2000;136:734-738. [PubMed] |
| 178. | Lirussi F, Azzalini L, Orando S, Orlando R, Angelico F. Antioxidant supplements for non-alcoholic fatty liver disease and/or steatohepatitis. Cochrane Database Syst Rev. 2007;CD004996. [PubMed] |
| 179. | Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485-2490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 483] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 180. | Nobili V, Manco M, Devito R, Di Ciommo V, Comparcola D, Sartorelli MR, Piemonte F, Marcellini M, Angulo P. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: a randomized, controlled trial. Hepatology. 2008;48:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 293] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 181. | Balmer ML, Siegrist K, Zimmermann A, Dufour JF. Effects of ursodeoxycholic acid in combination with vitamin E on adipokines and apoptosis in patients with nonalcoholic steatohepatitis. Liver Int. 2009;29:1184-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 182. | Kadayifci A, Merriman RB. Metformin, vitamin E, and diet for patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2006;101:1396; author reply 1396-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 183. | Ersöz G, Günşar F, Karasu Z, Akay S, Batur Y, Akarca US. Management of fatty liver disease with vitamin E and C compared to ursodeoxycholic acid treatment. Turk J Gastroenterol. 2005;16:124-128. [PubMed] |
| 184. | Bernal-Reyes R, Escudero RB. [Treatment of non-alcoholic steatohepatitis (NASH). A comparative study of ursodeoxycholic acid and alpha-tocopherol. A preliminary report]. Rev Gastroenterol Mex. 2002;67:70-75. [PubMed] |
| 185. | Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, Zala JF, Helbling B, Steuerwald M, Zimmermann A. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006;4:1537-1543. [PubMed] |
| 186. | Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1614] [Cited by in RCA: 1399] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 187. | Gerss J, Köpcke W. The questionable association of vitamin E supplementation and mortality--inconsistent results of different meta-analytic approaches. Cell Mol Biol (Noisy-le-grand). 2009;55 Suppl:OL1111-OL1120. [PubMed] |
| 188. | Parker HM, Johnson NA, Burdon CA, Cohn JS, O’Connor HT, George J. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;56:944-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 407] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 189. | Koppe SW, Sahai A, Malladi P, Whitington PF, Green RM. Pentoxifylline attenuates steatohepatitis induced by the methionine choline deficient diet. J Hepatol. 2004;41:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 190. | Duman DG, Ozdemir F, Birben E, Keskin O, Ekşioğlu-Demiralp E, Celikel C, Kalayci O, Kalayci C. Effects of pentoxifylline on TNF-alpha production by peripheral blood mononuclear cells in patients with nonalcoholic steatohepatitis. Dig Dis Sci. 2007;52:2520-2524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 191. | Lee YM, Sutedja DS, Wai CT, Dan YY, Aung MO, Zhou L, Cheng CL, Wee A, Lim SG. A randomized controlled pilot study of Pentoxifylline in patients with non-alcoholic steatohepatitis (NASH). Hepatol Int. 2008;2:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 192. | Sturm N, Bronowicki JP, Maynard-Muet M, Tran A, Heluwaert F, Plages A, Zarski JP. Metformin plus pentoxifylline versus prescriptive diet in non-alcoholic steatohepatitis (NASH): a randomized controlled pilot trial. Gastroenterol Clin Biol. 2009;33:984-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 193. | Satapathy SK, Garg S, Chauhan R, Sakhuja P, Malhotra V, Sharma BC, Sarin SK. Beneficial effects of tumor necrosis factor-alpha inhibition by pentoxifylline on clinical, biochemical, and metabolic parameters of patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1946-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 146] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 194. | Satapathy SK, Sakhuja P, Malhotra V, Sharma BC, Sarin SK. Beneficial effects of pentoxifylline on hepatic steatosis, fibrosis and necroinflammation in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2007;22:634-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 195. | Adams LA, Zein CO, Angulo P, Lindor KD. A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:2365-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 196. | Li W, Zheng L, Sheng C, Cheng X, Qing L, Qu S. Systematic review on the treatment of pentoxifylline in patients with non-alcoholic fatty liver disease. Lipids Health Dis. 2011;10:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 197. | Buranawati W, Thoun-U-Thaisri , Pramoolsinsup C, Wisedopas N, Atamasirikul K, Udomsubpayakul U. Pentoxifylline for Treatment of Nonalcoholic Fatty Liver Disease (NAFLD): A Randomized, Placebo-Controlled Study. Thai J Gastroenterol. 2007;8:57-63. |
| 198. | Zein CO, Gogate P, Yerian L, Kirwan JP, McCullough A. Pentoxifylline Improves Nonalcoholic Steatohepatitis: Results of a Doubleblinded, Randomized, Placebo-Controlled Trial. Am J Gastroenterol. 2010;105:S114. |
| 199. | Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE, McCullough AJ. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610-1619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 275] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 200. | Van Wagner LB, Koppe SW, Brunt EM, Gottstein J, Gardikiotes K, Green RM, Rinella ME. Pentoxifylline for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. Ann Hepatol. 2011;10:277-286. [PubMed] |
| 201. | Orlando R, Azzalini L, Orando S, Lirussi F. Bile acids for non-alcoholic fatty liver disease and/or steatohepatitis. Cochrane Database Syst Rev. 2007;CD005160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 202. | Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, Lymp JF, Burgart L, Colin P. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770-778. [PubMed] |
| 203. | Leuschner UF, Lindenthal B, Herrmann G, Arnold JC, Rössle M, Cordes HJ, Zeuzem S, Hein J, Berg T. High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a double-blind, randomized, placebo-controlled trial. Hepatology. 2010;52:472-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 230] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 204. | Kahn R, Davidson MB. The reality of type 2 diabetes prevention. Diabetes Care. 2014;37:943-949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 205. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2611] [Article Influence: 200.8] [Reference Citation Analysis (1)] |
| 206. | FDA. Information on Orlistat. 2010; Available from: http://www.fda.gov/. |
| 207. | Stinton LM, Loomba R. Recommendations for liver biopsy evaluation in non-alcoholic fatty liver disease. Minerva Gastroenterol Dietol. 2014;60:5-13. [PubMed] |
| 208. | Teschke R, Frenzel C. Drug induced liver injury: do we still need a routine liver biopsy for diagnosis today? Ann Hepatol. 2013;13:121-126. [PubMed] |
| 209. | Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:475-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 370] [Cited by in RCA: 466] [Article Influence: 42.4] [Reference Citation Analysis (2)] |
| 210. | Mosko JD, Nguyen GC. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:897-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 211. | Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2564] [Cited by in RCA: 2880] [Article Influence: 240.0] [Reference Citation Analysis (0)] |
| 212. | Dasarathy S, Dasarathy J, Khiyami A, Yerian L, Hawkins C, Sargent R, McCullough AJ. Double-blind Randomized Placebo-controlled Clinical Trial of Omega 3 Fatty Acids for the Treatment of Diabetic Patients With Nonalcoholic Steatohepatitis. J Clin Gastroenterol. 2014;Feb 27; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 213. | Corrado RL, Torres DM, Harrison SA. Review of treatment options for nonalcoholic fatty liver disease. Med Clin North Am. 2014;98:55-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 214. | Du J, Ma YY, Yu CH, Li YM. Effects of pentoxifylline on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2014;20:569-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 85] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 215. | Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1317] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 216. | Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935-2959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1815] [Cited by in RCA: 1998] [Article Influence: 181.6] [Reference Citation Analysis (0)] |
| 217. | Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2960-2984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 802] [Cited by in RCA: 828] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 218. | Scaglioni F, Marino M, Ciccia S, Procaccini A, Busacchi M, Loria P, Lonardo A, Malavolti M, Battistini NC, Pellegrini M. Short-term multidisciplinary non-pharmacological intervention is effective in reducing liver fat content assessed non-invasively in patients with nonalcoholic fatty liver disease (NAFLD). Clin Res Hepatol Gastroenterol. 2013;37:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 219. | Tendler D, Lin S, Yancy WS, Mavropoulos J, Sylvestre P, Rockey DC, Westman EC. The effect of a low-carbohydrate, ketogenic diet on nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci. 2007;52:589-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 220. | Thomas EL, Brynes AE, Hamilton G, Patel N, Spong A, Goldin RD, Frost G, Bell JD, Taylor-Robinson SD. Effect of nutritional counselling on hepatic, muscle and adipose tissue fat content and distribution in non-alcoholic fatty liver disease. World J Gastroenterol. 2006;12:5813-5819. [PubMed] |
| 221. | Okita M, Hayashi M, Sasagawa T, Takagi K, Suzuki K, Kinoyama S, Ito T, Yamada G. Effect of a moderately energy-restricted diet on obese patients with fatty liver. Nutrition. 2001;17:542-547. [PubMed] |
| 222. | Knobler H, Schattner A, Zhornicki T, Malnick SD, Keter D, Sokolovskaya N, Lurie Y, Bass DD. Fatty liver--an additional and treatable feature of the insulin resistance syndrome. QJM. 1999;92:73-79. [PubMed] |
| 223. | Eriksson S, Eriksson KF, Bondesson L. Nonalcoholic steatohepatitis in obesity: a reversible condition. Acta Med Scand. 1986;220:83-88. [PubMed] |