Published online Sep 14, 2014. doi: 10.3748/wjg.v20.i34.12171
Revised: May 23, 2014
Accepted: June 21, 2014
Published online: September 14, 2014
Processing time: 178 Days and 6.4 Hours
AIM: To evaluate the significance of KL-6/MUC1 (a type of MUC1) glycosylation in pancreatic cancer progression.
METHODS: KL-6/MUC1 expression was detected by immunohistochemistry in 48 patients with pancreatic duct cell carcinoma. The N-/O-glycosylation inhibitors (tunicamycin and benzyl-N-acetyl-α-galactosaminide) were then used to interfere with KL-6/MUC1 glycosylation in two pancreatic carcinoma cell lines, and the effects on KL-6/MUC1 expression, and cell adhesion and invasion were determined. In addition, protein expression of epithelial-mesenchymal transition markers, E-cadherin and vimentin, were evaluated in cells after treatment with glycosylation inhibitors.
RESULTS: Overexpression of KL-6/MUC1 was found in all pancreatic cancer tissues, but not in the surrounding normal pancreatic tissues. The expression profile of KL-6/MUC1 was significantly decreased after treatment with the inhibitors. The adhesion and invasive ability of cancer cells were significantly decreased after drug treatment, and increased E-cadherin and decreased vimentin expression were found.
CONCLUSION: KL-6/MUC1 glycosylation is involved in pancreatic cancer metastasis and invasion. Therapeutic strategies which target this may help control the aggressive behavior of pancreatic cancer cells.
Core tip: Aberrant glycosylation of MUC1 is associated with aggressive tumor behavior in many carcinomas. This study evaluated the significance of KL-6/MUC1 glycosylation in pancreatic cancer progression. The results indicate the important involvement of KL-6/MUC1 glycosylation in pancreatic cancer metastasis and invasion, which may be related to the epithelial-mesenchymal transition process. Therapeutic strategies which target glycosylation of KL-6/MUC1 may be useful for controlling the aggressive behavior of pancreatic cancer cells.
- Citation: Xu HL, Zhao X, Zhang KM, Tang W, Kokudo N. Inhibition of KL-6/MUC1 glycosylation limits aggressive progression of pancreatic cancer. World J Gastroenterol 2014; 20(34): 12171-12181
- URL: https://www.wjgnet.com/1007-9327/full/v20/i34/12171.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i34.12171
MUC1 is a polymorphic, highly glycosylated, type I transmembrane glycoprotein expressed by ductal epithelial cells of secretory organs, including the pancreas, breast, lung, and gastrointestinal tract, and is aberrantly glycosylated in most cases[1]. The deduced amino acid sequence of MUC1 mucin has four distinct domains: the NH2-terminal domain consisting of a hydrophobic signal sequence, a highly O-glycosylated tandem-repeat domain, a transmembrane domain, and a cytoplasmic domain[1,2]. Many studies have suggested that aberrant glycosylation of MUC1 plays an important role in cancer metastasis and MUC1 seems to be an attractive target for cancer treatment[3].
KL-6/MUC1, a type of MUC1 categorized as cluster 9, is recognized by the KL-6 antibody[4]. This mucin was first recognized in the serum of patients with intestinal pneumonia, but has recently been detected in various cancer tissues[5,6]. Our previous studies suggested that aberrant expression of KL-6/MUC1 in cancer tissues was associated with worse tumor behavior, such as invasion and metastasis in ampullary carcinoma, primary colorectal carcinoma, and cholangiocarcinoma[6-9].
Pancreatic duct cell carcinoma (PDC) is one of the most malignant gastrointestinal tumors, with a five-year overall survival rate of less than 5%. Once PDC is clinically evident, it rapidly metastasizes and this event often occurs by the time of diagnosis[10]. Thus, control of micrometastatic pancreatic cancer is a major objective in PDC treatment. It was shown that the aberrant expression of MUC1, detected using CD227, DF3, and CA15-3 antibodies, plays an important role in pancreatic cancer metastasis and invasion[11,12]. Thus, in this study, we first performed an immunocytochemical analysis to evaluate KL-6/MUC1 expression in PDC tissues using the KL-6 antibody, and then evaluated the significance of KL-6/MUC1 glycosylation in pancreatic cancer cell lines.
PDC specimens were collected from 48 patients: 28 men and 20 women, average age 66.1 years (range: 41-80 years). Intraductal papillary mucinous tumor (IPMT) tissues were resected from 12 patients: 8 men and 4 women, average age 45.6 years (range: 40-67 years). These patients underwent surgical resection at the Hepato-Biliary-Pancreatic Surgery Division of the Department of Surgery, Graduate School of Medicine, University of Tokyo between January 2000 and December 2005. This study was approved by the Institutional Review Board of the University of Tokyo. Written informed consent was obtained from each patient. The clinicopathologic characteristics of the patients were evaluated according to the general rules for the study of pancreatic cancer by the Japan Pancreas Society[13].
KL-6/MUC1 expression was detected by immunohistochemical staining as described previously[7]. Briefly, 5-μm-thick sections were cut from archival formalin-fixed paraffin-embedded tissue blocks, deparaffinized, and dehydrated. Endogenous peroxidase activity was halted by the addition of 0.3% hydrogen peroxide/methanol for 30 min. The sections were blocked with normal goat serum for 30 min, and then incubated with or without KL-6 antibody (1:200 dilution; Eisai Co., Ltd, Tokyo, Japan) for 60 min. Following incubation with biotin-labeled secondary antibody, KL-6/MUC1 was detected using the Histofine SAB-PO kit (Nichirei Corporation, Tokyo, Japan). 3,3’-diaminobenzidine was used as the chromogen, and hematoxylin was used as a counterstain. Phosphate-buffered saline was used instead of primary antibody as a negative control. The percentage of stained cells was determined in ten random microscopic fields of each tissue sample or in the entire region if the tissue sample consisted of fewer than ten fields (magnification × 200). In the evaluation of KL-6/MUC1 staining, cases with positivity observed in more than 10% of cancer cells were considered positive according to a previous report[7].
Panc-1 and Capan-1 cell lines were obtained from ATCC (Rockville, MD, United States). Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum in a humidified atmosphere with 5% CO2 at 37 °C. For maintenance and subculture, cells in the exponential phase were treated with 0.25% trypsin solution containing 0.02% ethylenediaminetetraacetic acid. Inhibitors were dissolved in dimethyl sulfoxide and diluted with culture medium to a final concentration of < 0.1%.
Cells were reseeded in 96-well plates (3 × 103 cells/well) and incubated with tunicamycin (0.5, 1.0, 2.5, 5.0, and 10 mg%) and benzyl-N-acetyl-α-galactosaminide (BAG; 2.5, 5.0, 10, 20, and 40 mmol/L) for 24, 48, 72, and 96 h. The cells without drug treatment were used as controls. Cell proliferation was assessed by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay. Absorbance was detected at 550 nm with 750 nm as a reference wavelength.
KL-6/MUC1 expression in Panc-1 and Capan-1 cells with or without glycosylation inhibitor treatment was detected by immunocytochemistry. Briefly, cells (1 × 104) were seeded on MAS-coated slides (Matsunami Inc., Osaka, Japan) and incubated overnight. Forty-eight hours after treatment with 2.0 mg% tunicamycin and 5.0 mmol/L BAG, the cells were fixed with 3.7% paraformaldehyde for 30 min followed by incubation in a solution of 0.2% Triton X-100 for 10 min. The cells were blocked with normal goat serum for 30 min at room temperature, and then incubated with or without KL-6 antibody (1:200 dilution) for 60 min at room temperature. Following incubation with biotin-labeled secondary antibody, KL-6/MUC1 was detected by the biotin streptavidin-peroxidase complex method using the Histofine SAB-PO kit with 3,3’-diaminobenzidine as the chromogen and hematoxylin as a counterstain.
The cell adhesion assay was performed in Biocoat Matrigel Matrix culture dishes (Thin Layer 100 mg/cm2, 35 mm; BD Biosciences, San Jose, CA, United States). Dishes were rehydrated for 30 min at 37 °C with 1 mL of DMEM supplemented with 10% heat-inactivated fetal bovine serum. Cells for adhesion assays were seeded at a density of 5 × 104 cells/well in culture dishes containing 2.0 mg% tunicamycin or 5.0 mmol/L BAG in non-serum culture medium. Dishes without drug treatment were used as controls. Cells were allowed to adhere for 48 h at 37 °C. Non-adherent cells were removed by washing three times with phosphate-buffered saline. Adherent cells were fixed and stained with the Diff-Quik kit (Dade Behring of Siemens AG, Munich, Germany), according to the manufacturer’s instructions. Stained cells were counted under a microscope at 400 × magnification and the mean cell numbers in eight random fields were calculated. Data were calculated from three separate experiments performed in triplicate.
The motility and invasive ability of cells were assessed in 24-well Transwell plates (Corning Inc., Corning, NY, United States). Cells were pre-incubated with or without 2.0 mg% tunicamycin or 5.0 mmol/L BAG for 24 h at 37 °C in a CO2 incubator and then detached and resuspended in serum-free DMEM. Dishes without drug treatment were used as controls. A suspension of cells (2 × 105 cells) with or without drug treatment was placed in the upper chambers. The lower chambers were filled with 0.75 mL of medium supplemented with 5% fetal bovine serum. Cells were allowed to migrate for 22 h at 37 °C. Migration was terminated by removing the cells from the upper compartment of the filter with a cotton swab. Cells that had invaded the Matrigel and reached the lower surface of the filter were stained using the Diff-Quik kit (Dade Behring). The migrated cells were quantified by counting the number of cells in eight random microscopic fields per filter (magnification × 200).
Immunofluorescence staining was used to detect the expression of KL-6/MUC1 and E-cadherin. Briefly, 48 h after treatment with 2.0 mg% tunicamycin and 5.0 mmol/L BAG, cells were fixed with 3.7% formaldehyde for 10 min, rinsed and permeabilized with a solution of 0.2% Triton-X 100 for 30 min. After blocking, the cells were incubated with a mixture of KL-6 (1:200) and E-cadherin (1:200; BD Biosciences) antibodies overnight at 4 °C. After rinsing, the cells were incubated at 37 °C for 1 h with diluted secondary antibody (1:200 anti-mouse IgG1-FITC and anti-mouse IgG2a-PE; BD Biosciences). The cells were then fixed and covered with mounting medium with DAPI. Fluorescence images were obtained using a fluorescence microscope (model BZ-9000; Keyence, Osaka, Japan).
Following drug treatment for 48 h, cell lysates were prepared using radio-immunoprecipitation assay (RIPA) lysis buffer. Equal amounts of protein (20 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and then electrotransferred onto polyvinylidene difluoride membranes. After blocking with 5% bovine serum albumin overnight at 4 °C, the membranes were incubated with different antibodies (E-cadherin, 1:1000; vimentin, 1:1000; β-actin, 1:1000; BD Biosciences) for 1 h, followed by washing three times. After incubation with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature, the reactivity was visualized by enhanced chemiluminescence using the ECL Western Blotting Starter Kit.
Treatments were compared to controls using Student’s two-tailed t-tests, with P < 0.05 indicating significance.
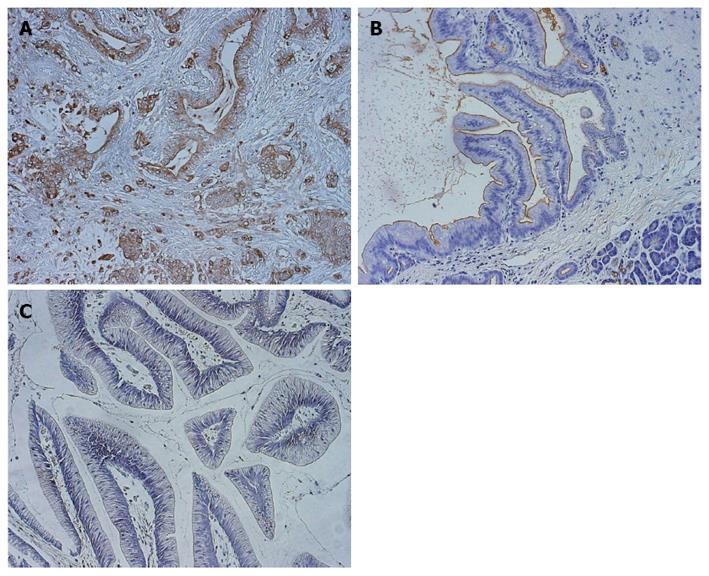
Immunohistochemical analysis was performed to determine the expression profile of KL-6/MUC1 in pancreatic cancer tissues. All 48 cases of PDC showed positive staining for KL-6/MUC1. Tumor-cell cytoplasm and luminal surfaces, as well as the inner surfaces of glands in cancer regions, were strongly stained (Figure 1A and B). The population of KL-6/MUC1-positive tumor cells in these cancer regions varied among the samples (mean 73.6%, range: 17.0%-95.0%). Positive staining was rarely observed in the surrounding normal pancreatic tissues (Figure 1B). In all 48 cases of PDC, venous invasion, lymphatic invasion, as well as liver or lymph node metastasis were found. Positive staining was not found in any of the IPMT tissues (0/12, 0%). None of the IPMT cases had venous or lymphatic invasion, or liver or lymph node metastasis (Figure 1C).
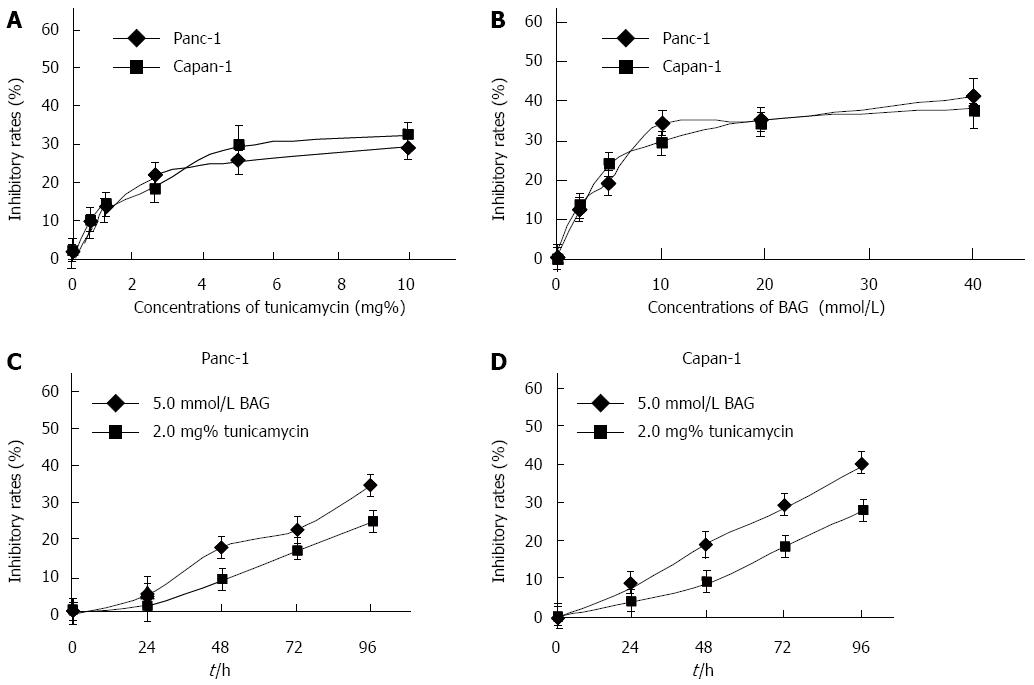
The effects of tunicamycin and BAG on Panc-1 and Capan-1 cell proliferation were analyzed by MTT assays. The results show that tunicamycin and BAG inhibited the proliferation of both cell lines in a concentration-dependent manner (Figure 2A and B). Significant inhibition of Panc-1 and Capan-1 cell proliferation was not observed in the presence of < 2.0 mg% tunicamycin and < 5.0 mmol/L BAG, with an inhibitory rate of < 20%. A time-dependent inhibition of cell proliferation was also observed in the presence of 2.0 mg% tunicamycin and 5 mmol/L BAG in both cell lines (Figure 2C and D). Thus, in subsequent experiments, the cells were treated with 2.0 mg% tunicamycin or 5 mmol/L BAG for 48 h.
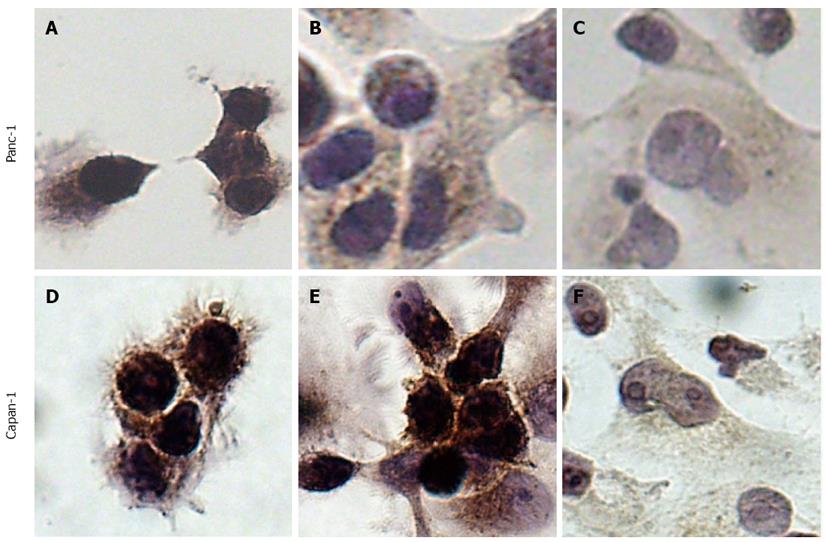
To examine subcellular expression of KL-6/MUC1, Panc-1 and Capan-1 cells with or without tunicamycin and BAG treatment were subjected to immunocytochemical detection (Figure 3). Before glycosylation inhibitor treatment of Panc-1 and Capan-1 cells, intense staining was observed throughout the entire cell, including cell membrane and cytoplasm (Figure 3A and D), indicating high expression of KL-6/MUC1 in both cell lines. After tunicamycin treatment, a subtle decrease in KL-6/MUC1 staining in both the cell membrane and cytoplasm of Panc-1 and Capan-1 cells was observed (Figure 3B and E). After BAG treatment, KL-6/MUC1 staining was significantly decreased in both cell lines, especially in the cell membrane (Figure 3C and F).
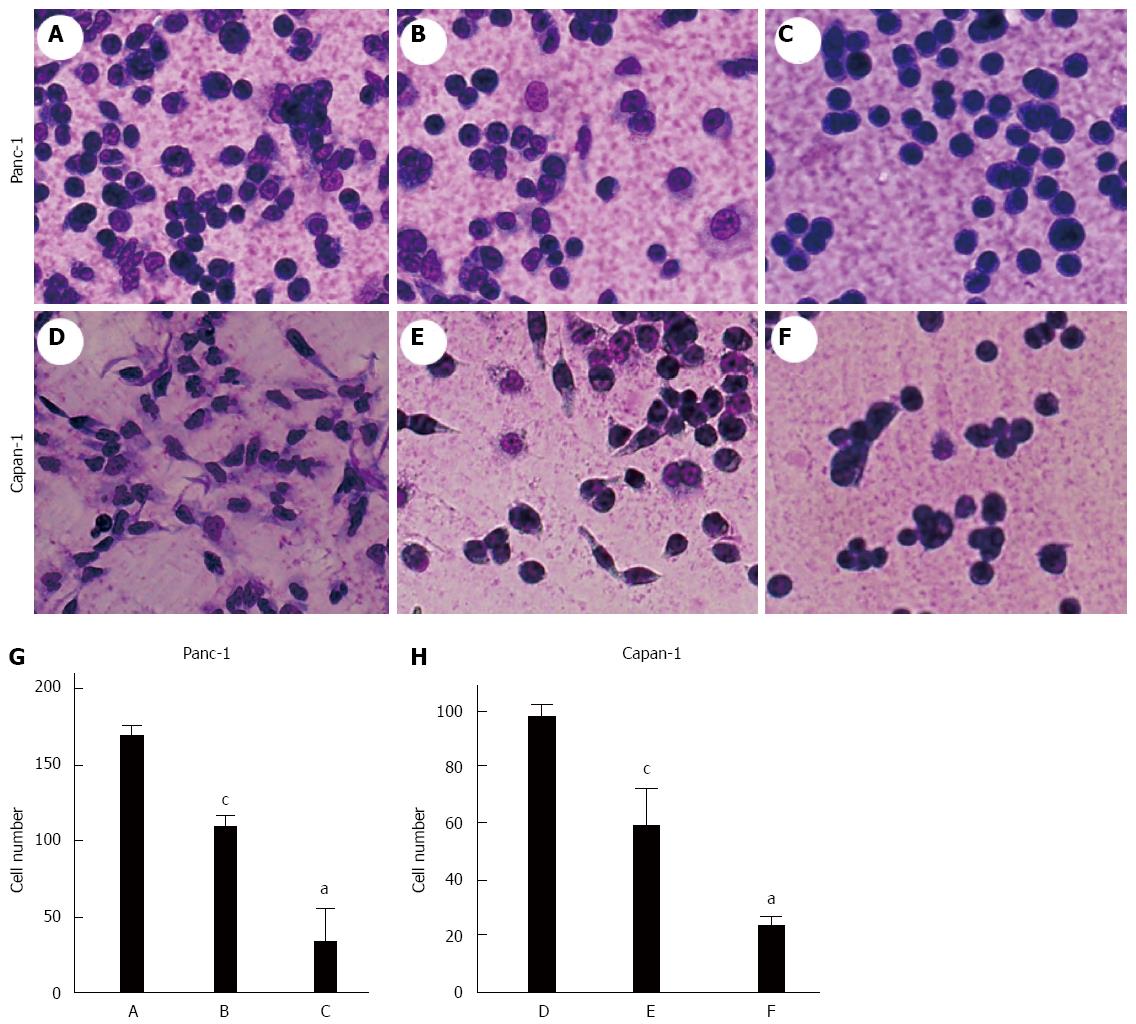
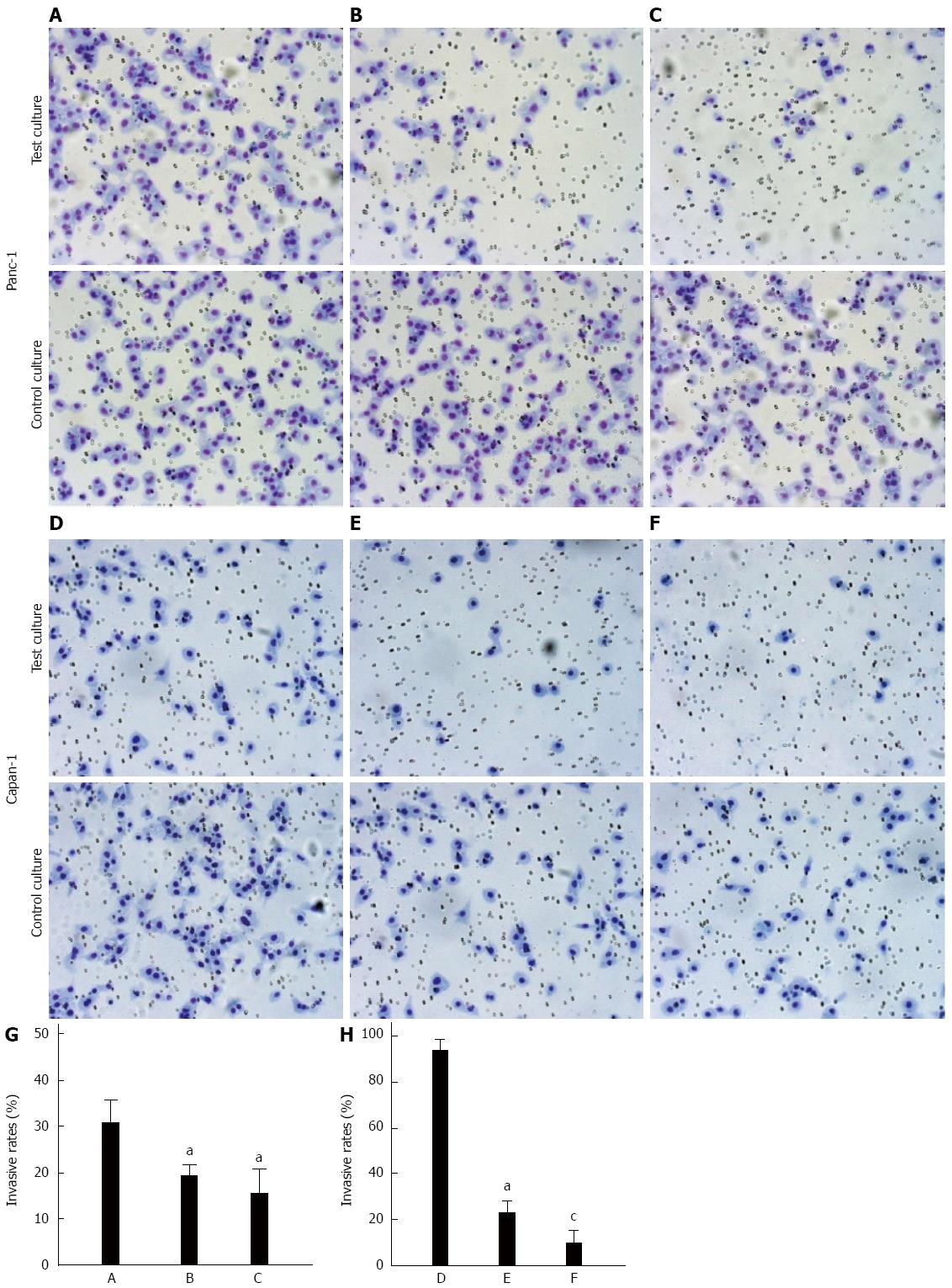
Microcscopic examination of Panc-1 and Capan-1 cells revealed that treatment with BAG or tunicamycin significantly reduced the number of cells adhered to the Matrigel-coated dishes compared with the control group (Ps < 0.05) (Figure 4). Significant differences were also found between the tunicamycin-treated and BAG-treated groups (P < 0.05). In addition, dramatic changes in cell morphology were observed, as cells exposed to BAG were more often spherical and individualized (Figure 4B-E).
One of the earliest steps in metastasis is the invasion of basement membrane. We further evaluated the motility and invasive ability of pancreatic carcinoma cells through a Matrigel-coated polycarbonate membrane. The invasive potential of both cell lines was significantly decreased after a 48 h pre-incubation with either tunicamycin ormM BAG (Ps < 0.05) (Figure 5). A significant difference was also found between the tunicamycin-treated and BAG-treated Capan-1 cells (P < 0.05).
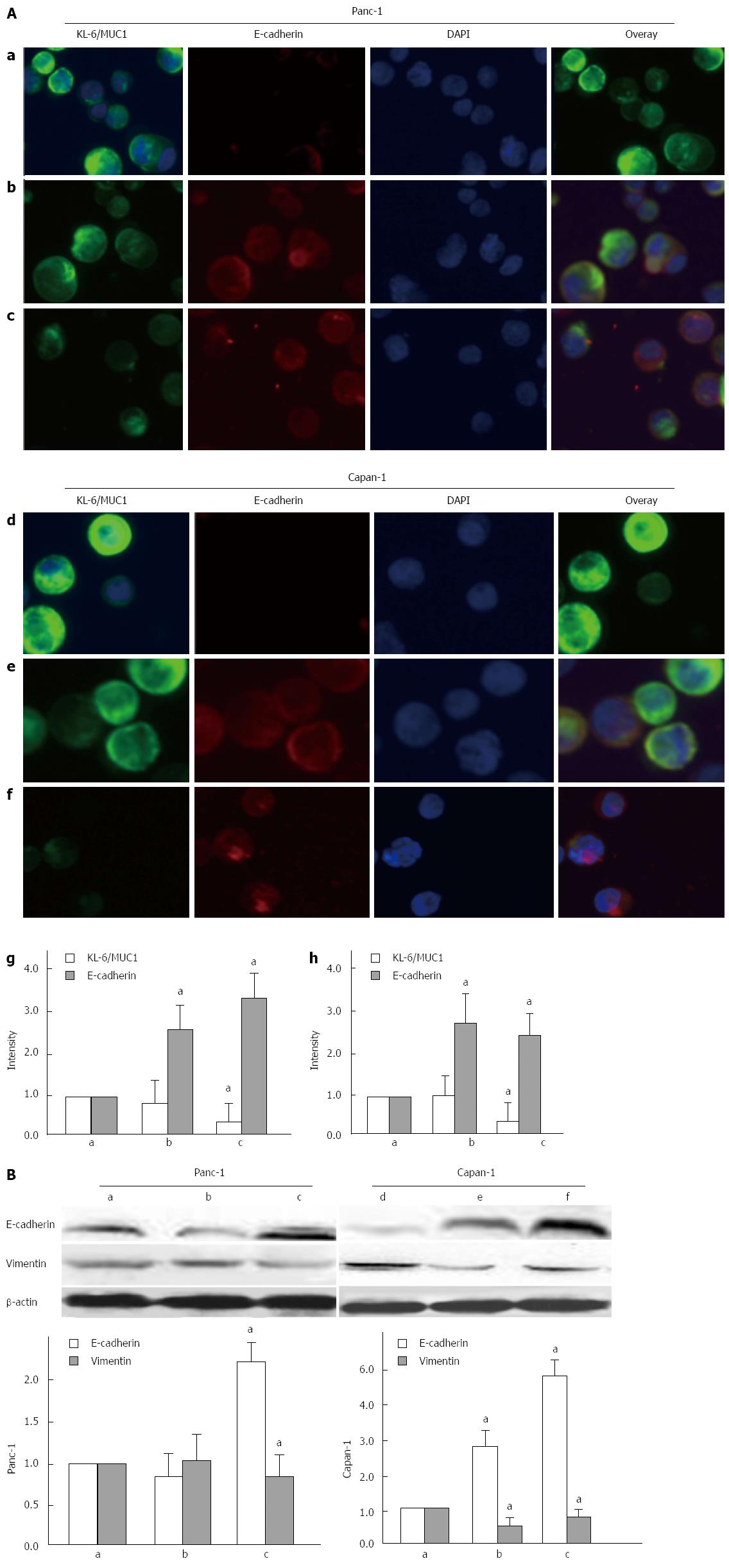
As epithelial-mesenchymal transition (EMT) is critical for cancer adhesion and invasion, we evaluated whether decreased KL-6/MUC1 expression was associated with this process by examining the expression of the epithelial marker E-cadherin before and after glycosylation inhibitor treatment. Both Panc-1 and Capan-1 cells showed strong immunofluroescence staining for KL-6/MUC1, indicating relatively high expression levels of KL-6/MUC1 (Figure 6Aa, d). A subtle decrease in KL-6/MUC1 expression was observed in cells treated with tunicamycin (Figure 6Ab, e), whereas a significant decrease was observed in cells treated with BAG (P < 0.05) (Figure 6Ac, f), which confirmed the results of immunocytochemical staining. Staining with E-cadherin showed that there was higher E-cadherin expression in both Panc-1 and Capan-1 cells treated with tunicamycin (Figure 6Ab, e) and BAG (Figure 6Ac, f) (P < 0.05), while no expression was detected in untreated cells (Figure 6Aa, d).
Western blotting showed that the expression of E-cadherin was significantly increased and the expression of the mesenchymal marker, vimentin, was significantly decreased after BAG treatment (P < 0.05) (Figure 6B). However, tunicamycin treatment did not affect the expression of E-cadherin or vimentin in Panc-1 cells as determined by Western blotting.
PDC is an extremely aggressive malignancy that rapidly progresses and metastasizes and carries a dismal prognosis. The control of micrometastatic pancreatic cancer remains a major objective in PDC treatment. Previous histologic studies have suggested that aberrant expression of KL-6/MUC1 in cancer tissues is associated with worse tumor behavior such as invasion and metastasis in ampullary carcinoma, primary colorectal carcinoma, and cholangiocarcinoma[6-8]. In the present study, we demonstrate that all PDC tissues show positive staining for KL-6/MUC1, while the surrounding normal pancreatic tissues do not. IPMT tissue, a slowly growing tumor with rare stromal invasion and a good prognosis[14], was rarely stained. These results indicate that overexpression of KL-6/MUC1 in pancreatic cancer tissues may be associated with aggressive tumor behavior or worse tumor staging of PDC.
The structures of atypical glycans may greatly contribute to the phenotype and biology of cancer tissue. The involvement of N- and/or O-linked glycans was documented by studies using mutation, deletion or addition of glycosylation sites, or using drugs affecting the processing of oligosaccharide chains[15-17]. BAG is a synthetic analogue of N-acetylgalactosamine (GalNAc) and inhibits elongation of O-glycans. This inhibition is competitive and instead of monosaccharide transfer to the GalNAc bound to serine or threonine, the elongation of the O-glycan chain occurs on BAG molecules[17,18]. Tunicamycin is a drug which blocks the transfer of GalNAc-P from UDP-GalNAc to dolichol phosphate[19]; it inhibits N-glycosylation and indirectly O-glycosylation, when N-glycosylation is a prerequisite. Tunicamycin also inhibits intracellular protein transport and reduces glycoprotein incorporation into the cellular membrane[19].
As the epitope recognized by the KL-6 antibody is a sialylated carbohydrate exposed on the surface of the MUC1 molecule, we used an O-glycosylation inhibitor (BAG) and an N-glycosylation inhibitor (tunicamycin) to affect the KL-6/MUC1 glycosylation profile and investigate how this affects tumor progression. Using the KL-6 antibody, we first detected KL-6/MUC1 expression in Panc-1 and Capan-1 cells. The Panc-1 cell line was established from human primary pancreatic carcinoma, whereas the Capan-1 cell line was obtained from a liver metastasis of a 40-year-old man with adenocarcinoma in the head of the pancreas. Overexpression of KL-6/MUC1 was found in both cell lines, suggesting that cancer clones that escape from primary tumors do not lose the KL-6 antigen. Immunocytochemical results also suggested that the glycosylation inhibitors altered the glycosylation profiles of KL-6/MUC1, although further studies are needed to investigate the detailed mechanisms of the two glycosylation inhibitors on KL-6/MUC1 glycosylation. The effects of tunicamycin treatment were not as obvious as the effects of BAG, possibly because KL-6/MUC1 is a more highly O-glycosylated cell surface glycoprotein.
An adhesion test and Transwell chamber assay were performed to investigate the relationship between KL-6/MUC1 glycosylation and adhesive and invasive properties of pancreatic carcinoma cells. Tumor cells interact with extracellular matrix components and the basement membrane, which is an essential initial event during the process of invasion and metastasis. It was proposed that there is a delicate balance between adhesion and anti-adhesion forces in MUC1 expressing cells, and that this balance plays an important role in the metastatic process[20,21]. Following glycosylation inhibitor treatment, the adhesion and invasion potential of the cells decreased. In addition, the O-glycosylation inhibitor caused more obvious changes in the adhesive and invasive potential of the cells. These results suggest that KL-6/MUC1 glycosylation, especially O-glycosylation, may play an important role in these biologic functions of KL-6/MUC1.
MUC1 and E-cadherin have both been implicated in pancreatic cancer development[22,23]. Our previous study showed that MUC1 knockdown led to increased E-cadherin-mediated cell-cell adhesion, suggesting that the cytoplasmic domain of MUC1 competes with E-cadherin for binding to β-catenin[24]. In this study, we observed that reduced KL-6/MUC1 glycosylation also increased E-cadherin, suggesting that the highly glycosylated tandem-repeat domain of MUC1 is also involved in cancer metastasis. However, significant changes in β-catenin expression were not found (data not shown). Additionally, the effects of tunicamycin on KL-6/MUC1 expression were not as apparent as with BAG, again suggestive of higher O-glycosylation of KL-6/MUC1.
As a feature of aggressive tumors, EMT is characterized by reduced E-cadherin and increased vimentin expression, contributing to increased tumor cell motility and invasive properties[25]. In this study, we showed that reduced KL-6/MUC1 expression was accompanied by increased E-cadherin expression and decreased vimentin expression in Capan-1 cells, but not in Panc-1 cells. The reason for this may be that Capan-1 cells have more metastatic potential than Panc-1 cells. These results indicate that the function of KL-6/MUC1 glycosylation in pancreatic cancer metastasis and invasion may be related to the EMT process.
This study indicates the important involvement of KL-6/MUC1 glycosylation in pancreatic cancer metastasis and invasion, which may be related to the EMT process. Therapeutic strategies which target glycosylation of KL-6/MUC1 may be useful for controlling the aggressive behavior of pancreatic cancer cells. Further studies are needed to investigate the detailed mechanism of KL-6/MUC1 glycosylation involved in metastatic progression of pancreatic carcinoma cells, as well as the possibility of using specific KL-6/MUC1 glycosylation inhibitors in the treatment of pancreatic carcinomas.
Pancreatic duct cell carcinoma (PDC) is an extremely aggressive malignancy, which rapidly metastasizes and carries a dismal prognosis. The control of micrometastatic pancreatic cancer remains a major objective in PDC treatment. Previous histologic studies have suggested that aberrant expression of KL-6/MUC1 in cancer tissues is associated with worse tumor behavior such as invasion and metastasis in ampullary carcinoma, primary colorectal carcinoma, and cholangiocarcinoma.
MUC1 is a polymorphic, highly glycosylated, type I transmembrane glycoprotein expressed by ductal epithelial cells of secretory organs, including the pancreas, breast, lung, and gastrointestinal tract. Many studies have suggested that aberrant glycosylation of MUC1 plays an important role in cancer metastasis and MUC1 seems to be an attractive target in cancer treatment. It was shown that the aberrant expression of MUC1, detected using CD227, DF3, and CA15-3 antibodies, plays an important role in the process of pancreatic cancer metastasis and invasion. KL-6/MUC1, a type of MUC1 categorized as cluster 9, is recognized by the KL-6 antibody.
This study showed that KL-6 was expressed in PDC, but not in intraductal papillary mucinous tumors (IPMT). These results indicate that overexpression of KL-6/MUC1 in pancreatic cancer tissues may be associated with aggressive tumor behavior or worse tumor staging of PDC. The structures of atypical glycans may greatly contribute to the phenotype and biology of cancer tissue. As the epitope recognized by KL-6 antibody is a sialylated carbohydrate exposed on the surface of the MUC1 molecule, the authors used two inhibitors to affect the KL-6/MUC1 glycosylation profile, and investigated how this affected tumor progression. The authors concluded that targeting glycosylation may be useful in controlling aggressive tumor behaviors.
This study indicated that glycosylation of KL-6/MUC1 in pancreatic cancer plays an important role in tumor metastasis and invasion, which may be related to the epithelial-mesenchymal transition process. Therapeutic strategies which target glycosylation of KL-6/MUC1 may be useful for controlling the aggressive behavior of pancreatic cancer cells.
IPMT of the pancreas is a rare tumor characterized by proliferation of the epithelium lining the pancreatic ducts. IPMT must be shown to connect to the pancreatic ductal system and have specific histologic features, which is quite different from other mucin-producing pancreatic cancers. Epithelial-mesenchymal transition the conversion of polarized, immotile epithelial cells to motile mesenchymal cells. This process is critical in embryonic morphogenesis, and in the progression of primary tumors to metastases.
In this paper, the authors studied the presence of a KL-6/MUC1 epitope in two types of pancreatic cancer, and found that KL-6 is expressed in PDC but not in IPMT. Then they inhibited O- and N-glycosylation to determine if either type of glycosylation is related to biologic properties of pancreatic cancer cells. They found differences that suggest that targeting glycosylation may be useful to control aggressive behavior of the tumor. This is an interesting study on the role of MUC1 glycosylation in pancreatic malignancies.
P- Reviewer: Seuningen I, Voutsadakis VI S- Editor: Qi Y L- Editor: AmEditor E- Editor: Ma S
| 1. | Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1360] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 2. | Pillai K, Pourgholami MH, Chua TC, Morris DL. MUC1 as a Potential Target in Anticancer Therapies. Am J Clin Oncol. 2013;Apr 19; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Madsen CB, Wandall HH, Pedersen AE. Potential for novel MUC1 glycopeptide-specific antibody in passive cancer immunotherapy. Immunopharmacol Immunotoxicol. 2013;35:649-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Kohno N, Inoue Y, Hamada H, Fujioka S, Fujino S, Yokoyama A, Hiwada K, Ueda N, Akiyama M. Difference in sero-diagnostic values among KL-6-associated mucins classified as cluster 9. Int J Cancer Suppl. 1994;8:81-83. [PubMed] |
| 5. | Kohno N. [Clinical usefulness of KL-6 as a serum marker of idiopathic interstitial pneumonia]. Nihon Kyobu Shikkan Gakkai Zasshi. 1996;34 Suppl:186-189. [PubMed] |
| 6. | Inagaki Y, Xu H, Nakata M, Seyama Y, Hasegawa K, Sugawara Y, Tang W, Kokudo N. Clinicopathology of sialomucin: MUC1, particularly KL-6 mucin, in gastrointestinal, hepatic and pancreatic cancers. Biosci Trends. 2009;3:220-232. [PubMed] |
| 7. | Xu HL, Inagaki Y, Seyama Y, Sugawara Y, Kokudo N, Nakata M, Wang FS, Tang W. Expression of KL-6 mucin, a human MUC1 mucin, in intrahepatic cholangiocarcinoma and its potential involvement in tumor cell adhesion and invasion. Life Sci. 2009;85:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Guo Q, Tang W, Inagaki Y, Midorikawa Y, Kokudo N, Sugawara Y, Nakata M, Konishi T, Nagawa H, Makuuchi M. Clinical significance of subcellular localization of KL-6 mucin in primary colorectal adenocarcinoma and metastatic tissues. World J Gastroenterol. 2006;12:54-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Xu H, Inagaki Y, Tang W, Guo Q, Wang F, Seyama Y, Midorikawa Y, Gai R, Kokudo N, Sugawara Y. Elevation of serum KL-6 mucin levels in patients with cholangiocarcinoma. Hepatogastroenterology. 2008;55:2000-2004. [PubMed] |
| 10. | Schneider G, Siveke JT, Eckel F, Schmid RM. Pancreatic cancer: basic and clinical aspects. Gastroenterology. 2005;128:1606-1625. [PubMed] |
| 11. | Cascio S, Farkas AM, Hughey RP, Finn OJ. Altered glycosylation of MUC1 influences its association with CIN85: the role of this novel complex in cancer cell invasion and migration. Oncotarget. 2013;4:1686-1697. [PubMed] |
| 12. | Kovjazin R, Horn G, Smorodinsky NI, Shapira MY, Carmon L. Cell surface-associated anti-MUC1-derived signal peptide antibodies: implications for cancer diagnostics and therapy. PLoS One. 2014;9:e85400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Isaji S, Kawarada Y, Uemoto S. Classification of pancreatic cancer: comparison of Japanese and UICC classifications. Pancreas. 2004;28:231-234. [PubMed] |
| 14. | Zamora C, Sahel J, Cantu DG, Heyries L, Bernard JP, Bastid C, Payan MJ, Sielezneff I, Familiari L, Sastre B. Intraductal papillary or mucinous tumors (IPMT) of the pancreas: report of a case series and review of the literature. Am J Gastroenterol. 2001;96:1441-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Remmers N, Anderson JM, Linde EM, DiMaio DJ, Lazenby AJ, Wandall HH, Mandel U, Clausen H, Yu F, Hollingsworth MA. Aberrant expression of mucin core proteins and o-linked glycans associated with progression of pancreatic cancer. Clin Cancer Res. 2013;19:1981-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 16. | Ho JJ, Jaituni RS, Crawley SC, Yang SC, Gum JR, Kim YS. N-glycosylation is required for the surface localization of MUC17 mucin. Int J Oncol. 2003;23:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Delacour D, Gouyer V, Leteurtre E, Ait-Slimane T, Drobecq H, Lenoir C, Moreau-Hannedouche O, Trugnan G, Huet G. 1-benzyl-2-acetamido-2-deoxy-alpha-D-galactopyranoside blocks the apical biosynthetic pathway in polarized HT-29 cells. J Biol Chem. 2003;278:37799-37809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Huang J, Byrd JC, Yoon WH, Kim YS. Effect of benzyl-alpha-GalNAc, an inhibitor of mucin glycosylation, on cancer-associated antigens in human colon cancer cells. Oncol Res. 1992;4:507-515. [PubMed] |
| 19. | Delacour D, Gouyer V, Zanetta JP, Drobecq H, Leteurtre E, Grard G, Moreau-Hannedouche O, Maes E, Pons A, André S. Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells. J Cell Biol. 2005;169:491-501. [PubMed] |
| 20. | Satoh S, Hinoda Y, Hayashi T, Burdick MD, Imai K, Hollingsworth MA. Enhancement of metastatic properties of pancreatic cancer cells by MUC1 gene encoding an anti-adhesion molecule. Int J Cancer. 2000;88:507-518. [PubMed] |
| 21. | Horm TM, Schroeder JA. MUC1 and metastatic cancer: expression, function and therapeutic targeting. Cell Adh Migr. 2013;7:187-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 22. | Roy LD, Sahraei M, Subramani DB, Besmer D, Nath S, Tinder TL, Bajaj E, Shanmugam K, Lee YY, Hwang SI. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene. 2011;30:1449-1459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 222] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 23. | Yamada S, Fuchs BC, Fujii T, Shimoyama Y, Sugimoto H, Nomoto S, Takeda S, Tanabe KK, Kodera Y, Nakao A. Epithelial-to-mesenchymal transition predicts prognosis of pancreatic cancer. Surgery. 2013;154:946-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | Xu H, Inagaki Y, Seyama Y, Du G, Wang F, Kokudo N, Tang W. Expression of KL-6/MUC1 in pancreatic cancer tissues and its potential involvement in tumor metastasis. Oncol Rep. 2011;26:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Ponnusamy MP, Seshacharyulu P, Lakshmanan I, Vaz AP, Chugh S, Batra SK. Emerging role of mucins in epithelial to mesenchymal transition. Curr Cancer Drug Targets. 2013;13:945-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |