INTRODUCTION
Patients with alcoholic liver disease (ALD) frequently display iron overload and an increase in intestinal iron absorption, as determined by whole-body retention studies[1]. Iron has been shown to induce tumor necrosis factor (TNF)α expression in experimental ALD models and act as a secondary risk factor for alcoholic liver injury[2-4]. Hepcidin is a circulatory antimicrobial peptide synthesized by the liver[5,6]. It acts as the key regulator of iron homeostasis by inhibiting intestinal iron transport as well as the export of iron from macrophages[7,8]. Our laboratory and others have demonstrated that both acute and chronic alcohol exposure suppresses hepcidin expression in the liver[9-13]. Patients with ALD also display reduced hepcidin expression[14]. Besides being the key iron-regulator, hepcidin is an acute phase protein, and is induced by endotoxin (lipopolysaccharide, LPS) and inflammatory cytokines, interleukin (IL)-1 and IL-6[15,16]. Chronic alcohol consumption increases the level of gut-derived endotoxin in the portal blood of patients with ALD[17]. This leads to the activation of Kupffer cells, and the release of proinflammatory cytokines contributing to ALD pathogenesis[18-20]. The mechanisms by which alcohol can suppress hepcidin expression in the presence of inflammatory signals in the liver are however unknown.
Toll-like receptors (TLR), members of the pattern-recognition receptor family, are important for innate immune responses[21]. LPS is the ligand for TLR4 and TLR4 is expressed by various cells of the liver[21]. The activation of TLR4 induces the assembly of adaptor proteins, which propagate TLR4 signaling by two different pathways[22]. In the MyD88-dependent pathway, MyD88 and TIRAP (Mal) adaptor complexes activate IL-1 receptor-associated kinases, IRAK1 and IRAK4, and associate with the E3 ubiquitin ligase, TRAF6. The ubiquinated TRAF6 activates the TAK1 kinase complex leading to the activation of the IκB kinase complex (IKKα, IKKβ, NEMO), and the subsequent phoshorylation and degradation of the nuclear factor (NF)-κB inhibitor, IκBα. Freed of its inhibitor, the transcription factor, NF-κB translocates into the nucleus. In the MyD88-independent pathway, adaptor proteins TRIF and TRAM associate with serine-threonine kinase, RIP1 or alternatively recruit TRAF6 and TAK1 to induce NF-κB activation[22].
Alcohol activates both MyD88-dependent and independent pathways[23,24]. Activation of NF-κB leads to the production of inflammatory cytokines[22]. IL-6 activates acute phase response genes through the transcription factors, Stat3 and C/EBPs[25]. Stat3 is involved in activation of hepcidin gene transcription by IL-6 and LPS[26-28]. We have previously reported that alcohol-induced oxidative stress inhibits the activity of the transcription factor, C/EBPα leading to the suppression of hepcidin expression and iron accumulation in vivo[9]. The hepcidin gene promoter also contains consensus binding sites for the transcription factor, NF-κB but its role in the regulation of hepcidin expression is unclear.
Small heterodimer partner (SHP, NROB2), an orphan member of the nuclear receptor family of transcription factors, acts as an inducible co-repressor and is highly expressed in the liver[29,30]. SHP has been reported to interact with Stat3 and inhibit its activity in hepatocytes[31]. Recently, a role for SHP in the regulation of TLR4 signaling has been suggested[32]. The involvement of SHP in the pathogenesis of ALD is unknown.
Both iron and alcohol are associated with infection and inflammation, and act synergistically to induce tissue injury. It is therefore important to understand the role of TLR4 signaling in the regulation of hepcidin expression by alcohol in the liver.
MATERIALS AND METHODS
Animal experiments
Animal experiments were approved by the animal ethics committee at the University of Nebraska Medical Center. TLR4 mutant mice on the C3H/HeJ background and wildtype counterpart on the C3H/HeOuJ background (Jackson Laboratories) were pair-fed with regular and ethanol-containing Lieber De Carli liquids diets (Dyets, Inc., cat no: 710027, 710260, respectively), as described previously[11,33]. 5 male mice were included in each group per experiment (n = 2). The ethanol content of the diet was gradually increased over a 9 d period to 5 % and mice were exposed to 5 % ethanol for 4 wk[33].
RNA isolation, cDNA synthesis and Real-time quantitative polymerase chain reaction analysis
RNA isolation, cDNA synthesis and quantitative polymerase chain reaction (PCR) using an ABI Prism 7700 Sequence Detection System (Applied Biosystems) were performed, as published previously[34,35]. Primers (sense 5’-ACTCGGACCCAGGCTGC-3’; antisense 5’-AGATAGGTGGTGCTGCTCAGG-3’) and Taqman fluorescent probe (5’ 6-[FAM]-TGTCTCCTGCTTCTCCTCCTTGCCA-3’ [TAMRA-Q]) flanking about 70 base pairs of mouse hepcidin gene open reading frame sequence were designed by the Primer Express 1.5 program (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase (gapdh) gene probes (Applied Biosystems) were used as the endogenous controls.
Co-immunoprecipitation and Western Blotting:
To isolate cytosol and nuclear fractions, livers were homogenized in buffer A [25 mmol/L Tris/HCl (pH 7.5), 50 mmol/L KCl, 2 mmol/L MgCl2, 1 mmol/L EDTA, 5 mmol/L DTT, 1 mmol/L PMSF, CompleteR protease inhibitor cocktail (Roche Diagnostics Corp.), phosphatase inhibitor cocktail A (Santa Cruz , sc-45044)] and incubated on ice for 20 min. Following centrifugation (3000 g for 5 min at 4 °C), supernatants were kept as cytoplasm and pellets were washed once with buffer A. Cell pellets were then incubated in Buffer B [10 mmol/L Tris/HCl (pH 7.5), 0.42 mol/L NaCl, 1.5 mmol/L MgCl2, 0.5 mmol/L EDTA, 1 mmol/L DTT, 25% sucrose, 1 mmol/L PMSF, CompleteR protease inhibitor cocktail (Roche Diagnostics Corp.), phosphatase inhibitor cocktail A (Santa Cruz, sc-45044)] for 15 min. to isolate nuclear proteins. For immunoprecipitations, 1 mg of whole liver lysate protein was incubated with equal amounts (3 μg) of SHP antibody or normal rabbit IgG (Santa Cruz) and protein A-Agarose pre-blocked with BSA (Santa Cruz). SHP immunocomplexes eluted by non-reducing SDS buffer were resolved on 8% polyacrylamide gels and immunoblotted with anti-p65 (Cell Signaling) antibody. Alkaline phosphatase-conjugated anti-rabbit (SouthernBiotech) light chain-specific immunoglobulins were used as secondary antibodies. Western blots were performed using commercial antibodies (Santa Cruz, Cell Signaling), as described previously[33,34]. Immune-reactive bands were detected by the ImmunStar™-anti-rabbit-AP or -anti-mouse-HRP kits (Bio-Rad Laboratories).
Chromatin immunoprecipitation
Chromatin immunoprecipitation was performed, as described[33]. Chromatin isolated from formalin-fixed mouse liver was sheared by sonication and immunoprecipitated by using control IgG or anti-p50 antibody and protein A-Agarose (Santa Cruz). Eluted DNA were analyzed by PCR using primers (forward 5’-GCCATACTGAAGGCACTGA-3’; reverse 5’-GTGTGGTGGCTGTCTAGG-3’) to amplify a 307 bp mouse hepcidin-1 gene promoter region harboring NF-κB DNA-binding sites.
Statistical analysis
Statistical analysis of differences in treatment groups was performed by using the non-parametric Mann-Whitney test and Student’s t test.
RESULTS
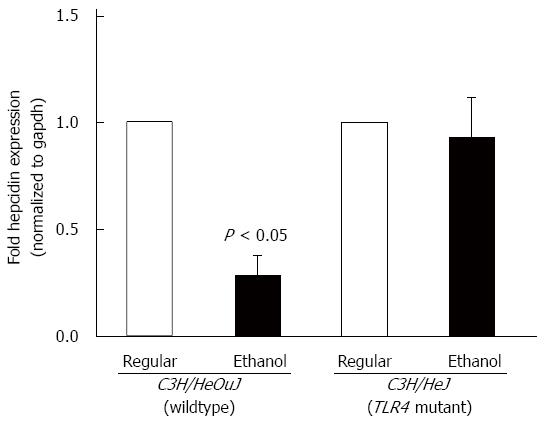
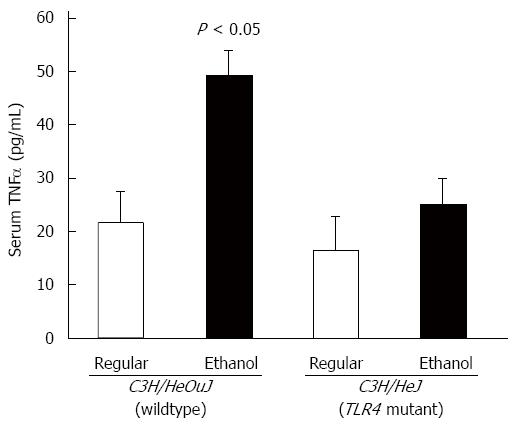
C3H/HeJ mice harbor a missense mutation in the third exon of the toll-like receptor 4 (TLR4) gene and express a non-functional mutant TLR4 rendering them resistant to endotoxin signaling[36]. For chronic alcohol studies, C3H/HeJ mice and wildtype control counterpart, C3H/HeOuJ mice were pair-fed with regular and ethanol-containing Lieber De Carli liquid diets, as described in Materials and Methods. Alcohol administration significantly inhibited hepcidin mRNA expression in the livers of C3H/HeOuJ wildtype mice compared to C3H/HeOuJ mice pair-fed with the regular diet (Figure 1). In contrast, alcohol did not suppress hepcidin expression in the livers of TLR4 mutant C3H/HeJ mice compared to C3H/HeJ mice pair-fed with the regular diet (Figure 1). To study alcohol-induced inflammation, we measured serum levels of pro-inflammatory cytokine, TNFα by ELISA assays. TNFα levels were significantly higher in alcohol-fed wildtype, but not in TLR4 mutant, mice compared to respective pair-fed controls fed with the regular diet (Figure 2).
Figure 1 Hepcidin mRNA expression.
cDNA, synthesized from RNA isolated from the livers of toll-like receptor 4 mutant (C3H/HeJ) and wildtype (C3H/HeOuJ) mice pair-fed with regular and ethanol-containing diets, was employed in real-time polymerase chain reaction to measure hepcidin mRNA expression, as described in Methods. Hepcidin expression in ethanol-fed mice was expressed as fold expression of that in the respective mice pair-fed with the regular diet (mean ± SE; n = 10 per group).
Figure 2 Tumor necrosis factor α expression.
The level of tumor necrosis factor (TNF)α in sera of toll-like receptor 4 mutant (C3H/HeJ) and wildtype control (C3H/HeOuJ) mice, pair-fed with regular and ethanol-containing diets was determined by ELISA assays, using a commercial kit (BioLegend), according to the manufacturer’s instructions, and expressed as picogram cytokine per ml of serum (mean ± SE; n = 10 per group).
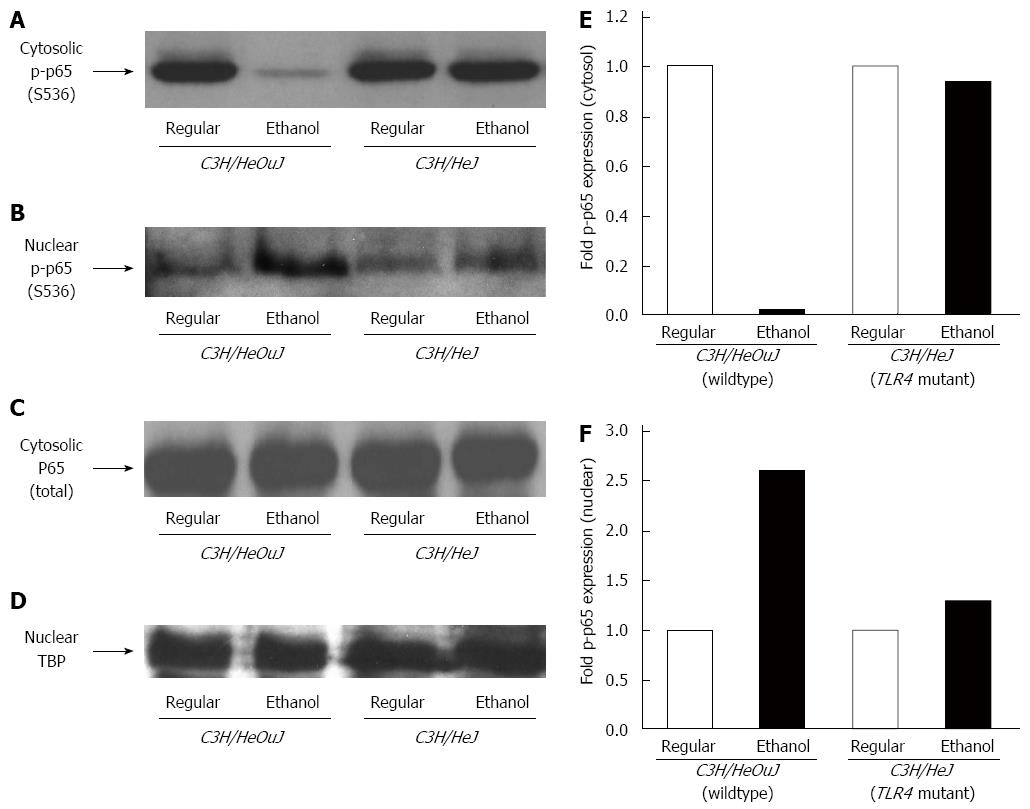
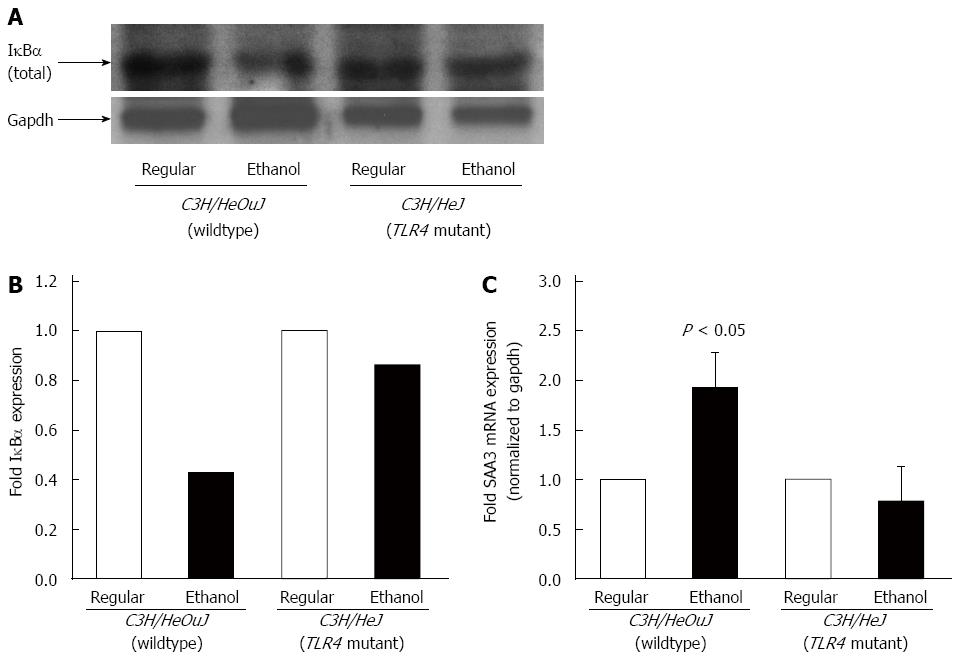
The activation of TLR4 signaling leads to the activation of NF-κB[22]. The phosphorylation of NF-κB p65 subunit protein was decreased 50-fold in the cytosolic and increased 3-fold in the nuclear, fractions isolated from the livers of alcohol-fed wildtype mice, as compared to that in wildtype mice pair-fed with the regular diet (Figure 3). In contrast, alcohol did not significantly alter the phosphorylation or nuclear translocation of NF-κB p65 subunit protein in the livers of C3H/HeJ mice expressing a non-functional mutant TLR4 (Figure 3). Following alcohol exposure, the protein expression of NF-κB inhibitor, IκBα in the cytosol of the livers isolated from wildtype, but not from TLR4 mutant, mice was significantly reduced (Figure 4A and B). To further confirm NF-κB activation, the expression of serum amyloid A-3 (SAA3), which is a well-known target gene of NF-κB, was determined[37]. Accordingly, alcohol up-regulated SAA3 gene expression in the livers of wildtype, but not TLR4 mutant, mice (Figure 4C).
Figure 3 Nuclear factor-κB phosphorylation.
Cytosolic (A, C) and nuclear (B, D) protein fractions isolated from the livers of C3H/HeJ and C3H/HeOuJ mice pair-fed with regular and ethanol-containing diets were used for western blots to detect phosphorylated (serine 536) p65 (p-p65) expression. Anti-total p65 (p65) (C) and anti-TATA-binding protein (TBP) (D) antibodies were used as controls. Cytosolic (E) and nuclear (F) p-p65 expression was quantified by densitometric analysis and normalized to total p65 or TBP protein expression, respectively. Normalized p-p65 expression in ethanol-fed mice was expressed as fold expression of that in the respective mice pair-fed with the regular diet (mean; n = 3 per group).
Figure 4 IκBα expression and SAA3 expression.
A: Cytosolic protein fractions isolated from the livers of C3H/HeOuJ and C3H/HeJ mice pair-fed with regular and ethanol-containing diets were used for western blots to detect IκBα expression. An anti-gapdh antibody was used as control; B: IκBα expression was quantified by densitometric analysis and normalized to gapdh expression. Normalized IκBα expression in ethanol-fed mice was expressed as fold expression of that in the respective mice pair-fed with the regular diet (mean; n = 3 per group); C: cDNA, synthesized from RNA isolated from the livers of these mice, was employed in real-time polymerase chain reaction to measure SAA3 mRNA expression. SAA3 expression in ethanol-fed mice was expressed as fold expression of that in the respective mice pair-fed with the regular diet (mean ± SE; n = 10 per group).
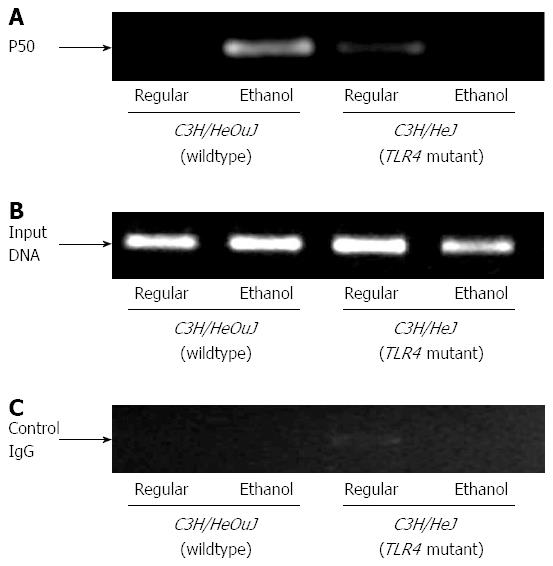
In order to determine the binding of NF-κB to hepcidin gene promoter, we performed chromatin immunoprecipitation assays using antibodies for NF-κB p50 subunit DNA-binding protein, as described in Materials and Methods. In accordance with p65 activation, the binding of p50 to hepcidin gene promoter was induced by alcohol in the livers of wildtype, but not TLR4 mutant, mice (Figure 5A). This interaction was specific because no binding was observed when chromatin samples were incubated with control IgG (Figure 5C).
Figure 5 Nuclear factor-κB binding to hepcidin gene promoter.
Chromatin isolated from the livers of control or ethanol-fed C3H/HeJ and C3H/HeOuJ mice was immunoprecipitated with an anti-p50 nuclear factor-κB antibody (A) or control IgG (C). Immunoprecipitated DNAs (A, C) and input DNA control (B) were subjected to polymerase chain reaction, as described in Methods.
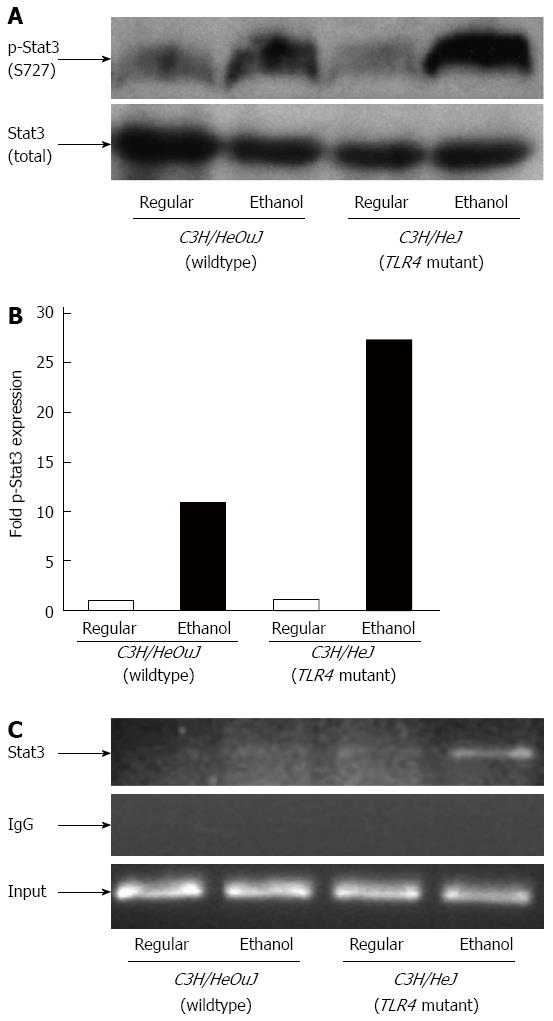
Similar to NF-κB, Stat3 is also important in the regulation of inflammatory processes in the liver[25]. Stat3 has also been shown to be involved in the induction of hepcidin gene expression by inflammation[26-28]. In contrast to NF-κB, alcohol-mediated activation of Stat3 was weaker in wildtype mice. The level of phosphorylated Stat3 protein in the livers of alcohol-fed wildtype mice was 10-fold higher compared to wiltype mice pair-fed with the regular diet (Figure 6A and B). Phospho-Stat3 protein expression was however increased by 27-fold in the livers of TLR4 mutant mice following alcohol exposure compared to mutant mice pair-fed with the regular diet (Figure 6A and B). Furthermore, Stat3 binding to hepcidin gene promoter was observed in the livers of TLR4 mutant, but not wildtype, mice following alcohol exposure, as shown by chromatin immunoprecipitation assays (Figure 6C). No such binding was observed when chromatin samples were incubated with control IgG (Figure 6C).
Figure 6 Stat3 activation and binding to hepcidin gene promoter.
A: Phospho-(p-Stat3) and total Stat3 protein expression in whole liver cell lysates isolated from C3H/HeJ and C3H/HeOuJ mice pair-fed with regular and ethanol-containing diets was detected by western blotting; B: p-Stat3 expression in each sample was quantified by densitometric analysis and normalized to total Stat3 protein expression. Normalized p-Stat3 expression in ethanol-fed mice was expressed as fold expression of that in the respective mice pair-fed with the regular diet (mean; n = 3 per group); C: Chromatin isolated from the livers of control or ethanol-fed C3H/HeJ and C3H/HeOuJ mice was immunoprecipitated with an anti-Stat3 antibody or control IgG. Immunoprecipitated DNAs and input DNA control were subjected to polymerase chain reaction, as described in Methods.
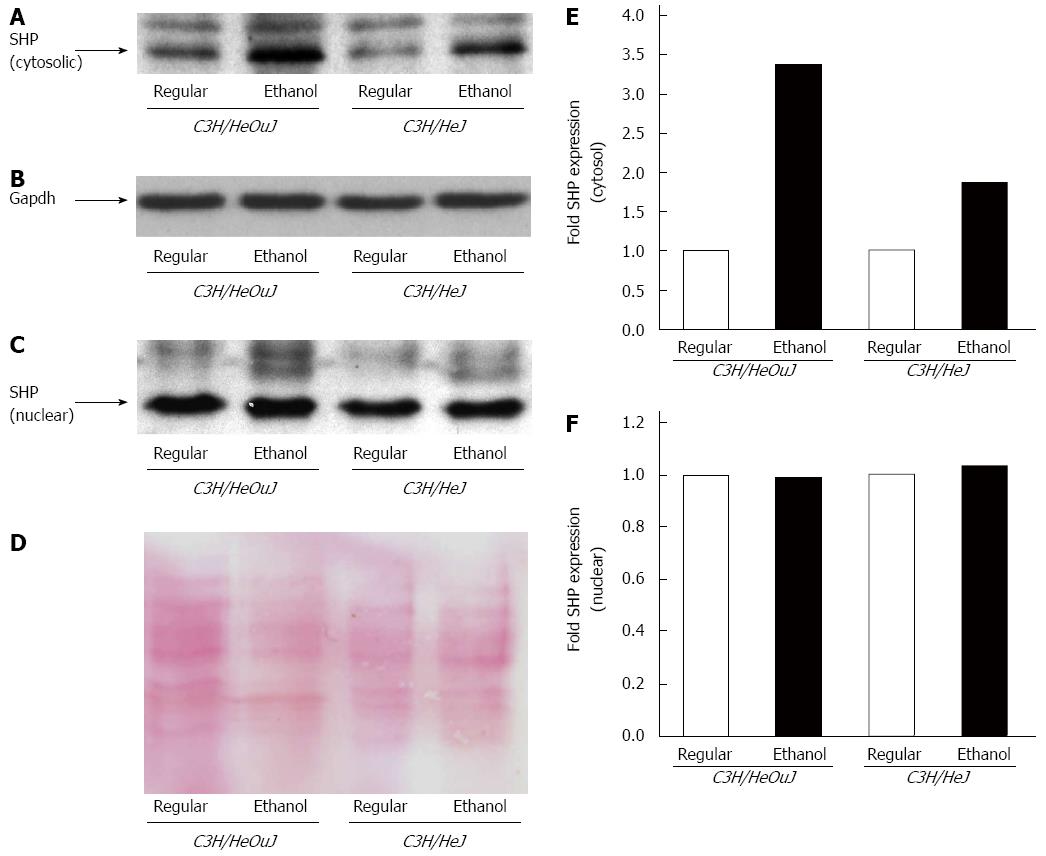
A role for small heterodimer partner (SHP) protein, which is a transcriptional co-repressor, has recently been suggested in TLR4 signaling[32]. The effect of alcohol on SHP expression is however unknown. We therefore determined the expression of SHP in both cytosolic and nuclear fractions isolated from the livers of C3H/HeOuJ wildtype and C3H/HeJ TLR4 mutant mice pair-fed with ethanol-containing and regular Lieber De Carli diets. Following alcohol exposure, wildtype mice exhibited a 3-fold elevated level of cytosolic SHP expression compared to wildtype mice pair-fed with the regular diet. Alcohol induced a 1.8-fold increase in cytosolic SHP expression in TLR4 mutant mice compared to mutant mice pair-fed with the regular diet (Figure 7A, B and E). In contrast to cytosol, SHP protein expression in the nucleus was not significantly affected by alcohol both in wildtype and mutant mice (Figure 7C, D and F). The basal level of nuclear SHP expression was however higher than that in the cytosol (Figure 7A and C).
Figure 7 Small heterodimer partner protein expression.
Small heterodimer partner (SHP) protein expression was detected by western blots using cytosolic (A, B) and nuclear (C, D) fractions isolated from the livers of C3H/HeJ and C3H/HeOuJ mice pair-fed with regular and ethanol-containing diets. An anti-gapdh antibody (B) was used as a cytosolic protein loading control; D: Nuclear proteins on PVDF blotting membranes were visualized by Ponceau S staining to evaluate equal protein loading. Cytosolic (E) and nuclear (F) SHP expression was quantified by densitometric analysis and normalized to control protein expression. Normalized SHP expression in ethanol-fed mice was expressed as fold expression of that in the respective mice pair-fed with the regular diet (mean; n = 3 per group).
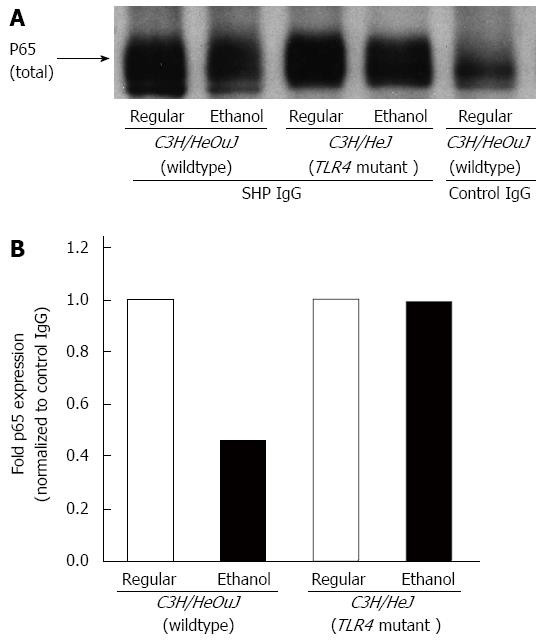
In order to study the interaction of SHP with NF-κB in the liver and the effect of alcohol on this interaction, we performed immunoprecipitation experiments followed by western blotting. Whole cell lysate proteins, isolated from the livers of C3H/HeOuJ wildtype and C3H/HeJ TLR4 mutant mice pair-fed with ethanol-containing and regular Lieber De Carli diets, were immunoprecipitated with either an anti-SHP antibody or anti-rabbit IgG, as control. The immune complexes were subsequently blotted with an antibody for NF-κB p65 subunit protein, as described in Methods. A protein-protein interaction was detected between SHP and p65 in the liver (Figure 8A, lanes 1-4). This interaction was specific because no significant level of p65 protein was detected in immune complexes incubated with control IgG (Figure 8A, lane 5). The livers of wildtpe mice fed with the regular diet exhibited a stronger interaction of SHP with p65 protein compared to ethanol-fed wildtype mice (Figure 8A, lanes 1 and 2 and Figure 8B). Alcohol-mediated attenuation of the interaction between SHP and p65 was however not observed in TLR4 mutant mice (Figure 8A, lanes 3 and 4 and Figure 8B).
Figure 8 Effect of alcohol on small heterodimer partner and nuclear factor-κB interaction.
A: Whole cell lysates isolated from the livers of C3H/HeJ and C3H/HeOuJ mice pair-fed with regular and ethanol-containing diets were immunoprecipitated with an anti-small heterodimer partner (SHP) antibody or anti-rabbit IgG, as control. The expression of nuclear factor-κB in the immune complexes was determined by western blotting using an anti-p65 antibody, as described in Methods; B: p65 protein expression in SHP immune complexes was quantified by densitometric analysis and normalized to control IgG immune complexes. Normalized p65 expression in ethanol-fed mice was expressed as fold expression of that in the respective mice pair-fed with the regular diet (mean; n = 3 per group).
DISCUSSION
Inflammation plays a key role in the pathogenesis of alcoholic liver disease[20,38]. Alcohol also increases intestinal iron uptake and serum iron indices leading to iron overload[1,39]. We and others have shown that the inhibition of hepcidin by alcohol might play a role in this process[9-12,14]. Besides being the key iron regulator, hepcidin is also an acute phase protein induced by inflammation[15,16]. The underlying mechanisms by which alcohol can inhibit hepcidin in the presence of inflammatory signals are unclear.
Our studies clearly demonstrate a role for alcohol-induced TLR4 signaling in the inhibition of hepcidin expression in the liver. Unlike wildtype mice, mice with non-functional TLR4 receptor did not display suppression of hepcidin expression following chronic alcohol intake. Of note, alcohol elevated serum levels of proinflammatory cytokine, TNFα only in wildtype mice with intact TLR4 signaling. Activation of the transcription factor, NF-κB plays a central role in TLR4 signaling. Upon activation, NF-κB is phosphorylated and translocates to the nucleus[22]. Accordingly, the phoshorylation and nuclear translocation of NF-κB p65 protein subunit by alcohol was induced in wildtype, but not in TLR4 mutant, mice. Accordingly, a significant decrease in cytosolic IκBα protein levels was observed in wildtype, but not in TLR4 mutant, mice. Following alcohol administration, mRNA expression of SAA3, a well-established NF-κB-responsive gene, was induced only in wildtype mice indicating the functional inhibition of NF-κB in TLR4 mutant mice. Taken together, these findings suggest a correlation between alcohol-induced activation of NF-κB and the inhibition of hepcidin expression in the liver. This is further supported by chromatin immunoprecipitation studies. Namely, the occupancy of hepcidin gene promoter by NF-κB p50 subunit protein was elevated by alcohol administration in wildtype, but not TLR4 mutant, mice. Alcohol however did not induce the binding of NF-κB p65 subunit protein to hepcidin promoter (data not shown). This might be due to our experimental system or it is feasible that chronic alcohol exposure interferes with the nuclear interaction of p65 with p50 in the liver.
Activation of NF-κB leads to the production of inflammatory cytokines but alcohol has also been shown to interfere with IL-6-mediated activation of Stat3 in hepatocytes, in vitro[40]. Of note, Stat3 plays a role in the induction of hepcidin transcription by endotoxin and inflammatory cytokines[26-28]. A cross-talk between Jak/Stat pathway and TLR4 signaling has been implicated[41]. MyD88 has been shown to play a role in LPS-induced activation of Stat3 in the liver and hypothalamus[42]. In contrast, TLR4 signaling has been reported to inhibit Stat3 in mesenchymal stem cells[43]. To further understand the role of alcohol-induced TLR4 signaling in the regulation of hepcidin, we investigated the activation of Stat3 in TLR4 mutant and wildtype mice. Besides the phoshorylation at tyrosine by Jak kinases, Stat3 is also phoshorylated at serine, which is important for its transcriptional activity[44,45]. By using phospho-specific antibodies, we have shown that chronic alcohol intake attenuates the activation of Stat3 in the livers of wildtype, but not TLR4 mutant, mice. The binding of Stat3 to hepcidin promoter was observed in alcohol-fed TLR4 mutant, but not wildtype, mice. Although weak, this Stat3 binding might be involved in reversing the inhibitory effect of alcohol on hepcidin expression in TLR4 mutant mice. Our results therefore strongly suggest that the presence of a functional TLR4 receptor and intact TLR4 signaling is required for alcohol to inhibit Stat3, and thereby hepcidin transcription in the liver.
A relationship between small heterodimer partner (SHP), a co-repressor belonging to the nuclear receptor family of transcription factors, and NF-κB has been suggested but the findings are inconclusive. SHP has been reported to activate NF-κB in macrophages treated with oxidized lipoproteins[46]. In contrast, SHP has been shown to inhibit TRAF6 and NF-κB in bone marrow-derived mouse macrophages[32]. Alcohol is well known to interact with other classes of nuclear receptors but its effect on SHP is however unknown[47]. We show here that alcohol induces the expression of SHP protein in the liver, which is stronger in mice with intact TLR4 signaling. Furthermore, the stimulatory effect of alcohol on SHP expression is mainly observed in the cytoplasm, but not in the nucleus. To further understand the effect of alcohol on SHP, we investigated the interaction of SHP with NF-κB by co-immunoprecipitation studies. A strong complex between SHP and NF-κB proteins has been observed in both wildtype and TLR4 mutant mice, which were fed with the control diet. This suggests that a functional TLR4 receptor is not required for the basal level of protein-protein interaction between SHP and NF-κB in the liver. Interestingly, this interaction was attenuated following chronic alcohol consumption mainly in wildtype mice. Yuk et al[32] reported an interaction of SHP with NF-κB in untreated bone marrow-derived mouse macrophages, which was significantly decreased upon LPS stimulation. Further investigations are required to understand whether the interaction of cytosolic SHP with NF-κB in the liver might play a role in the regulation of TLR4 signaling and inflammatory processes by alcohol. SHP has also been shown to form a nuclear complex with Stat3 in an IL-6-dependent manner in HepG2 hepatoma cells overexpressing SHP and Stat3[31]. Kim et al[31] have also suggested a role for SHP in the inhibition of Stat3 in primary rat hepatocytes treated with the antidiabetic drug metformin. Since chronic alcohol exposure did not alter the expression of nuclear SHP expression, it is unclear whether SHP, as a co-repressor, is involved in the inhibition of Stat3 by alcohol in the liver. Of note, we observed binding of Stat3 to hepcidin promoter in alcohol-fed TLR4 mutant, but not in wildtype, mice. The role of SHP in the regulation of Stat3 and hepcidin expression by alcohol in the liver will be addressed in future studies.
Collectively, the findings in this study strongly suggest that the activation of TLR4 signaling and NF-κB are part of the mechanisms by which alcohol can suppress hepcidin expression despite the presence of inflammatory signals in the liver. The inhibition of hepcidin, which causes iron overload, together with inflammation will exacerbate liver injury, as observed with chronic alcohol intake.
COMMENTS
Background
Chronic alcohol consumption leads to the release of gut-derived endotoxins into the portal circulation and inflammation in the liver. The authors have previously shown that alcohol suppresses hepcidin expression in the liver. Hepcidin is both a key iron-regulatory protein and an acute phase protein induced by inflammation. However, it is unclear how alcohol can suppress hepcidin expression in the presence of inflammatory signals in the liver.
Research frontiers
Patients with alcoholic liver disease (ALD) exhibit both inflammation and altered iron homeostasis. Activation of toll-like receptor signaling and nuclear factor (NF)-κB in the liver also plays a role in the pathogenesis of ALD. It is therefore important to understand the regulation of hepcidin transcription by these signaling pathways induced by chronic alcohol intake.
Innovations and breakthroughs
The authors have previously demonstrated a causal relationship between alcohol-mediated inhibition of hepcidin in the liver and the increase in intestinal iron transporter expression. Several independent studies have clearly highlighted the importance of toll-like receptor signaling, NF-κB signaling and proinflammatory cytokines in the pathogenesis of ALD. This study investigates the question as to how alcohol can both induce inflammation and suppress hepcidin expression, which is known to be induced by endotoxin and inflammatory cytokines, in the liver.
Applications
The findings in this study suggest that induction of toll-like receptors (TLR) 4 and NF-κB signaling and the inhibition of Stat3 signaling are all involved in the suppression of hepcidin transcription by chronic alcohol consumption. These findings will help us to further understand the mechanisms by which alcohol can induce inflammation and iron accumulation by altering hepcidin expression in the liver. This might ultimately lead to the development of novel diagnostic and treatment strategies for ALD.
Terminology
Inflammation plays a key role in both ALD pathogenesis and iron homeostasis. TLR are important for innate immune responses and inflammation. Endotoxin (LPS) binds to TLR4 receptor and induces the activation of NF-κB signaling and proinflammatory cytokine production. Proinflammatory cytokines activate Jak/Stat pathway including the transcription factor, Stat3.
Peer review
The authors investigated the implication of TLR4 signaling in the activation of hepcidin in mice chronically fed with alcohol. They showed that suppression of hepcidin mRNA levels by alcohol in wild type mice was lost in TLR4 mutant ones.
P- Reviewer: Starkel P, Yang SC S- Editor: Ma N L- Editor: Logan S E- Editor: Ma S