Published online Sep 7, 2014. doi: 10.3748/wjg.v20.i33.11856
Revised: April 29, 2014
Accepted: May 25, 2014
Published online: September 7, 2014
Processing time: 200 Days and 2.5 Hours
AIM: To investigate the diagnostic performance of multi-detector computed tomography (MDCT) in detecting biliary complications after orthotopic liver transplantation (OLT).
METHODS: Eighty-three consecutive OLT recipients, who presented with clinical or biochemical signs of biliary complications, underwent MDCT examination. Two experienced radiologists assessed MDCT images in consensus to determine biliary complications. Final confirmation was based on percutaneous transhepatic cholangiography or endoscopic retrograde cholangiography in 58 patients, surgery in four patients, liver biopsy in 10, and clinical and sonography follow-up in 11 patients.
RESULTS: Biliary complications were eventually confirmed in 62 of 83 patients (74.7%), including anastomotic biliary strictures in 32, nonanastomotic biliary strictures in 21, biliary stones in nine (5 with biliary strictures), anastomotic bile leak in five, and biloma in six (all with nonanastomotic strictures, and 2 with biligenic hepatic abscess). Twenty-one patients had no detection of biliary complications. The sensitivity, specificity, accuracy, positive predictive value and negative predictive value of MDCT for detecting biliary strictures were 90.6%, 86.7%, 89.2%, 92.3% and 83.9%, respectively. For detecting biliary stones, anastomotic bile leak and biloma, the sensitivity, specificity, accuracy, positive predictive value and negative predictive value of MDCT were all 100%.
CONCLUSION: MDCT is a useful screening tool for detecting biliary complications after OLT.
Core tip: The value of multi-detector computed tomography (MDCT) in detecting biliary complications after orthotopic liver transplantation (OLT) is conflicting. This study, with 83 OLT recipients suspected of biliary complications, suggests that MDCT is a useful screening tool for detecting biliary complications after OLT. MDCT presented a sensitivity of 90.6% and specificity of 86.7% for biliary strictures, and both sensitivity and specificity of 100% for biliary stones, anastomotic bile leak and biloma. So far, this is the largest sample population to investigate the diagnostic accuracy of MDCT for biliary complications after OLT, which will help us make a treatment decision.
- Citation: Meng XC, Huang WS, Xie PY, Chen XZ, Cai MY, Shan H, Zhu KS. Role of multi-detector computed tomography for biliary complications after liver transplantation. World J Gastroenterol 2014; 20(33): 11856-11864
- URL: https://www.wjgnet.com/1007-9327/full/v20/i33/11856.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i33.11856
Orthotopic liver transplantation (OLT) is a widely accepted treatment for end-stage liver disease and selected cases of hepatocellular carcinoma. Despite significant improvements in surgical techniques, biliary complications after OLT are still associated with high morbidity and mortality, and still constitute one of the main causes of graft dysfunction or loss of recipients[1]. According to available data, the reported rates of biliary complications range from 5.8% to 30% of liver transplants[2-6], and early detection of these complications and adequate management are crucial for graft and patient survival.
However, early detection of biliary complications after OLT is challenging. Clinical manifestations (such as fever, abdominal complaints, and signs of cholangitis) or biochemical findings are often nonspecific[7], which may relate to a wide range of potential complications, including graft rejection, hepatic arterial thrombosis or stenosis[8,9]. Imaging techniques play a key role in differentiating and evaluating biliary complications after OLT[8-11]. Direct cholangiographic procedures, including endoscopic retrograde cholangiography (ERC) and percutaneous transhepatic cholangiography (PTC), are widely accepted as the gold standards for the diagnosis of biliary complications, which could offer potential therapeutic options simultaneously[9]. However, they are both invasive and carry potential risk of complications, such as bleeding, perforation, sepsis, and even death[3]. Magnetic resonance cholangiography (MRC) is a noninvasive imaging technique, which has been considered as an alternative to invasive cholangiography in many clinical settings for its detailed visualization of the bile duct tree without exogenous contrast[8-13]. However, in some cases, MRC cannot be used for routine examination, especially for patients in poor condition who need to carry monitoring equipment or who cannot hold their breath well. Besides these, the scanning techniques are also important factors that can affect the imaging quality of MRC and the diagnostic accuracy of biliary strictures[3]. These limit the application of MRC in the follow-up of OLT recipients.
With fast scanning speed and high imaging quality, multi-detector computed tomography (MDCT) is considered as the main choice for detecting vascular complications after liver transplantation[11,14,15], which reveals the biliary system at the same time. So, there is the potential to detect biliary complications in one-step MDCT examination, which may help with early detection of biliary complications. However, to the best of our knowledge, there are only a few reports focusing on the role of MDCT for biliary complications after liver transplantation[16,17], and the number of patients was small. Therefore, the aim of the present study was to evaluate MDCT for detecting biliary complications following liver transplantation and comparing findings with ERC and PTC.
Between October 2003 and October 2012, 83 consecutive OLT recipients, who presented with clinical or biological signs of biliary complications, underwent contrast-enhancement MDCT examination at our institution. All patients presented with abnormal liver function tests and a variety of clinical symptoms such as fever and cholangitis. Among these 83 patients, 64 were men and 19 were women, with an age range of 27-63 years (mean, 46.2 years). This study was approved by our Institutional Review Board. Informed consent was obtained from all patients.
In liver transplantation procedures, bile duct continuity was established in all cases by a primary duct-to-duct anastomosis (choledocho-choledochostomy) without T-tube stent. The interval between OLT and clinical onset of biliary complications ranged from 7 d to 68 mo (mean, 13 mo). Indications for liver transplantation were as follows: hepatitis B liver cirrhosis (n = 54), hepatitis C liver cirrhosis (n = 3), hepatocellular carcinoma (n = 21), sclerosing cholangitis (n = 2), idiopathic cirrhosis (n = 2), and Budd-Chiari syndrome (n = 1).
From October 2003 to December 2008, 46 patients underwent CT scans on a GE LightSpeed Qx/I CT scanner (General Electric Medical Systems, Milwaukee, WI, USA) (early-stage MDCT). After December 2008, 37 patients underwent CT on a Toshiba Aquilion ONE CT scanner (Toshiba Medical Systems, Otawara, Tochigi, Japan) (late-stage MDCT). Patients routinely received plain and double-phase contrast-enhanced CT covering the entire craniocaudal extent of the liver and all the vascular anastomoses. After the plain scan, non-ionic iodinated contrast medium (iopromide, Ultravist 300; Schering, Berlin, Germany) was injected via an antecubital vein at a dose of 2 mL/kg at a rate of 4 mL/s through a power injector. Contrast-enhanced CT images were obtained during the hepatic arterial phase with a 25-s delay and the portal venous phase with a 65-s delay after initiation of contrast injection.
Technical parameters for the CT examinations on GE LightSpeed Qx/I were as follows: beam collimation 16 mm, high-speed scan mode (pitch, 6), 5.0-mm slice thickness, 2.5-mm reconstruction slice thickness, and 1.0-mm intervals. Technical parameters on the Toshiba Aquilion ONE CT scanner were as follows: beam collimation 16 mm, 0.5-mm slice thickness and intervals, 1.0-mm reconstruction slice thickness, and 0.5-mm intervals. All CT scanning was performed in the supine position at 240 mA and 120 kV with a standard algorithm.
Further data processing was performed on an Advantage workstation 4.0 (General Electric Medical Systems) or a prototype workstation (Toshiba) equipped with software allowing generation of the oblique reformat. One radiologist experienced in the oblique reformat processed all images to reveal the biliary system. All these images were stored on the hard disk memory of the workstation for subsequent image analysis.
The MDCT transverse images and the oblique reformat images were interpreted in conference by two experienced abdominal radiologists who were blinded to patient identification and all the surgical, pathological and cholangiographic findings. All images were retrospectively evaluated to detect the presence of biliary dilatation, biliary strictures, intra- or extrahepatic fluid collection, and biliary stones. Intrahepatic bile ducts were considered dilated if the maximum diameter was > 3 mm[10,13]. Extrahepatic bile ducts (common hepatic and common bile ducts) were considered dilated if measuring > 8 mm[10,13]. For biliary strictures, the site (anastomotic or nonanastomotic) was decided. A round or irregular slightly high or high-density focus inside the bile duct was termed biliary stones. In the case of inter-observer disagreement, a final diagnosis was reached by consensus.
On the basis of MDCT findings, biliary complications were classified as biliary strictures, anastomotic bile leakage, biloma, biliary stones, and other complications. Biliary strictures were further subclassified as anastomotic and nonanastomotic. Anastomotic strictures were defined as a focal stricture at the site of the biliary anastomosis[8,13]. Nonanastomotic strictures were defined as one or multiple strictures of the extrahepatic bile duct, intrahepatic bile ducts (including the hepatic duct confluence), or both[8,13]. Anastomotic bile leakage was defined as a variable amount of pericholedochal fluid collection having direct communication with biliary anastomosis. Biloma was defined as an intrahepatic fluid collection communicating with dilated intrahepatic bile ducts.
Diagnostic confirmation was obtained by direct cholangiography in 58 patients (PTC in 35 and ERC in 23); surgery in four (retransplantation in 2 and hepaticojejunostomy in 2); liver biopsy in 10; and clinical and sonography follow-up of at least 6 mo in 11 patients. The ERC and PTC images were interpreted in conference by two other observers (a radiologist and a surgeon), who used all the clinical, laboratory, endoscopic and PTC imaging data during the review.
The MDCT findings were compared with the PTC, ERC, surgical, liver biopsy or imaging follow-up results and defined as true positives when they correctly detected biliary complications confirmed by the final diagnosis reference standards; false positives when they were not confirmed by PTC, ERC, surgery, liver biopsy or imaging follow-up; false negatives when complications were detected by PTC, ERC, surgery, liver biopsy or imaging follow-up were not detected by MDCT; or true negatives when the absence of complications was confirmed by PTC, ERC, surgery, liver biopsy or imaging follow-up. The sensitivity, specificity, accuracy, positive predictive value and negative predictive value of the MDCT findings were determined by the reviewers.
A total of 62 biliary complications were eventually confirmed in the 83 patients (74.7%) by PTC, ERC, surgery, liver biopsy or clinical and sonography follow-up, including anastomotic strictures in 32 patients (38.6%), nonanastomotic strictures in 21 (25.3%), biliary stones in nine (10.8%; 5 with biliary strictures), anastomotic bile leakage in five (6.0%), and biloma in six (7.2%) (2 of the 6 patients with nonanastomotic strictures developed biligenic hepatic abscess). Twenty-one patients had no detection of OLT-related biliary complications: seven showed no abnormality, and 14 had other clinical problems, which raised clinical suspicion of a biliary condition, including graft rejection (n = 6), recurrent viral hepatitis (n = 4), transplantation-related lymphoproliferation of hilar and portal area (n = 1), fluid collections in hepatic hilar and portal area (n = 2), and pancreatitis (n = 1) (Table 1).
| Diagnosis | MDCT (83 patients) | Final (83 patients) |
| Normal | 21 | 7 |
| Anastomotic strictures | 34 | 32 |
| Nonanastomotic strictures | 18 | 21 |
| Anastomotic bile leakage | 5 | 5 |
| Biliary stones | 4 | 4 |
| Other complications | ||
| Rejection of the transplanted liver | 0 | 6 |
| Recurrent viral hepatitis | 0 | 4 |
| Transplantation-related lymphoproliferation | 0 | 1 |
| Postoperative hilar and portal fluid | 0 | 2 |
| Pancreatitis | 1 | 1 |
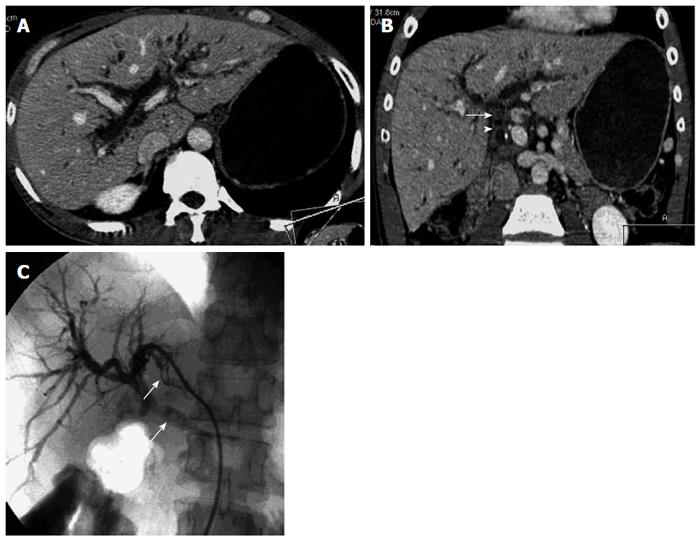
Biliary strictures: In 32 patients with anastomotic strictures, 30 were correctly detected by MDCT, who presented with a short stricture at the anastomosis, with notable biliary dilation above the stricture (Figure 1). The site of the dilated bile duct was intrahepatic in 30 patients (93.8%) and extrahepatic in 27 (84.4%). Two patients with anastomotic strictures were missed at early-stage MDCT (false-negative) due to the absence of biliary dilation. However, ERC confirmed both of them. False-positive findings also occurred in two patients in early-stage MDCT, who presented with mild intra- and extrahepatic biliary dilation and fluid collection in the hilar and portal area. Among 32 patients with anastomotic strictures, five (15.6%) had biliary stones concomitantly, and three (9.4%) were complicated with hepatic artery stenosis.
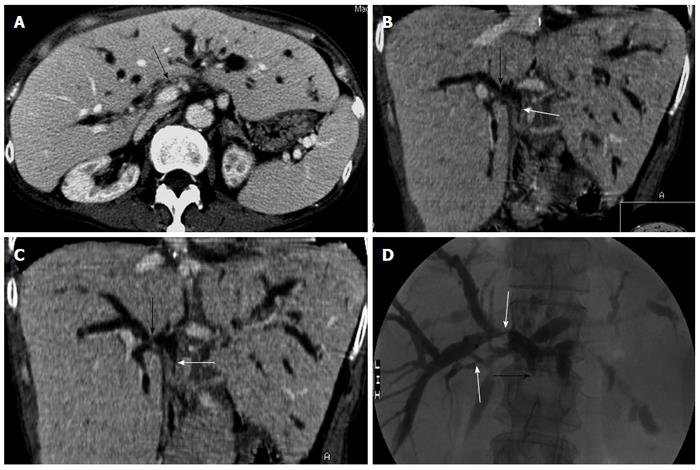
MDCT correctly identified 16 of the 21 cases with nonanastomotic strictures (Figure 2) who presented with one or multiple strictures at the extrahepatic bile duct, the intrahepatic bile ducts (including hepatic confluence), or both. MDCT detected dilation of the intrahepatic bile ducts in 18 patients (85.7%), extrahepatic bile ducts in seven (33.3%), and none in three. Three patients without dilatation were missed (false-negative) by MDCT (one of them occurred in early-stage examination), whose strictures only involved the confluence sites of bile ducts with or without biliary sludge in ERC. Two patients with extrahepatic nonanastomotic strictures were interpreted as anastomotic strictures in early-stage MDCT. There were two false-positive results in two patients with normal bile ducts, who presented with slight intrahepatic biliary dilation in early-stage MDCT. Of the 21 patients with nonanastomotic strictures, 6 (28.6%) were complicated with biloma, and 12 (57.1%) had hepatic artery stenosis.
Biliary stones: MDCT correctly detected all of the cases with biliary stones (n = 9). The stones were typically seen as a round or irregular slightly high or high-density focus inside the dilated bile ducts (Figure 3), and were identified alone (n = 4) or associated with anastomotic strictures (n = 5).
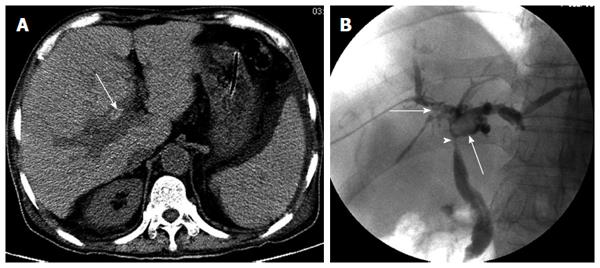
Anastomotic bile leakage: In five patients with anastomotic bile leakage, the observers accurately described the presence of fluid collection adjacent to the anastomotic region (Figure 4), but could not demonstrate active leakage. Anastomotic bile leakage was eventually confirmed by PTC in all five patients.
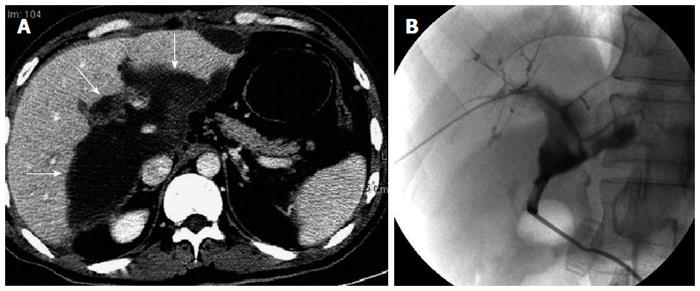
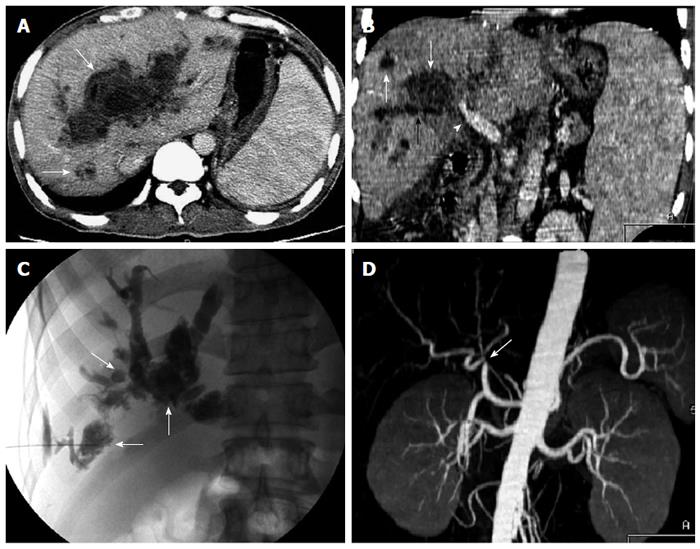
Biloma and biligenic hepatic abscess: Biloma was found in six patients. It was an intrahepatic bile collection (bile lakes), which appeared as a well-circumscribed rounded lesion with fluid-density of variable size on MDCT. MDCT revealed the communication between the biloma and the dilated bile ducts. Among the six patients with biloma, two developed biligenic hepatic abscess due to biliary infection (Figure 5). All six patients with biloma had nonanastomotic biliary strictures and concomitant hepatic arterial stenosis.
Of the 53 patients with biliary strictures, 48 were correctly identified by MDCT (sensitivity, 90.6%) and 5 were missed (false-negative). MDCT correctly identified the absence of biliary strictures in 26/30 patients (specificity, 86.7%) and 4 were misdiagnosed (false-positive). The accuracy, positive predictive value and negative predictive value of MDCT in detecting biliary strictures were 89.2%, 92.3% and 83.9%, respectively. In differentiating anastomotic from nonanastomotic strictures, 46 of 53 patients (86.8%) with biliary strictures were correctly identified by MDCT. Missed diagnosis occurred in 5 patients. The other 2 patients with nonanastomotic strictures were misdiagnosed as anastomotic strictures by MDCT. In evaluating other biliary complications, including biliary stones, anastomotic bile leak and biloma, the sensitivity, specificity, accuracy, positive predictive value and negative predictive value of MDCT were all 100%. The value of MDCT in detecting biliary complications in OLT patients is shown in Table 2.
| Biliary complications | True-positive(n) | True-negative(n) | False-positive(n) | False-negative(n) | Sensitivity | Specificity | Accuracy | Positive predictive value | Negative predictive value |
| Biliary strictures | 48 | 26 | 4 | 5 | 90.6% | 86.7% | 89.2% | 92.3% | 83.9% |
| Biliary stones | 9 | 74 | 0 | 0 | 100% | 100% | 100% | 100% | 100% |
| Anastomotic bile leakage | 5 | 78 | 0 | 0 | 100% | 100% | 100% | 100% | 100% |
| Biloma | 6 | 77 | 0 | 0 | 100% | 100% | 100% | 100% | 100% |
Biliary strictures are the most common type of late biliary complications after transplantation, which account for 85.5% (53/62) of biliary complications in our study. Elevation of liver enzymes and hyperbilirubinemia in OLT recipients may be caused by a variety of reasons, such as graft rejection, recurrence of underlying liver disease, biliary strictures and/or biliary stones. Imaging evaluation is always necessary to rule out biliary strictures in these patients. Usually, biliary strictures occur from 3 to 5 mo after transplantation, so direct cholangiography via postsurgical T-tube is always impossible. Although sonography is a simple method for noninvasive screening test, its low sensitivity and high rate of false-negative results in detecting biliary strictures[18-20] limit its usefulness in evaluating biliary complications for OLT recipients. Sensitivity and specificity of MRC for post-transplant biliary complications are reported to be high, however, its diagnostic performance in detecting biliary strictures is often affected by the technique and patients’ conditions[8-13]. It has been reported that MDCT can demonstrate biliary complications after liver transplantation, however, the detailed results are not available[11,14]. To the best of our knowledge, only one study reported the diagnostic value of CT on biliary strictures after liver transplantation, however, the small study population could not sufficiently illuminate the potential role of CT[16]. Therefore, the performance of MDCT in detecting biliary strictures needs to be further established.
In our study, MDCT presented a sensitivity of 90.6%, specificity of 86.7%, accuracy of 89.2%, positive predictive value of 92.3%, and negative predictive value of 83.9% for the detection of biliary strictures in 83 post-OLT recipients who received MDCT examination. Compared with Zoepf et al[16], our study revealed a higher sensitivity and specificity. This may be attributed to the improvements of MDCT techniques, which allow the thinner scans and post-processing of bile ducts in any desired planar with oblique reformat. The combination of the transverse MDCT images and oblique reformat images can help to visualize the bile duct tree and the sites of biliary strictures.
Suprastenotic dilation of the bile ducts is an important sign for the detection of biliary strictures on MDCT. In this study, among the 53 patients with biliary strictures, 48 (90.6%) presented with suprastenotic biliary dilatation with a variable degree on MDCT. However, the absence of dilatation on MDCT could not completely deny the existence of biliary strictures. All five patients who were missed (false-negative) by MDCT in this study did not present with biliary dilation. This may have been due to the fact that the marked thickened bile duct wall limited its dilation, or that a denervated donor liver that developed reperfusion or ischemic injury during preservation may show no physiological response to the intraductal pressure[21-23]. Some recipients with biliary strictures do not develop bile duct dilation even in high-grade stenosis after OLT[23]. Therefore, if biliary strictures are highly suspected and MDCT does not provide any evidence of biliary dilation, direct cholangiography (ERC or PTC) should be performed to rule out biliary strictures. It is worth noting that, in some patients, slight biliary dilation is not associated with biliary obstruction. In our study, four patients with slight biliary dilation on MDCT were misdiagnosed as biliary strictures. Fluid collection in the hilar area may be attributed to two of them, which affected the visualization of biliary anastomosis. The cause of biliary dilatation in the other two cases was unclear. Some researchers presumed that this might result from papillary dyskinesia due to devascularization or denervation of the papilla of Vater during transplantation[24].
Biliary strictures are classified as anastomotic or nonanastomotic strictures according to their site. The identification of the type of biliary strictures can help us determine the therapeutic schedule. For the patients only with anastomotic strictures, balloon dilation or stent placement through endoscopic or percutaneous transhepatic methods often can bring good therapeutic results[25,26]. However, for most of the patients with nonanastomotic strictures, endoscopic and/or percutaneous transhepatic biliary drainage or balloon dilation may not reverse the bile duct injuries, and retransplantation may be eventually required for a large portion of them[27,28]. In our study, MDCT could correctly identify the type of strictures in 46 of 53 patients (86.8%), including 30 patients with anastomotic strictures and 16 with nonanastomotic strictures, which provided useful information for the treatment.
Our study further demonstrated that hepatic artery stenosis was an important cause of biliary strictures. In our series, MDCT found hepatic artery stenosis in 57.1% of patients with nonanastomotic strictures, and in 15.6% of patients with anastomotic strictures. MDCT can accurately detect hepatic artery complications, with a sensitivity of 100%, specificity of 89%, and accuracy of 93%[29]. Early diagnosis of hepatic artery stenosis by MDCT may allow successful treatment, with urgent surgical revascularization of the graft or with percutaneous angioplasty, and avoid retransplantation before the development of severe hepatic failure[30-32].
Biliary stones are another important complication after OLT, which usually occur with biliary strictures. MDCT was effective in detecting biliary stones, whose sensitivity, specificity and accuracy were all 100% in our study.
Most anastomotic bile leakages occur in the early period after OLT, and > 70% occur within the first month. In our study, all the anastomotic bile leakage appeared as pericholedochal circumscribed fluid collections on MDCT. It was difficult to distinguish a circumscribed perianastomotic ascitic or postoperative fluid from bile leakage. However, if a fluid collection adjacent to the anastomotic region is detected, anastomotic bile leakage should be considered and ERC or PTC performed for the straight demonstration of active leakage[8,10]. In our study, five patients with anastomotic bile leakage were eventually confirmed by PTC.
Biloma mainly relates to the necrosis of bile duct wall due to hepatic arterial insufficiency, which usually occurs with biliary strictures. In this study, MDCT correctly revealed all six patients with biloma and their complicated nonanastomotic strictures and hepatic arterial stenosis. Two of them presented with biligenic hepatic abscesses due to secondary infection of biloma, and both received retransplantation after CT.
The major drawback of MDCT is that MDCT cannot directly display the bile duct tree and the sites of biliary strictures, as MRC, ERC or PTC can. However, this does not affect the ability of MDCT in the diagnosis of biliary strictures, especially for a CT scanner with 0.5-mm quantum detector, which can achieve 0.35 mm × 0.35 mm × 0.35 mm isotropic voxel size for fine-detail imaging. In our study, for all patients who received late-stage MDCT, raw data were acquired with 0.5-mm slice thickness and intervals. Based on those, bile duct reconstruction on any desired planar with oblique reformat could clearly visualize the bile duct tree and the sites of biliary strictures, although they are not as intuitive as those on MRC images. This might be the reason why false-positive and false-negative cases of biliary strictures were fewer in late-stage than early-stage MDCT (7 patients vs 2 patients). Similar to MRC, it is still difficult to measure precisely the length and degree of strictures based on MDCT images. In contrast, ERC and PTC demonstrate more advantages in showing the site, length and grade of the biliary strictures. Besides these, the inherent drawbacks of MDCT are the applied radiation dose and intravenous application of iodinated contrast medium, which are harmful to the recipients, especially in those with graft or renal dysfunction after transplantation.
In conclusion, MDCT is a useful imaging procedure in the detection of biliary complications after liver transplantation. Biliary dilation is an important sign for the detection of biliary strictures on MDCT, but the absence of dilation cannot rule out biliary strictures. For those who present with symptoms of biliary obstruction but without evidence of biliary dilation on MDCT, ERC or PTC should be considered as early as possible. Fluid collection of bile leakage, biliary stones and biloma can be clearly detected by MDCT, but it is difficult to reveal active leakage.
Multi-detector computed tomography (MDCT) is considered as the main choice for detecting vascular complications after orthotopic liver transplantation (OLT), which also demonstrates biliary complications in one-step examination. However, the value of MDCT in the detection of biliary complications after OLT is conflicting.
Early detection of biliary complications after OLT is challenging. Clinical manifestations or biochemical findings are often nonspecific. This study suggests that MDCT is a useful screening tool for detecting biliary complications after OLT, with high rates of sensitivity and specificity.
This is the largest sample population to investigate the diagnostic accuracy of MDCT for biliary complications after OLT.
This study suggests the performance of MDCT in detecting biliary complications after OLT, which will provide useful information for guiding the treatment of biliary complications.
Biliary complications after OLT usually are classified as biliary strictures, anastomotic bile leakage, biliary stones, biloma, biligenic hepatic abscess, and other complications. The incidence ranges from 5.8% to 30% of liver transplants, and early detection of these complications and adequate management are crucial for graft and patient survival.
This was a retrospective study regarding the value of MDCT for the detection of post-OLT biliary complications in 83 OLT recipients. The main value of this study is its large sample population and the point that MDCT can reveal biliary complications with high rates of sensitivity and specificity. The images are also educational even for clinicians not directly involved in liver transplantation.
P- Reviewer: Fourtounas C, Salvadori M S- Editor: Nan J L- Editor: Kerr C E- Editor: Ma S
| 1. | Porayko MK, Kondo M, Steers JL. Liver transplantation: late complications of the biliary tract and their management. Semin Liver Dis. 1995;15:139-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Hernandez Q, Ramirez P, Munitiz V, Piñero A, Robles R, Sanchez-Bueno F, Rodriguez JM, Lujan J, Acosta F, Miras M. Incidence and management of biliary tract complications following 300 consecutive orthotopic liver transplants. Transplant Proc. 1999;31:2407-2408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Jorgensen JE, Waljee AK, Volk ML, Sonnenday CJ, Elta GH, Al-Hawary MM, Singal AG, Taylor JR, Elmunzer BJ. Is MRCP equivalent to ERCP for diagnosing biliary obstruction in orthotopic liver transplant recipients? A meta-analysis. Gastrointest Endosc. 2011;73:955-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Qian YB, Liu CL, Lo CM, Fan ST. Risk factors for biliary complications after liver transplantation. Arch Surg. 2004;139:1101-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Thethy S, Thomson BNj, Pleass H, Wigmore SJ, Madhavan K, Akyol M, Forsythe JL, James Garden O. Management of biliary tract complications after orthotopic liver transplantation. Clin Transplant. 2004;18:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 173] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Enestvedt CK, Malik S, Reese PP, Maskin A, Yoo PS, Fayek SA, Abt P, Olthoff KM, Shaked A. Biliary complications adversely affect patient and graft survival after liver retransplantation. Liver Transpl. 2013;19:965-972. [PubMed] [DOI] [Full Text] |
| 7. | Verdonk RC, Buis CI, Porte RJ, Haagsma EB. Biliary complications after liver transplantation: a review. Scand J Gastroenterol Suppl. 2006;89-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Girometti R, Cereser L, Como G, Zuiani C, Bazzocchi M. Biliary complications after orthotopic liver transplantation: MRCP findings. Abdom Imaging. 2008;33:542-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Katz LH, Benjaminov O, Belinki A, Geler A, Braun M, Knizhnik M, Aizner S, Shaharabani E, Sulkes J, Shabtai E. Magnetic resonance cholangiopancreatography for the accurate diagnosis of biliary complications after liver transplantation: comparison with endoscopic retrograde cholangiography and percutaneous transhepatic cholangiography - long-term follow-up. Clin Transplant. 2010;24:E163-E169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Novellas S, Caramella T, Fournol M, Gugenheim J, Chevallier P. MR cholangiopancreatography features of the biliary tree after liver transplantation. AJR Am J Roentgenol. 2008;191:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Zamboni GA, Pedrosa I, Kruskal JB, Raptopoulos V. Multimodality postoperative imaging of liver transplantation. Eur Radiol. 2008;18:882-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Xu YB, Bai YL, Min ZG, Qin SY. Magnetic resonance cholangiography in assessing biliary anatomy in living donors: a meta-analysis. World J Gastroenterol. 2013;19:8427-8434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Valls C, Alba E, Cruz M, Figueras J, Andía E, Sanchez A, Lladó L, Serrano T. Biliary complications after liver transplantation: diagnosis with MR cholangiopancreatography. AJR Am J Roentgenol. 2005;184:812-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Quiroga S, Sebastià MC, Margarit C, Castells L, Boyé R, Alvarez-Castells A. Complications of orthotopic liver transplantation: spectrum of findings with helical CT. Radiographics. 2001;21:1085-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Marubashi S, Kobayashi S, Wada H, Kawamoto K, Eguchi H, Doki Y, Mori M, Nagano H. Hepatic artery reconstruction in living donor liver transplantation: risk factor analysis of complication and a role of MDCT scan for detecting anastomotic stricture. World J Surg. 2013;37:2671-2677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Zoepf T, Maldonado-Lopez EJ, Hilgard P, Dechêne A, Malago M, Broelsch CE, Schlaak J, Gerken G. Diagnosis of biliary strictures after liver transplantation: which is the best tool? World J Gastroenterol. 2005;11:2945-2948. [PubMed] |
| 17. | Barton P, Maier A, Steininger R, Mühlbacher F, Lechner G. Biliary sludge after liver transplantation: 1. Imaging findings and efficacy of various imaging procedures. AJR Am J Roentgenol. 1995;164:859-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Hussaini SH, Sheridan MB, Davies M. The predictive value of transabdominal ultrasonography in the diagnosis of biliary tract complications after orthotopic liver transplantation. Gut. 1999;45:900-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Campbell WL, Sheng R, Zajko AB, Abu-Elmagd K, Demetris AJ. Intrahepatic biliary strictures after liver transplantation. Radiology. 1994;191:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Scatton O, Meunier B, Cherqui D, Boillot O, Sauvanet A, Boudjema K, Launois B, Fagniez PL, Belghiti J, Wolff P. Randomized trial of choledochocholedochostomy with or without a T tube in orthotopic liver transplantation. Ann Surg. 2001;233:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 182] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Shaw AS, Ryan SM, Beese RC, Sidhu PS. Ultrasound of non-vascular complications in the post liver transplant patient. Clin Radiol. 2003;58:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Kok T, Van der Sluis A, Klein JP, Van der Jagt EJ, Peeters PM, Slooff MJ, Bijleveld CM, Haagsma EB. Ultrasound and cholangiography for the diagnosis of biliary complications after orthotopic liver transplantation: a comparative study. J Clin Ultrasound. 1996;24:103-115. [PubMed] [DOI] [Full Text] |
| 23. | St Peter S, Rodriquez-Davalos MI, Rodriguez-Luna HM, Harrison EM, Moss AA, Mulligan DC. Significance of proximal biliary dilatation in patients with anastomotic strictures after liver transplantation. Dig Dis Sci. 2004;49:1207-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Fulcher AS, Turner MA. Orthotopic liver transplantation: evaluation with MR cholangiography. Radiology. 1999;211:715-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Cantù P, Tenca A, Donato MF, Rossi G, Forzenigo L, Piodi L, Rigamonti C, Agnelli F, Biondetti P, Conte D. ERCP and short-term stent-trial in patients with anastomotic biliary stricture following liver transplantation. Dig Liver Dis. 2009;41:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Weber A, Prinz C, Gerngross C, Ludwig L, Huber W, Neu B, Ebert MP, Meining A, Weidenbach H, Schmid RM. Long-term outcome of endoscopic and/or percutaneous transhepatic therapy in patients with biliary stricture after orthotopic liver transplantation. J Gastroenterol. 2009;44:1195-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Barriga J, Thompson R, Shokouh-Amiri H, Davila R, Ismail MK, Waters B, Tombazzi CR. Biliary strictures after liver transplantation. Predictive factors for response to endoscopic management and long-term outcome. Am J Med Sci. 2008;335:439-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Verdonk RC, Buis CI, van der Jagt EJ, Gouw AS, Limburg AJ, Slooff MJ, Kleibeuker JH, Porte RJ, Haagsma EB. Nonanastomotic biliary strictures after liver transplantation, part 2: Management, outcome, and risk factors for disease progression. Liver Transpl. 2007;13:725-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Boraschi P, Donati F. Complications of orthotopic liver transplantation: imaging findings. Abdom Imaging. 2004;29:189-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Saad WE, Davies MG, Sahler L, Lee DE, Patel NC, Kitanosono T, Sasson T, Waldman DL. Hepatic artery stenosis in liver transplant recipients: primary treatment with percutaneous transluminal angioplasty. J Vasc Interv Radiol. 2005;16:795-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Huang M, Shan H, Jiang Z, Li Z, Zhu K, Guan S, Qian J, Chen G, Lu M, Yang Y. The use of coronary stent in hepatic artery stenosis after orthotopic liver transplantation. Eur J Radiol. 2006;60:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Hamby BA, Ramirez DE, Loss GE, Bazan HA, Smith TA, Bluth E, Sternbergh WC. Endovascular treatment of hepatic artery stenosis after liver transplantation. J Vasc Surg. 2013;57:1067-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |