Published online Sep 7, 2014. doi: 10.3748/wjg.v20.i33.11793
Revised: May 5, 2014
Accepted: May 26, 2014
Published online: September 7, 2014
Processing time: 185 Days and 22 Hours
AIM: To investigate the association of the functional monocyte chemotactic protein-1 (MCP-1) promoter polymorphism (A-2518G) with spontaneous bacterial peritonitis (SBP).
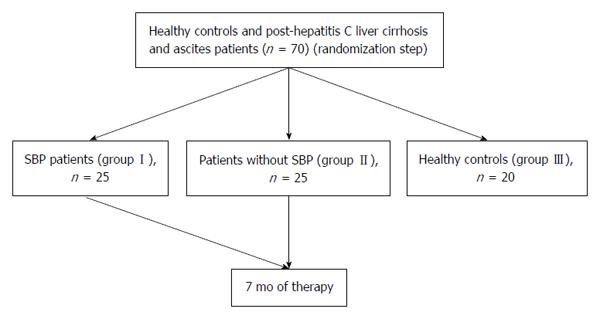
METHODS: Fifty patients with post-hepatitis C liver cirrhosis and ascites were categorized into two groups; group I included 25 patients with SBP and group II included 25 patients free from SBP. In addition, a group of 20 healthy volunteers were included. We assessed the MCP-1 gene polymorphism and gene expression as well as interleukin (IL)-10 levels in both blood and ascitic fluid.
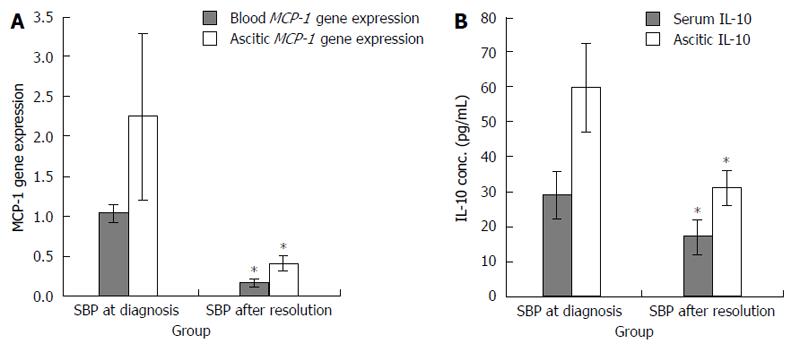
RESULTS: A significant MCP-1 gene polymorphism was detected in groups I and II (P = 0.001 and 0.02 respectively). Group I was associated with a significantly higher frequency of AG genotype [control 8 (40%) vs SBP 19 (76.0%), P < 0.001], and group II was associated with a significantly higher frequency of GG genotype when compared to healthy volunteers [control 1 (5%) vs cirrhotic 16 (64%), P < 0.001]. Accordingly, the frequency of G allele was significantly higher in both groups (I and II) [control 10 (25%) vs SBP 27 (54%), P < 0.001 and vs cirrhotic 37 (74.0%), P < 0.001, respectively]. The total blood and ascetic fluid levels of IL-10 and MCP-1 gene expression were significantly higher in group I than in group II. Group I showed significant reductions in the levels of MCP-1 gene expression and IL-10 in the whole blood and ascetic fluid after therapy.
CONCLUSION: MCP-1 GG genotype and G allele may predispose HCV infected patients to a more progressive disease course, while AG genotype may increase the susceptibility to SBP. Patients carrying these genotypes should be under supervision to prevent or restrict further complications.
Core tip: Monocyte chemotactic protein-1 (MCP-1) polymorphism was investigated in hepatitis C virus (HCV) infected patients because of the higher susceptibility of cirrhosis and ascites patients to bacterial infections. MCP-1 secretion is up-regulated during chronic hepatitis and correlates with the severity of hepatic inflammation. Inheritance of MCP-1 GG genotype and MCP-1 G allele may predispose HCV infected patients to a more progressive disease course, while AG genotype may be a risk factor for spontaneous bacterial peritonitis (SBP) in patients with decompensated post-hepatitis C cirrhosis. MCP-1 expression and elevated IL-10 levels may be related to the development of SBP. HCV cirrhotic and SBP patients carrying the above genotypes should be under supervision and monitoring.
- Citation: Salama MK, Sabry D, Al-Ghussein MA, Ahmed R, AbdAllah S, Taha FM, Fathy W, Wadie MS, Nabih M, Abul-Fotouh A, Darwish T. Molecular detection of monocyte chemotactic protein-1 polymorphism in spontaneous bacterial peritonitis patients. World J Gastroenterol 2014; 20(33): 11793-11799
- URL: https://www.wjgnet.com/1007-9327/full/v20/i33/11793.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i33.11793
Patients with cirrhosis and ascites show higher susceptibility to bacterial infections, mainly because of the inadequate defense mechanisms[1-3]. Factors influencing the development of spontaneous bacterial peritonitis (SBP) in patients with liver cirrhosis are poorly understood. Previous studies have indicated that peritoneal macrophages of cirrhotic patients might contribute to the control of SBP or influence its associated pathology in human cirrhosis by producing high quantities of angiogenic peptides and nitric oxide[4,5]. SBP can be caused by many reasons due to the alterations of the immune system that are very common in patients with end-stage liver disease and associated with an increased risk of infection and death[6,7]. Consequently, elevated concentrations of pro-inflammatory cytokines are found in ascitic fluid of these patients[8,9]. In addition, hepatitis C virus (HCV) infection is associated with increased hepatic expression of monocyte chemotactic protein-1 (MCP-1)[10].
MCP-1 acts as a chemotactic factor for monocytes/macrophages, activated lymphocytes and neutrophils during infections[11,12]; thus, these cells migrate to the ascitic fluid. Monocytes and macrophages release TNF-α and other cytokines, which in turn induce the expression of adhesion molecules on endothelial cells, thereby mediating a systemic reaction to the infection[11,12]. TNF-α has been shown to be elevated in the ascitic fluid of SBP patients, stimulating the release of interleukin-8 (IL-8), growth-related oncogene-α (GRO-α), and MCP-1 by mononuclear cells or endothelial cells. This release propagates the inflammatory reaction[13]. MCP-1 secretion is up-regulated during chronic hepatitis and correlates with the severity of hepatic inflammation[14,15]. Furthermore, a previous study showed elevated MCP-1 levels in ascitic fluid of cirrhotic patients with SBP compared to patients without SBP[13].
The aim of this work was to study the association of the functional MCP-1 promoter polymorphism (A-2518G) with SBP and investigate the expression of MCP-1 in blood and ascites as well as serum and ascitic IL-10 levels.
The case-control study protocol was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. After being approved by the Institutional Review Board of Kasr El-Aini Hospital, the present study was conducted on 50 patients with post-hepatitis C liver cirrhosis and ascites attending the Kasr El-Aini Cairo University Hospital from February 2012 to September 2012. The study population is illustrated in Figure 1. Patients were categorized into two groups according to the presence of SBP or not as follows; group I (n = 25) included patients with SBP proved by ascitic fluid polymorphonuclear leukocyte (PMN) count ≥ 250 cells/mm3, and group II (n = 25) included patients without SBP. Patients with alcoholic liver cirrhosis, Wilson’s disease, hemochromatosis, glycogen storage disease and malignant or tuberculous ascites were excluded from this study. As an additional control group (group III), 20 healthy volunteers (15 males and 5 females) with a mean age of 48.28 ± 4.56 years were included in the study, and they were recruited from the members of the Medical Biochemistry Department, Faculty of Medicine.
Written informed consent to participate in the study was obtained from all participants. After that, they were subjected to a detailed medical history assessment and laboratory investigation (complete blood count, liver and renal function tests). Serum IL-10 level assessment, quantitative assessment of MCP-1 gene expression in blood and detection of MCP-1 gene polymorphism were performed. The ascitic fluid of patients of both groups I and II was analysed for IL-10 level and the quantitative assessment of MCP-1 gene expression. Appropriate antibiotic medication therapy was prescribed for patients of group I and after the ascitic fluid PMN count became less than 250 cells/mm3, they were reassessed by measuring the MCP-1 gene expression in the whole blood and in the ascitic fluid in addition to the IL-10 level in both serum and ascitic fluid.
Genomic DNA was prepared from venous blood samples using the Innu PREP blood DNA mini kit (Analytic Jena, Germany) following the manufacturer’s instructions. The identification of the polymorphism was carried out using polymerase chain reaction (PCR), followed by a restriction fragment length polymorphism (RFLP) assay, using a PvuII site, which is introduced by the presence of the G nucleotide. The regulatory region of the MCP-1 gene (from -2746 at -1817) was amplified by PCR using a forward primer (5′-CCGAGATGTTCCCAGCACAG-3′) and a reverse primer (5′-CTGCTTTGCTTGTGCCTCTT-3′)[16].
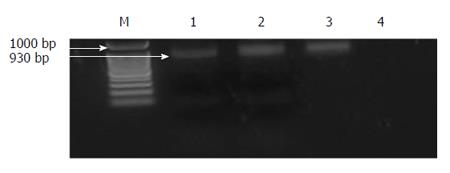
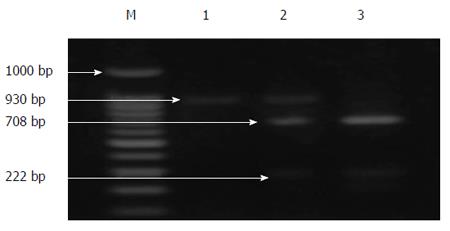
PCR was performed in a 40 μL reaction system containing 10 × buffer (10 mmol/L Tris-HCl pH 9, 2.0 mmol/L MgCl2, 50 mmol/L KCl), 200 μm dNTPs, 2.5 pmole of each primer, 5 μL of DNA, 0.5 U Taq polymerase (Amersham Pharmacia Biotech, Piscataway, NJ, USA) and ddH2O. The following thermal profiles were run: 95 °C for 40 s, 56 °C for 30 s, and 72 °C for 4 min. After a final extension of 10 min at 72 °C, 7 μL of the PCR products were resolved on 2% agarose gels and stained with ethidium bromide to visualize the expected 930-bp band. After visualization, 8 μL of the PCR products were digested with 10 U of PvuII in 10× buffer and H2O up to a final volume of 20 μL at 37 °C for 2 h. The resulting products were separated by electrophoresis on 1.5% agarose gels, containing ethidium bromide at a final concentration of 0.5 g/mL. Samples showing only a 930-bp band were assigned as A/A, those showing two bands at 708 and 222 bp were considered G/G and those showing three bands at 930, 708 and 222 bp were typed as A/G.
RNA extraction from blood and ascitic fluid samples: SV total RNA isolation system (Promega, USA) was used to extract RNA.
Primer design and selection: All primers were designed based on target sequences obtained from the reference[17].
cDNA synthesis: The extracted RNA was reverse transcribed into cDNA using RT-PCR kit (Stratagene USA)[18].
Real-time quantitative PCR (qPCR) amplification and analysis were performed using an Applied Biosystem with software version 3.1 (StepOne™, USA). The qPCR assay with the primer sets were optimized at the annealing temperature. All cDNA including previously prepared samples, internal control (for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene expression as housekeeping gene), and non-template control (water to confirm the absence of DNA contamination in the reaction mixture), were in duplicate. The sequences of the primers used for amplification of the GAPDH gene were forward, 5’CGCTCTCTGCTCCTCCTGTT 3’ and reverse, 5’ CCATGGTGTCTGAGCGATGT 3’[19].
IL-10 was analysed using kits produced by Orgenium Laboratories Business Unit (Vantaa, Finland)[20].
The results were analysed using the SPSS software package version 9.0 (Chicago, IL, USA). Quantitative data are expressed as mean ± standard deviation (SD). Differences between two groups were compared by the Student’s t-test. Genotype and allele frequencies were reported as percentages and the differences between groups were determined by χ2 test. Correlations between data were performed using Pearson and Spearman correlation tests as required. Differences were considered significant at P < 0.05.
The demographic and clinical data of the studied groups are presented in Table 1. Patients of both groups and the healthy controls were age and sex matched. There was no statistically significant difference between both studied groups of patients regarding the studied laboratory data, except for significantly higher levels of the MCP-1 gene expression in whole blood (cirrhotic 0.112 ± 0.046 vs SBP 1.04 ± 0.119, P < 0.001) and serum IL-10 in SBP patients (cirrhotic 15.91 ± 4.53 vs SBP 29.26 ± 7.037, P < 0.001).
| Control group(n = 20) | Group I (SBP)(n = 25) | Group II (cirrhotic)(n = 25) | |
| Age (yr) | 48.28 ± 4.56 | 51.24 ± 9.3 | 47.08 ± 12.9 |
| Sex (male) | 15 (75) | 18 (72) | 15 (60) |
| BMI (kg/m2) | 28.42 ± 2.33 | 27.94 ± 2.1 | 28.2 ± 1.8 |
| DM (Yes) | 0 | 5 (20) | 8 (32) |
| GIT bleeding (Yes) | 0 | 5 (20) | 5 (20) |
| Hepatic encephalopathy (Yes) | 0 | 9 (36) | 7 (28) |
| Duration of liver cirrhosis (yr) | 0 | 4.46 ± 5 | 4.04 ± 3.26 |
| Duration of ascites (yr) | 0 | 1 ± 2.01 | 1.71 ± 1.54 |
| Hemoglobin (g/dL) | 12.6 ± 1.6 | 10.27 ± 1.95b | 9.5 ± 2.22b |
| Platelets (103/μL) | 158.4 ± 12.8 | 146.6 ± 90.2 | 118.6 ± 35.9b |
| TLC (103/μL) | 6.3 ± 0.97 | 5.82 ± 3.05 | 6.7 ± 2.58 |
| Serum albumin (g/dL) | 4.34 ± 0.62 | 2.26 ± 0.39b | 2.3 ± 0.46b |
| Total bilirubin (mg/dL) | 1.036 ± 0.064 | 5.42 ± 8.7b | 2.69 ± 2.65b |
| Direct bilirubin (mg/dL) | 0.176 ± 0.078 | 3.07 ± 5.3b | 1.44 ± 1.58b |
| Urea (mg/dL) | 17.4 ± 3.3 | 59.45 ± 26.6ab | 42.8 ± 24.49b |
| Creatinine (mg/dL) | 0.86 ± 0.208 | 1.96 ± 1.79b | 1.5 ± 0.95b |
| AST (IU/L) | 47.96 ± 7.7 | 69.86 ± 38.03a | 85.8 ± 52.99b |
| ALT (IU/L) | 23.79 ± 7.5 | 39.7 ± 16.03b | 40.17 ± 24.76a |
| ALP (IU/L) | 95.5 ± 19.8 | 167.9 ± 69.49b | 101.4 ± 39.67 |
| INR | 0.996 ± 0.13 | 1.84 ± 0.59b | 1.66 ± 0.40b |
| MCP-1 gene expression in whole blood | 0.131 ± 0.0367 | 1.04 ± 0.119bd | 0.112 ± 0.046 |
| Serum IL-10 (pg/mL) | 14.48 ± 3.29 | 29.26 ± 7.037bd | 15.91 ± 4.53 |
| PMN count in ascites (cells/mm3) | - | 1194.6 ± 1187.6d | 110.3 ± 60.89 |
| Serum-Ascites Albumin gradient (SAAG) (g/dL) | - | 1.34 ± 0.107d | 1.67 ± 0.32 |
| Ascitic IL-10 (pg/mL) | - | 60.07 ± 12.67d | 16.86 ± 5.2 |
| Ascitic MCP-1 gene expression | - | 2.251 ± 1.039d | 1.5 ± 0.59 |
MCP-1 polymorphism in all the studied groups is presented in Table 2. Our results showed that the genotype frequencies in the healthy controls did not depart from those expected on the basis of Hardy-Weinberg equilibrium (P = 0.76). However, in cirrhotic patients without SBP (group II) and those with SBP (group I), the observed and expected frequencies were significantly different (P = 0.02 and 0.001, respectively). When compared to normal healthy volunteers, a significantly higher frequency of the GG genotype was reported in cirrhotic patients without SBP (group II) [control 1 (5%) vs cirrhotic 16 (64%), P < 0.001], while a significantly higher frequency of the AG genotype was reported with cirrhotic patients with SBP (group I) [control 8 (40%) vs SBP 19 (76.0%), P < 0.001]. When comparing the two groups of patients with each other, a significantly higher frequency of the GG genotype was reported with cirrhotic patients without SBP (group II) [SBP 4 (16%) vs cirrhotic 16 (64%), P < 0.001], while a significantly higher frequency of the AG genotype was reported with cirrhotic patients with SBP (group I) [SBP 19 (76.0%) vs cirrhotic 5 (20%), P <0.001]. Accordingly, there was a significantly higher frequency of the G allele in both groups of patients (I and II) when compared to healthy volunteers (control 10 (25%) vs SBP 27 (54%), P < 0.001 and vs cirrhotic 37 (74.0%), P < 0.001 respectively). When comparing both groups of patients with each other, it was revealed that the G allele represented 54% in those with SBP (group I) vs 74% in those without SBP (group II) [SBP 27 (54.0%) vs cirrhotic 37 (74%), P < 0.001], while the A allele represented 46% in those with SBP (group I) vs 26% in those without SBP (group II) [SBP 23(46.0%) vs cirrhotic 13 (26%), P < 0.001], and these differences were statistically significant.
Results are presented in Table 1. Our results revealed that the ascitic fluid levels of the IL-10 and MCP-1 gene expression were significantly higher in patients with SBP (group I) than those without SBP (group II).
Cirrhotic patients with SBP (group I) showed significant reductions in the levels of MCP-1 gene expression and IL-10 in the whole blood and ascitic fluid after therapy (Figure 2A and B). In cirrhotic patients with SBP a significant positive relationship was detected between the MCP-1 gene expression in the whole blood and the duration of liver disease (r = 0.46, P = 0.02). Also, a significant positive relationship was detected between the serum IL-10 and both the SAAG and the serum albumin level (r = 0.623 and 0.472, P = 0.023 and 0.02, respectively). In addition, a significant positive relationship was detected between the ascitic MCP-1 gene expression and the total bilirubin level (r = 0.535, P = 0.03). Contrarily, a significant negative relationship was detected between the ascitic MCP-1 gene expression and the total leucocytic count (TLC) (r = 0.671, P = 0.003). However, these relationships were statistically insignificant in cirrhotic patients without SBP. On the other hand, a significant positive relationship was detected between the serum IL-10 and the urea level (r = 0.449, P = 0.036), as well as between the ascitic MCP-1 gene expression and the serum creatinine level (r = 0.57, P = 0.01). A significant negative relationship was detected between the ascitic IL-10 and the duration of the liver cirrhosis (r = 0.39, P = 0.048). PCR products for MCP-1 gene (930 bp) before digestion with restriction enzyme for different groups are shown in Figure 3. PCR products for MCP-1 gene (930 bp) after digestion with restriction enzyme in Figure 4 showed A/A genotype at 930 bp, A/G genotype at 930, 708 and 222 bp and G/G genotype at 708 and 222 bp.
Interestingly, a significant MCP-1 genotype polymorphism was observed in cirrhotic patients with and without SBP in our study, which was not observed in the healthy Egyptian volunteers. Further analysis showed that cirrhotic patients without SBP were associated with a higher frequency of GG genotype, while those with SBP were associated with a higher frequency of AG genotype. Also, it was found that the G allele frequency was significantly higher in both the cirrhotic patients with and without SBP than the healthy volunteers as well as being higher in the cirrhotic patients without SBP than in those with SBP. This result is in agreement with the finding by Gäbele et al[21], who reported that carriers of the G allele of the MCP-1 polymorphism were more frequent in patients with alcohol induced cirrhosis than in heavy drinkers without evidence of liver damage (controls). Also, in a previous study, carriers of the G allele were significantly more frequent in HCV patients with more advanced fibrosis and severe inflammation[14]. In vitro stimulated monocytes from individuals carrying a G allele at -2518 produced more MCP-1 than cells from A/A homozygous subjects[22]. Carriers of the G allele were significantly more frequent in HCV patients with more advanced fibrosis and severe inflammation[15].
In patients with SBP, MCP-1 acts as a chemotactic factor for monocytes and macrophages; thus, these cells migrate to the ascitic fluid. These monocytes and macrophages release TNF-α and other cytokines, which in turn induce the expression of adhesion molecules on endothelial cells, thereby mediating a systemic reaction to the infection[11,12]. This explains the significant increase, reported in our study, of the mean level of the MCP-1 gene expression in both blood and ascetic fluid of cirrhotic patients with SBP compared with cirrhotic patients without SBP, which was in agreement with previous studies[21-23]. These findings suggest that this potent chemokine plays a pathophysiological role during the development and the course of SBP. As well in our study, the SBP patients showed a significant increase in the mean level of PMN count compared with cirrhotic patients without SBP. In the present study, the mean level of MCP-1 gene expression in blood was higher in control subjects than in cirrhotic patients without SBP. However, this difference was not statistically significant. This is in concordance with what was reported by Nischalke et al[24], who found that the MCP-1 was markedly lower in HCV-infected patients than in controls, and it was explained by the down-regulation of MCP-1 expression by viral proteins and the inhibition of activity of the MCP-1 gene promoter by HCV core protein.
In agreement with the results of previous studies[13,25,26], our research reported a significant increase in the serum IL-10 level in the SBP patients than those in the healthy volunteers and cirrhotic patients without SBP. Also it was higher in cirrhotic patients without SBP than in healthy volunteers, but the difference was not statistically significant. This goes with the assumption that the elevated IL-10 levels in both cirrhotic patients with and without SBP have a regulatory role in the inflammatory process in liver cirrhosis patients[25].
Our study reported that mean level of serum ascites albumin gradient (SAAG) was significantly higher in cirrhotic patients than in SBP patients, and this is in agreement with the results of Khan et al[26] who found that the SAAG was higher in cirrhotic than SBP patients.
Changes in various cytokines levels after SBP treatment were previously observed, e.g., MCP-1 and IL-10 levels showed a significant decrease during follow-up after treatment[13], and this is in agreement with the result of our study that SBP patients showed significant decreases in the mean levels of blood and ascitic fluid MCP-1 gene expression and serum IL-10 after SBP treatment.
In conclusion, inheritance of MCP-1 GG genotype and MCP-1 G allele may predispose HCV infected patients to a more progressive disease course, while AG genotype may be a risk factor for SBP in patients with decompensated post-hepatitis C cirrhosis. MCP-1 expression and IL-10 levels in blood and ascitic fluid may be related to the development and the course of SBP. Further randomized controlled trials with greater sample size are recommended.
The authors wish to thank the Biochemistry and Molecular Biology Unit and Kasr El Aini University Hospital at the Faculty of Medicine, Cairo University.
The high susceptibility of hepatitis C patients with cirrhosis and ascites to bacterial infections correlates with peritoneal macrophages that might contribute to the control of spontaneous bacterial peritonitis (SBP) or influence its associated pathology. The chemotactic factor monocyte chemotactic protein-1 (MCP-1) secretion is up-regulated during chronic hepatitis and correlates with the severity of hepatic inflammation, and thus the functional MCP-1 promoter polymorphism (A-2518G) can be associated with cirrhosis and SBP.
The functional MCP-1 promoter polymorphism (A-2518G) genotypes distribution and allele frequencies were demonstrated as markers for cirrhosis and/or SBP susceptibility in HCV patients. Above and beyond, MCP-1 expression and level along with IL-10 level were evaluated as pre- and post-treatment monitoring indicators for such cases.
Several reports have highlighted that carriers of the G allele of the MCP-1 polymorphism were more frequent in patients with alcohol induced cirrhosis and HCV fibrosis and severe inflammation. This is the first study to report that inheritance of MCP-1 GG genotype and MCP-1 G allele may predispose HCV infected patients to cirrhosis, while AG genotype may be a risk factor for spontaneous bacterial peritonitis SBP in patients with decompensated cirrhosis. Additionally, our investigations would propose MCP-1 expression and IL-10 levels in blood and ascitic fluid to be correlated with the development and the course of SBP.
HCV infected patients carrying the G allele of the MCP-1 polymorphism should be under intensive observation, because MCP-1 GG genotype carriers may develop cirrhosis and AG genotype can be a high risk factor for spontaneous bacterial peritonitis. MCP-1 expression and IL-10 levels in blood and ascitic fluid should be investigated during the development of these cases.
MCP-1 is a signalling protein that acts as a chemotactic factor for monocytes and macrophages; thus, these cells migrate to the ascetic fluid. SBP is a peritoneal recurrent bacterial infection due to lower immunity state.
This paper has high scientific and methodological levels.
P- Reviewer: Skrypnyk IN S- Editor: Ding Y L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut. 2006;55 Suppl 6:vi1-v12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 208] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Vincent JL, Gustot T. Sepsis and cirrhosis: many similarities. Acta Gastroenterol Belg. 2010;73:472-478. [PubMed] |
| 3. | Pluta A, Gutkowski K, Hartleb M. Coagulopathy in liver diseases. Adv Med Sci. 2010;55:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Cejudo-Martín P, Ros J, Navasa M, Fernández J, Fernández-Varo G, Ruiz-del-Arbol L, Rivera F, Arroyo V, Rodés J, Jiménez W. Increased production of vascular endothelial growth factor in peritoneal macrophages of cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2001;34:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Bories PN, Campillo B, Scherman E. Up-regulation of nitric oxide production by interferon-gamma in cultured peritoneal macrophages from patients with cirrhosis. Clin Sci (Lond). 1999;97:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246-1256, 1256.e1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 837] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 7. | Gustot T, Durand F, Lebrec D, Vincent JL, Moreau R. Severe sepsis in cirrhosis. Hepatology. 2009;50:2022-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 326] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 8. | Andus T, Gross V, Holstege A, Schölmerich J. High interleukin-6 concentrations in hepatic ascites. Dig Dis Sci. 1994;39:219-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Andus T, Gross V, Holstege A, Ott M, Weber M, David M, Gallati H, Gerok W, Schölmerich J. High concentrations of soluble tumor necrosis factor receptors in ascites. Hepatology. 1992;16:749-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Narumi S, Tominaga Y, Tamaru M, Shimai S, Okumura H, Nishioji K, Itoh Y, Okanoue T. Expression of IFN-inducible protein-10 in chronic hepatitis. J Immunol. 1997;158:5536-5544. [PubMed] |
| 11. | Rollins BJ. Monocyte chemoattractant protein 1: a potential regulator of monocyte recruitment in inflammatory disease. Mol Med Today. 1996;2:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 305] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Kolattukudy PE, Niu J. Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway. Circ Res. 2012;110:174-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 13. | Kim JK, Chon CY, Kim JH, Kim YJ, Cho JH, Bang SM, Ahn SH, Han KH, Moon YM. Changes in serum and ascitic monocyte chemotactic protein-1 (MCP-1) and IL-10 levels in cirrhotic patients with spontaneous bacterial peritonitis. J Interferon Cytokine Res. 2007;27:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Mühlbauer M, Bosserhoff AK, Hartmann A, Thasler WE, Weiss TS, Herfarth H, Lock G, Schölmerich J, Hellerbrand C. A novel MCP-1 gene polymorphism is associated with hepatic MCP-1 expression and severity of HCV-related liver disease. Gastroenterology. 2003;125:1085-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3016] [Cited by in RCA: 2915] [Article Influence: 182.2] [Reference Citation Analysis (0)] |
| 16. | Martin G, Lawlor E. Non-toxic DNA extraction in a clinical setting. Leuk Res. 1994;18:469-471. [PubMed] |
| 17. | Shyy YJ, Li YS, Kolattukudy PE. Structure of human monocyte chemotactic protein gene and its regulation by TPA. Biochem Biophys Res Commun. 1990;169:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Coffin JM, Hughes SH, Varmus HE. Retroviruses. 1st ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press 1997; 2473-2477. |
| 19. | Ercolani L, Florence B, Denaro M, Alexander M. Isolation and complete sequence of a functional human glyceraldehyde-3-phosphate dehydrogenase gene. J Biol Chem. 1988;263:15335-15341. [PubMed] |
| 20. | Delves P, Roitt I. Encyclopedia of Immunology. 2nd ed. San Diego: Academic Press 1998; 332-334. |
| 21. | Gäbele E, Mühlbauer M, Paulo H, Johann M, Meltzer C, Leidl F, Wodarz N, Wiest R, Schölmerich J, Hellerbrand C. Analysis of monocyte chemotactic protein-1 gene polymorphism in patients with spontaneous bacterial peritonitis. World J Gastroenterol. 2009;15:5558-5562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Rovin BH, Lu L, Saxena R. A novel polymorphism in the MCP-1 gene regulatory region that influences MCP-1 expression. Biochem Biophys Res Commun. 1999;259:344-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 353] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 23. | Matsukawa A, Hogaboam CM, Lukacs NW, Lincoln PM, Strieter RM, Kunkel SL. Endogenous monocyte chemoattractant protein-1 (MCP-1) protects mice in a model of acute septic peritonitis: cross-talk between MCP-1 and leukotriene B4. J Immunol. 1999;163:6148-6154. [PubMed] |
| 24. | Nischalke HD, Nattermann J, Fischer HP, Sauerbruch T, Spengler U, Dumoulin FL. Semiquantitative analysis of intrahepatic CC-chemokine mRNas in chronic hepatitis C. Mediators Inflamm. 2004;13:357-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Rodríguez-Ramos C, Galan F, Díaz F, Elvira J, Martín-Herrera L, Girón-González JA. Expression of proinflammatory cytokines and their inhibitors during the course of spontaneous bacterial peritonitis. Dig Dis Sci. 2001;46:1668-1676. [PubMed] |
| 26. | Khan J, Pikkarainen P, Karvonen AL, Mäkelä T, Peräaho M, Pehkonen E, Collin P. Ascites: aetiology, mortality and the prevalence of spontaneous bacterial peritonitis. Scand J Gastroenterol. 2009;44:970-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |