Published online Sep 7, 2014. doi: 10.3748/wjg.v20.i33.11762
Revised: March 27, 2014
Accepted: April 21, 2014
Published online: September 7, 2014
Processing time: 278 Days and 20.3 Hours
AIM: To compare the performance of the Da-an real-time hepatitis B virus (HBV) DNA assay and Abbott RealTime HBV assay.
METHODS: HBV DNA standards as well as a total of 180 clinical serum samples from patients with chronic hepatitis B were measured using the Abbott and Da-an real-time polymerase chain reaction (PCR) assays. Correlation and Bland-Altman plot analysis was used to compare the performance of the Abbott and Da-an assays. The HBV DNA levels were logarithmically transformed for analysis. All statistical analyses were performed using SPSS for Windows version 18.0. The correlation between the two assays was analyzed by Pearson’s correlation and linear regression. The Bland-Altman plots were used for the analysis of agreement between the two assays. A P value of < 0.05 was considered statistically significant.
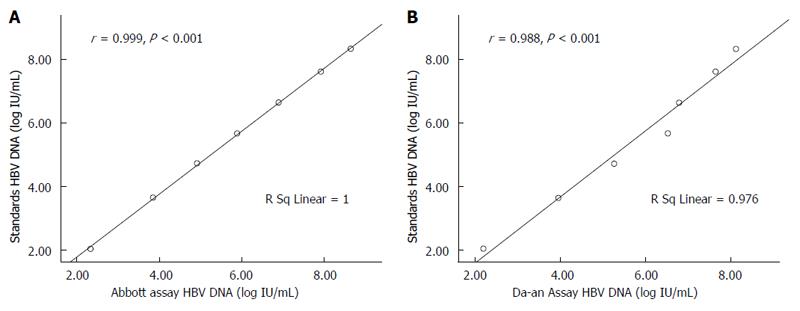
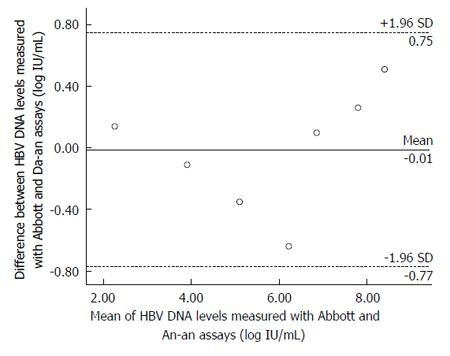
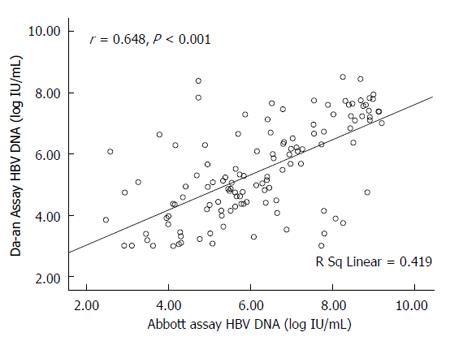
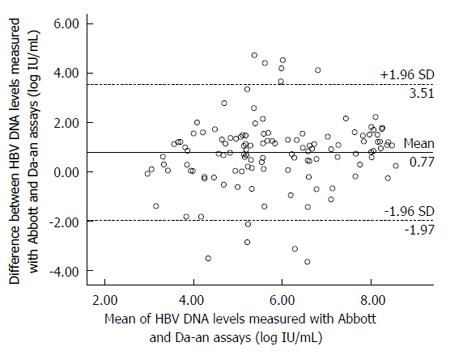
RESULTS: The HBV DNA values measured by the Abbott or Da-an assay were significantly correlated with the expected values of HBV DNA standards (r = 0.999, for Abbott; r = 0.987, for Da-an, P < 0.001). A Bland-Altman plot showed good agreement between these two assays in detecting HBV DNA standards. Among the 180 clinical serum samples, 126 were quantifiable by both assays. Fifty-two samples were detectable by the Abbott assay but below the detection limit of the Da-an assay. Moreover, HBV DNA levels measured by the Abbott assay were significantly higher than those of the Da-an assay (6.23 ± 1.76 log IU/mL vs 5.46 ± 1.55 log IU/mL, P < 0.001). A positive correlation was observed between HBV DNA concentrations determined by the two assays in 126 paired samples (r = 0.648, P < 0.001). One hundred and fifteen of 126 (91.3%) specimens tested with both assays were within mean difference ± 1.96 SD of HBV DNA levels.
CONCLUSION: The Da-an assay presented lower sensitivity and a narrower linear range as compared to the Abbott assay, suggesting the need to be improved.
Core tip: The hepatitis B virus (HBV) DNA values measured by the Abbott or Da-an real-time polymerase chain reaction assay were significantly correlated with the expected values of HBV DNA standards. A Bland-Altman plot showed good agreement between the assays. For clinical evaluation, HBV DNA levels derived from the Abbott assay were significantly higher than those of the Da-an assay.
- Citation: Qiu N, Li R, Yu JG, Yang W, Zhang W, An Y, Li T, Liu XE, Zhuang H. Comparison of Abbott and Da-an real-time PCR for quantitating serum HBV DNA. World J Gastroenterol 2014; 20(33): 11762-11769
- URL: https://www.wjgnet.com/1007-9327/full/v20/i33/11762.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i33.11762
An estimated 350 million people worldwide are chronically infected with hepatitis B virus (HBV) and about one-third of these live in China[1]. The spectrum of disease and natural history of chronic HBV infection are diverse and variable, ranging from an inactive carrier state to progressive chronic hepatitis B (CHB), which may evolve to cirrhosis and hepatocellular carcinoma (HCC)[2]. Longitudinal studies of untreated patients with CHB indicate that the 5-year cumulative incidence of developing cirrhosis ranges from 8% to 20% following diagnosis. The 5-year cumulative incidence of hepatic decompensation is approximately 20% for untreated patients with compensated cirrhosis. Untreated patients with decompensated cirrhosis have a poor prognosis, with only a 14%-35% probability of survival at 5 years. The annual incidence of HBV-related HCC in patients with cirrhosis ranges from 2% to 5%[2]. Antiviral therapy against HBV is an important measure for preventing and delaying progression of the disease from CHB to cirrhosis, end-stage liver disease, HCC, and death[3]. During therapy, quantification of HBV DNA plays a crucial role in the management of CHB by allowing criteria to be established for determining patient eligibility for antiviral therapy, monitoring treatment response, and identifying the emergence of resistance in order to adapt therapy[4].
Sensitive and accurate quantification of HBV DNA is necessary to monitor patients with CHB who are receiving antiviral therapy[5]. An assay with good sensitivity and accurate detection for quantitating HBV DNA level will contribute to optimal monitoring of antiviral therapy, early confirmation of drug resistance, and timely treatment adaption. Measurement of viral load is mostly accomplished by detection of HBV DNA in serum or plasma with nucleic acid amplification or signal amplification technologies. Real-time polymerase chain reaction (PCR), one of the target (HBV DNA) amplification techniques, has been developed with more sensitivity and broader dynamic range when compared to signal amplification technique[4]. Several real-time PCR-based commercially available tests for the quantitation of HBV DNA in serum or plasma specimens are routinely used in diagnostic laboratories in China. The Da-an real-time HBV DNA assay (Da-an Gene Co. Ltd, Sun Yat-Sen University, Guangdong, China) is one of them. However, internationally ubiquitous real-time assays, including the COBAS TaqMan HBV test (Roche Molecular Diagnostics, Pleasonton, CA, United States), Abbott RealTime HBV assay (Abbott Molecular, Des Plaines, IL, United States), and the Artus RealART HBV LC PCR kit (QIAGEN, Hamburg, Germany) are rarely used in clinical practice in China due to their high cost.
The present comparative study was carried out to explore the difference and correlation between a domestic assay (Da-an assay) and an internationally accepted assay (the Abbott assay). The performance of the two assays was evaluated, and the correlation and agreement between them were analyzed.
A total of 48 patients with chronic hepatitis B aged 17-65 years (35 male and 13 female) was enrolled in this study. These patients had received adefovir as antiviral therapy at the hospital since 2010. A hundred and eighty serum samples were obtained at week 0, 12, 24, 36 and 48 during antiviral treatment. All samples were stored in the laboratory at -70 °C.
This study was approved by the Ethics Committee of Peking University Health Science Center in accordance with the Helsinki Declaration. Informed consent was obtained from each patient.
A panel of reference sera for quantitating HBV DNA was provided by the Chinese National Institutes for Food and Drug Control. The HBV DNA reference panel was used to evaluate the sensitivity of the assays, which consisted of eight negative controls (N1-8), nine positive controls (P1-9), and seven sensitivity standards (L0-6). The sensitivity standards L0-6 were made from a dilution series of a single serum sample with HBV-marker-negative serum. This panel of reference sera was standardized by the WHO international standard for HBV DNA (NIBSC10/264) and was measured with several internationally accepted commercial assays for quantitating HBV DNA. The range of concentrations of the sensitivity sera L0-6 are listed in Table 1, and the logarithmic mean concentrations of standards L0-6 were 2.04, 3.64, 4.72, 5.67, 6.65, 7.62 and 8.34 log IU/mL, respectively. The HBV genotype of the sensitivity standard (L0-6) is genotype B. The panel was produced by the Chinese National Institutes for Food and Drug Control and data were provided by the package insert instruction of the panel. The panel was stored at -70 °C.
| Expected concentration of HBV DNA standard (log IU/mL) | Abbott assay (log IU/mL)(mean ± SD) | CV | Da-an assay (log IU/mL) (mean ± SD) | CV |
| L0: 8.34 (7.89-8.79) | 8.64 ± 0.07 | 0.8% | 8.13 ± 0.17 | 2.0% |
| L1: 7.62 (7.17-8.07) | 7.91 ± 0.04 | 0.5% | 7.65 ± 0.10 | 1.3% |
| L2: 6.65 (6.20-7.10) | 6.89 ± 0.05 | 0.7% | 6.79 ± 0.01 | 0.2% |
| L3: 5.67 (5.22-6.12) | 5.89 ± 0.01 | 0.2% | 6.53 ± 0.65 | 10.0% |
| L4: 4.72 (4.26-5.17) | 4.92 ± 0.08 | 1.7% | 5.27 ± 0.21 | 3.9% |
| L5: 3.64 (3.18-4.09) | 3.85 ± 0.24 | 1.1% | 3.96 ± 0.04 | 1.0% |
| L6: 2.04 (1.59-2.49) | 2.33 ± 0.07 | 3.0% | 2.19 ± 0.01 | 0.4% |
The serum samples were processed using m2000sp, an automatic nucleic acid extraction apparatus that uses a magnetic microparticle-based principal for DNA purification. Amplification was performed on an Abbott m2000rt real-time instrument. An initial serum volume of 200 μL was used for the nucleic acid extraction, and the final elution volume was 70 μL. Fifty microliters of elute was used as an amplification template. 15 IU/mL (1.18 log IU/mL, for 200-μL sample) of the limit of detection (LOD) in the Abbott assay was determined by testing dilutions of the WHO International Standard for HBV DNA (NIBSC97/746). The upper limit of quantitation for the Abbott assay was 109 IU/mL and the lower limit of quantitation was equivalent to LOD (15 IU/mL for 0.2-mL sample). A specimen with a result of “not detected” was defined to be negative and a result of “< 15 IU/mL” was presumed to be under the limit of detection. Samples with HBV DNA levels above the upper limit (109 IU/mL) were diluted and remeasured at appropriate concentrations.
The procedure of the Da-an real-time PCR HBV DNA assay (catalog number: DA-D051) consisted of extracting nucleic acid manually followed by DNA quantitation with real-time PCR. Nucleic acid extraction in detail: (1) serum sample with a starting volume of 100 μL was prepared in an Eppendorf tube, then 100 μL concentrated solution was added and mixed by pulse vortexing for 5 s; (2) centrifugation at 12000 rpm for 10 min; (3) discard the upper liquid, then add the extraction reagent 20 μL to the precipitation and mix by pulse vortexing for 5-10 s, centrifuge for 5 s; (4) the tube was placed in a water bath at 100 °C for 10 min; and (5) centrifugation at 12000 rpm for 5 min, and the final 20 μL of extracted nucleic acid was used as the template. Two microliters of the purified nucleic acid was added to the real-time PCR mixture (for a final volume of 45 μL) for amplifying the target HBV surface gene. The TaqMan probe was used in this real-time PCR amplification system, which was performed on a LightCycler 480 system (Roche) by incubating the reaction mixture at 93 °C for 2 min, followed by 40 cycles of PCR amplification at 93 °C for 5 s and 57 °C for 45 s. The dynamic range of the Da-an assay was 103-108 IU/mL for the first-generation products. The result of HBV DNA level “< 3 log IU/mL” was also reported and considered as an inaccurate result because it was beyond the linear (dynamic) range. It was reported as negative while the CT (the cycle of threshold) values of the samples were shown as 40, 0 or blank.
HBV DNA was extracted from 200 μL serum using a QIAamp DNA Blood Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions. Nested PCR was used to amplify the entire reverse transcriptase (RT) region of HBV. The PCR conditions and the sequences of the nested PCR primers were the same as described by Yang et al[6]. The product of PCR with approximately 1195 base pairs was visualized on 1% agarose gel, purified, and sequenced commercially (Shanghai Invitrogen Biotechnology Co. Ltd., Shanghai, China)
HBV genotyping was determined using the NCBI Viral Genotyping Tool (http://www.ncbi.nlm.nih.gov/projects/genotyping/formpage.cgi) and phylogenetic analysis with MEGA 4.0 software.
The HBV DNA levels were logarithmically transformed for analysis. All statistical analyses were performed using SPSS for Windows version 18.0 (SPSS, Chicago, IL, United States). The correlation between the two assays was analyzed by Pearson’s correlation and linear regression. The Bland-Altman plots were used for the analysis of agreement between the two assays. A P value of < 0.05 was considered statistically significant.
A total of eight negative references and nine positive references from the HBV DNA standard panel sera were correctly detected by the Abbott or Da-an assay. For the seven sensitivity references, as shown in Figure 1, HBV DNA values measured by the Abbott or Da-an assays were significantly correlated with the expected values of HBV DNA standards (r = 0.999, P < 0.01, for Abbott; r = 0.987, P < 0.01, for Da-an, respectively). Furthermore, good agreement between the results of the two assays for detecting HBV DNA standards was observed by the Bland-Altman analysis (Figure 2). All of the difference values of the paired viral loads were within the range of mean difference ± 1.96 SD (log IU/mL). The mean value of differences of the paired viral loads, 95% confidence interval, and maximal difference were -0.01 log IU/mL, (-0.77, 0.75) log IU/mL, and 0.64 log IU/mL, respectively, thus indicating that there was no significant difference between these two assays.
The reference sera L0-6 were tested three times with the Abbott and Da-an assay over a period of 3 d. All of the means of quantitative results obtained with the Abbott assay were within the range of the expected values with the inter-assay variation ranging from 0.2% to 3.0%. For HBV DNA levels detected with the Da-an assay, two of the seven mean values were out of the reference ranges with the inter-assay variation ranging from 0.2% to 10% (Table 1).
All of the 48 CHB patients were infected with HBV genotype C. This genotype dispersal conformed to the HBV genotype distribution profile in Northern China[3,6]. Among the 180 clinical serum samples, 126 had a detected value by the Da-an assay while 178 were quantitated by the Abbott assay. Comparison of HBV DNA levels measured by these two assays is shown in Table 2. The only discrepancy with the results is that one sample was below the detection limit of the Abbott assay, but was detectable when quantitated by the Da-an assay. This serum sample was taken at week 36 of adefovir therapy from patient No. 1. The HBV DNA levels of this patient at week 24, 36 and 48 of treatment were 4.77, < 1.18 IU/ and < 1.18 IU/mL, respectively, for the Abbott test, and 3.23, 4.99 and < 3 IU/mL for the Da-an test. It could be deduced that a false-positive result with the Da-an assay may have occurred.
| Abbott RealTime assay result(log IU/mL) | Da-an real-time HBV DNA assay result (log IU/mL) | |||
| ≥8.00 | ≥3.00-< 8.00 | < 3.00 (negative) | Total | |
| > 9.00 | 0 | 3 | 0 | 3 |
| ≥ 1.18- ≤ 9.00 | 3 | 120 | 52 | 175 |
| < 1.18 | 0 | 1 | 0 | 1 |
| Negative | 0 | 0 | 1 | 1 |
| Total | 3 | 124 | 53 | 180 |
The paired HBV DNA levels of the 126 samples detectable by both assays were analyzed. The mean logarithmic level of HBV DNA quantitated by the Abbott assay was significantly higher than that by the Da-an assay (6.23 ± 1.76 log IU/mL vs 5.46 ± 1.55 log IU/mL, P < 0.01). As shown in Figure 3, correlation analysis showed that a significantly positive correlation was obtained between HBV DNA concentrations (r = 0.648, P < 0.01). In addition, samples were divided into two groups (≥ 5.00 log IU/mL and < 5.00 log IU/mL) according to the viral load detected by the Abbott assay. There was a positive correlation between the paired HBV DNA levels in the group with a higher viral load (r = 0.665, P < 0.01), but no correlation in the group with the lower viral load (r = 0.321, P = 0.073).
The agreement analysis for the HBV DNA levels in 126 clinical samples tested by these two assays was shown in Figure 4. The largest differences in the HBV DNA values of the paired samples were located in the range of mean difference ± 1.96 SD (115/126). The proportions of specimens with < 1 log, 1-2 log, and > 2 log difference of HBV DNA levels between the assays were 44.5% (56/126), 42.0% (53/126) and 13.5% (17/126), respectively. The mean difference value of the paired viral loads, 95% confidence interval, and maximal difference were 0.77, (-1.97, 3.51) and 4.72 log IU/mL, respectively.
Fifty-two samples were detectable by the Abbott assay but below the detection limit of the Da-an assay. HBV DNA levels of these 52 samples ranged from 1.26 to 8.39 log IU/mL. The distribution of HBV DNA concentrations were 1.18-2 log IU/mL, 12 samples; 2-3 log IU/mL, eight samples; 3-4 log IU/mL, nine samples; 4-5 log IU/mL, four samples; 5-6 log IU/mL, three samples; 6-7 log IU/mL, 10 samples; 7-8 log IU/mL, four samples; and 8-9 log IU/mL, two samples; respectively.
At the baseline of adefovir dipivoxil therapy, the entire RT region of HBV in serum samples from 48 CHB patients was amplified and sequenced commercially. Lamivudine resistants (L180M, M204V and M204I) were found in two of the 48 patients (Nos. 23 and 41). The HBV DNA levels of patient No. 41 during treatment follow-up were 8.26 log, 3.91, 3.63, 3.91 and 2.57 log IU/mL for the Abbott assay; 6.32 log, < 3, < 3, < 3 and < 3 log IU/mL for the Da-an assay. The same trend was observed in another patient. Based on the dynamic changes of HBV DNA levels, adefovir dipivoxil was an effective antiviral drug for both of the lamivudine-resistant patients. At the same time, the differences of HBV DNA levels measured by both assays were also observed.
The three major liver societies, the American Association for the Study of Liver Diseases[7], the European Association for the study of the Liver[2], and the Asia-Pacific Association for the Study of the Liver[8], have all issued guidelines for the management of CHB that specify certain HBV DNA thresholds to determine which patients are candidates for antiviral treatment. Viral load measurement is used not only in the diagnosis of HBV but also in the monitoring of patients for effective antiviral treatment, although the respective guidelines differ in the recommended intervals for such testing. In China, HBV DNA levels should be detected every 3 mo in patients with CHB during antiviral therapy[3]. The quantitation of viral load is a routinely performed molecular test in clinical laboratories. The Da-an real-time HBV DNA test, produced domestically and approved by the State Food and Drug Administration, China for in vitro diagnostic use, is widely used in China. Therefore, it is necessary to compare the domestic assay to the international standard assay.
The real-time PCR-based commercial assays for HBV DNA quantitation used in clinical practice have been available worldwide for several years. There are some differences in sensitivity, specificity, dynamic range, and reproducibility among the assays. Based on our results, the Abbott RealTime HBV assay has a higher sensitivity as compared with the Da-an real-time HBV DNA assay (15 IU for 0.2 mL sample vs 1000 IU for 0.2 mL), and the dynamic range of the Abbott assay is also broader than that of Da-an assays (1.18-9.0 log IU/mL vs 3-8 log IU/mL). Among the 180 clinical serum samples, 126 were detected by both assays; 52 samples with viral load below the detection limit of the Da-an assay were detectable by the Abbott assay; one sample was below the detection limit for the Abbott assay but detectable by the Da-an assay; and one sample was negative for both assays. Numerous factors affect the accuracy of quantifying HBV DNA, such as sample volumes used for HBV DNA isolation, enzyme inhibitors in samples, different methods for extracting nucleic acid, and diversity in primers and fluorescent markers for real-time PCR. Several reasons for differences in sensitivity, the linear range and other differences between the two assays are analyzed in detail as follow.
The first difference between these two assays is that they use different methods to extract nucleic acid. A boiling method applied in the Da-an assay could affect the purity of nucleic acids and the efficiency of HBV DNA isolated. In addition, the manual specimen preparation is labor-intensive and can cause run-to-run variability and specimen-to-specimen contamination[9]. A false-positive result with the Da-an assay may be due to sample-to-sample contamination. For the Abbott assay, HBV DNA is extracted from serum samples by the m2000sp, an automated sample preparation system designed to use magnetic microparticle-based reagents for the purification of nucleic acids from samples. One of the major advantages of automating the HBV DNA extraction is the ability to provide a standardized process among the samples. At the same time, the automated sample preparation system (m2000sp) combined with the m2000rt analyzer significantly reduces hand-on work time and labor intensity while reducing the risk of contamination and human error[10].
Furthermore, factors include the differences in sample volumes, final elution volumes, template volumes, and PCR volumes: i.e., 100, 20, 2 and 45 μL for the Da-an assay, and 200, 70, 50 and 100 μL for the Abbott assay, respectively. Besides the sample and PCR volumes, the difference in the ratio of the final elution volume over the template volume also matters (70/50 μL for the Abbott and 20/2 μL for the Da-an), which means the lower the ratio of elution/template volume the higher HBV DNA concentration in the final PCR mixture. Thus a higher concentration of DNA template could enhance the detection rate. The effects of the sample volume and HBV DNA concentration in the template on the sensitivity of HBV DNA detection were also reported in a previous study[11].
Another concern is the target regions for PCR amplification. The Abbott assay selected the highly conserved region in the S gene of the HBV genome as the target region, which located in the N-terminal third of the S gene ensuring that the assay is not affected by YMDD mutants, HBsAg escape mutants, or drug-resistant mutants, because this region is essential for the assembly and secretion of subviral particles, and tolerates minor structural changes. Therefore, the Abbott assay provides for the detection of genotypes A-H. The Da-an assay also selected a relatively conserved target region within the S gene of the HBV genome. However, the manufacturer did not specify the range of genotypes for the assay.
As a final point, the effect of the internal control applied in monitoring PCR amplification was demonstrated. One prerequisite for the PCR-based quantitative approach is to avoid PCR inhibitory substances, such as hemoglobin or heparin, in clinical samples. There are no external controls that can adequately control for these conditions, thus, false-negative test results can be generated[12,13]. In the Abbott assay, a DNA sequence unrelated to the HBV target sequence is introduced into the sample preparation procedure and processed with the calibrators, controls, and specimens. It serves as an internal control to compensate for the differences in DNA extraction efficiency between specimens and possible PCR inhibition in the reaction mixtures[14], which further controls for target isolation and amplification. The Da-an assay does not include such an internal control, thus, it does not control for the loss of nucleic acid during the process of extraction, causing suboptimal amplification or false-negative results.
We showed a strong correlation and good agreement between HBV DNA levels quantitated with the Abbott and Da-an assays in HBV DNA standards but not in clinical samples. Especially, 52 serum samples were detected by the Abbott assay but not by the Da-an assay. The reason may be that the Da-an assay could perform relatively well when testing the HBV DNA standards whose genotype is B. For all clinical samples with genotype C, the Da-an assay performed poorly. The Abbott assay provides for detection of genotype A-H. However, the Da-an assay did not declare that. It is possible that the Da-an assay could perform well, testing some genotypes of HBV but poorly when testing other genotypes. In addition, sequences of HBV DNA may change during antiviral therapy. The Abbott assay selected the highly conserved region in the S gene of the HBV genome as the target region and ensured that the assay is not affected by YMDD mutants, HBsAg escape mutants, or drug-resistant mutants. The Da-an assay also selected a relatively conserved target region within the S gene but did not exclude the impact of HBV DNA mutation. Zheng et al[15] reported that 200 serum samples were measured by the three real-time PCR reagents. Six out of 200 serum samples were underestimated or undetected by the Da-an assay. The sequence of the fluorescence probe binding region (FPBR) in HBV DNA genome of six serum samples was determined and compared with the sequence of HBV wild type. The mutations of the FPBR sequence were found and clarified that the mutations affected the measurement of HBV DNA.
Based on the above analysis, the manufacturer producing the Da-an real-time PCR HBV assay should improve their molecular technique, increase the sensitivity, and extend the dynamic range of the assay. As the related guidelines for managing chronic hepatitis B mention, the current standard of care is to adapt antiviral therapy in patients with drug resistance as early as possible, namely, at the time of viral breakthrough, which is defined by an increase in HBV DNA level by 1 log IU/mL compared to the nadir value[2,7,8,16]. It is important to detect lower viral load accurately, especially in viral breakthrough occurring when HBV DNA levels change from 10 to 100 IU/mL. The Abbott assay, with a sensitivity of 15 IU/mL for 200-μL serum samples, will be adequate for the detection of viral breakthrough at an early stage and allow for the rapid addition of a rescue therapy before clinical breakthrough[17]. An assay with good sensitivity and wide detection range for quantitating HBV DNA concentration is a crucial measurement to allow optimal monitoring of antiviral therapy and timely treatment adaption. The Da-an assay is not suitable for this clinical application because it has inadequate sensitivity. Therefore, it is urgent to improve the quality of the Da-an assay for the manufacturer.
Although the Abbott assay has a higher sensitivity (15 IU vs 1000 IU), shorter assay time (4 h vs 8 h), and wider dynamic range (1.18-9.00 log IU/mL vs 3-8 log IU/mL) as compared with the Da-an assay, the costs of the Abbott assay are extremely high (approximately 50 US dollars/Abbott test vs 7 US dollars/Da-an test), which limits their routine use in clinical molecular laboratories in China. With respect to the Da-an assay, more improvements, including an automatic nucleic acid extraction apparatus and introducing an internal control, are needed for clinical practitioners. The disease burden of CHB is heavy in many developing countries. The patients with CHB receiving antiviral treatment are increasing annually. The domestic assays for quantitating HBV DNA were widely used not only in China but also in other developing countries. This comparative study would be helpful for manufacturers who have produced the products for HBV DNA quantitation in their countries.
In this study, the performance of the Da-an real-time HBV DNA assay and the Abbott RealTime HBV assay for quantitating HBV DNA levels were evaluated and compared. A strong correlation and good agreement between HBV DNA levels quantitated with the Abbott and Da-an assays was observed for testing the HBV DNA standard panel but not for clinical samples. As compared with the Abbott assay, the Da-an assay had lower sensitivity and a narrower linear range that needs further improvement.
The authors are grateful to Ms. Sandra Lester, Dept. of Microbiology, Immunology and Biochemistry, University of Tennessee Health Science Center and Dr. Baoying Liu, Laboratory of Immunology, NEI/NIH, the United States for proofreading the manuscript. The authors would like to thank Dr. Xing Wu, Department of Virology, the Chinese National Institutes for Food and Drug Control for providing a panel of reference sera
Sensitive and accurate quantification of hepatitis B virus (HBV) DNA is important to monitor patients with chronic hepatitis B during antiviral therapy. The domestic assays for quantitating HBV DNA are widely used in China and other developing countries. The comparative study was conducted to compare the performance of domestic and internationally accepted assays, to contribute valuable research data that would be helpful for improving the domestic assays in developing countries.
This is believed to be the first report on the comparison of the Abbott and Da-an real-time polymerase chain reaction assays for quantitating HBV DNA in serum. A strong correlation and good agreement between HBV DNA levels quantitated with the Abbott and Da-an assays was observed for testing the HBV DNA standard panel but not for clinical samples. The Da-an assay presented lower sensitivity and a narrower linear range as compared to the Abbott assay, and needs to be improved.
This comparative study will be helpful for domestic manufacturers in developing countries to improve the quality of their products.
The authors compared the sensitivity and linear range of two HBV DNA quantification assays, the Abbott assay presented a relative higher sensitivity and a wider linear range in clinical samples. The conclusions will be beneficial for physicians to select the appropriate assay to quantify HBV DNA levels in chronic HBV- infected patients, especially in developing countries.
P- Reviewer: Karatapanis S, Zhang XY S- Editor: Gou SX L- Editor: Kerr C E- Editor: Ma S
| 2. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2401] [Article Influence: 184.7] [Reference Citation Analysis (0)] |
| 3. | Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. [The guideline of prevention and treatment for chronic hepatitis B (2010 version)]. Zhonghua Ganzangbing Zazhi. 2011;19:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 83] [Reference Citation Analysis (0)] |
| 4. | Bowden DS, Thompson AJ. New developments in HBV molecular diagnostics and quantitative serology. Hepatol Int. 2008;2:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Yang JF, Lin YY, Huang JF, Liu SF, Chu PY, Hsieh MY, Lin ZY, Chen SC, Wang LY, Dai CY. Comparison of clinical application of the Abbott HBV PCR kit and the VERSANT HBV DNA 3.0 test to measure serum hepatitis B virus DNA in Taiwanese patients. Kaohsiung J Med Sci. 2009;25:413-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Yang JX, Liu BM, Li XG, Yan CH, Xu J, Sun XW, Wang YH, Jiao XJ, Yan L, Dong JP. Profile of HBV antiviral resistance mutations with distinct evolutionary pathways against nucleoside/nucleotide analogue treatment among Chinese chronic hepatitis B patients. Antivir Ther. 2010;15:1171-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, Tobias H. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008;6:1315-1341; quiz 1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 336] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 8. | Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Allice T, Cerutti F, Pittaluga F, Varetto S, Gabella S, Marzano A, Franchello A, Ghisetti V. Comparison of the Cobas Ampliprep/Cobas TaqMan HBV Test versus the Cobas Amplicor HBV monitor for HBV-DNA detection and quantification during antiviral therapy. New Microbiol. 2008;31:27-35. [PubMed] |
| 10. | Ronsin C, Pillet A, Bali C, Denoyel GA. Evaluation of the COBAS AmpliPrep-total nucleic acid isolation-COBAS TaqMan hepatitis B virus (HBV) quantitative test and comparison to the VERSANT HBV DNA 3.0 assay. J Clin Microbiol. 2006;44:1390-1399. [PubMed] |
| 11. | Ismail AM, Sivakumar J, Anantharam R, Dayalan S, Samuel P, Fletcher GJ, Gnanamony M, Abraham P. Performance characteristics and comparison of Abbott and artus real-time systems for hepatitis B virus DNA quantification. J Clin Microbiol. 2011;49:3215-3221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Shyamala V, Arcangel P, Cottrell J, Coit D, Medina-Selby A, McCoin C, Madriaga D, Chien D, Phelps B. Assessment of the target-capture PCR hepatitis B virus (HBV) DNA quantitative assay and comparison with commercial HBV DNA quantitative assays. J Clin Microbiol. 2004;42:5199-5204. [PubMed] |
| 13. | Burggraf S, Olgemöller B. Simple technique for internal control of real-time amplification assays. Clin Chem. 2004;50:819-825. [PubMed] |
| 14. | Sum SS, Wong DK, Yuen JC, Lai CL, Yuen MF. Comparison of the COBAS TaqMan HBV test with the COBAS Amplicor monitor test for measurement of hepatitis B virus DNA in serum. J Med Virol. 2005;77:486-490. [PubMed] |
| 15. | Zheng YW, Liang MW, Qian JL, Fang W. The influences of the HBV DNA results by the mutations of the fluorescence probe binding region sequences. Redai Yi xue Zazhi. 2012;12:837-839. |
| 16. | Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 841] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 17. | Thibault V, Pichoud C, Mullen C, Rhoads J, Smith JB, Bitbol A, Thamm S, Zoulim F. Characterization of a new sensitive PCR assay for quantification of viral DNA isolated from patients with hepatitis B virus infections. J Clin Microbiol. 2007;45:3948-3953. [PubMed] |