Published online Sep 7, 2014. doi: 10.3748/wjg.v20.i33.11700
Revised: March 21, 2014
Accepted: June 2, 2014
Published online: September 7, 2014
Processing time: 260 Days and 7.6 Hours
Malignant peritoneal mesothelioma (PM) is an infrequent disease which has historically been associated with a poor prognosis. Given its long latency period and non-specific symptomatology, a diagnosis of PM can be suggested by occupational exposure history, but ultimately relies heavily on imaging and diagnostic biopsy. Early treatment options including palliative operative debulking, intraperitoneal chemotherapy, and systemic chemotherapy have marginally improved the natural course of the disease with median survival being approximately one year. The advent of cytoreduction (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) has dramatically improved survival outcomes with wide median survival estimates between 2.5 to 9 years; these studies however remain largely heterogeneous, with differing study populations, tumor biology, and specific treatment regimens. More recent investigations have explored extent of cytoreduction, repeated operative intervention, and choice of chemotherapy but have been unable to offer definitive conclusions. CRS and HIPEC remain morbid procedures with complication rates ranging between 30% to 46% in larger series. Accordingly, an increasing interest in identifying molecular targets and developing targeted therapies is emerging. Among such novel targets is sphingosine kinase 1 (SphK1) which regulates the production of sphingosine-1-phosphate, a biologically active lipid implicated in various cancers including malignant mesothelioma. The known action of specific SphK inhibitors may warrant further exploration in peritoneal disease.
Core tip: Peritoneal mesothelioma (PM) historically has been associated with a very poor prognosis. Cytoreduction with hyperthermic intraperitoneal chemotherapy improved survival outcomes but carries significant morbidity. Increasingly, research has focused on identifying molecular targets and only a handful have been described; even fewer directed therapies have been evaluated. We review the role of sphingosine kinase 1 and sphingosine-1-phosphate (S1P) signaling in PM and discuss the possibility of targeting it with FTY720, a functional antagonist of S1P Receptor 1. Further investigation is warranted in this new avenue of interest.
- Citation: Raza A, Huang WC, Takabe K. Advances in the management of peritoneal mesothelioma. World J Gastroenterol 2014; 20(33): 11700-11712
- URL: https://www.wjgnet.com/1007-9327/full/v20/i33/11700.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i33.11700
Peritoneal mesothelioma (PM) represents the second most common site of malignant mesothelioma and accounts for 10% to 20% of reported cases[1,2]. Of the 10589 patients with mesothelioma identified in the SEER database between 1973 and 2005, 1112 or 10.5% had disease which was abdominal in origin. Modest differences in incidence rates have been reported among Western countries ranging between 0.5 to 3 cases per million[3]. In the United States, the overall incidence has remained unchanged but age adjusted rates have demonstrated a stepwise increase per decade with 15000 cases projected to occur by 2050[3-5]. Males constitute 56% of cases; compared to those with thoracic disease, patients with PM are more likely to be female[6] and younger[7]. No racial predilection has been recognized[2,4].
Asbestos exposure is the strongest known risk factor for the development of malignant mesothelioma[5]. The association with peritoneal disease, while observed in various epidemiological reports, however, is weaker and not conclusive[3,8]. Factors abating this relationship have included long latency periods of 20 to 50 years from exposure to disease and lack of pathogenesis directly implicating asbestos fibers[8,9]. There is some evidence, however, to suggest that cumulative exposure has been associated with increased prevalence. Berry et al[10] studied the exposure of crocidolite among miners over several decades. More than 67% of identified cases of peritoneal mesothelioma occurred with an exposure of greater than 50 fibers per mL years; this was in contrast to only 16% subjects who developed disease with less than 10 fibers per mL years of exposure. Likewise, no cases of PM were observed within 20 years of exposure. Various other environmental factors have been implicated and include thorotrast, erionite - volcanic ash, therapeutic radiation, and chronic peritonitis[3,11]. In the absence of environmental exposures, familial Mediterranean fever, mesothelioma genetic susceptibility syndrome with BRCA germline mutations, and simian vacuolating virus have been postulated to contribute to PM[2,12].
Symptoms and signs are non-specific with most relating increasing abdominal girth, ascites, or pain[12,13]. Other reported findings have included weight loss, fevers, night sweats, early satiety, anorexia, emesis, constipation, and presence of umbilical hernia; an abdominal mass was appreciated in 10%-30%[13,14]. Computed tomography (CT) imaging is the most common initial imaging modality and can reveal moderate to extensive ascites with peritoneal, visceral, or omental involvement. Yan et al[15] reported that CT radiographic findings of large tumor burden with significant bowel distortion or the presence of a small bowel obstruction to be predictive of incomplete resectability. MRI imaging may more accurately quantify the extent of disease, however, its routine use is not supported yet[16]. The role of PET scan is not well defined and may be have some limited use in the detection of recurrent disease[17]. Biopsy is required to establish a diagnosis and can be performed radiographically or surgically. Paracentesis with fluid cytology has a variable sensitivity of 32% to 76% with the major limitation being difficulty in distinguishing benign from malignant lesions[2,18]. Various serum tumor markers have also been explored. CA-125 and CA15.3 were noted to be elevated at baseline in 53.3% and 48.5% of patients; their role, however, may be more important in monitoring disease recurrence or progression than at initial diagnosis[19,20].
Traditional treatments for peritoneal mesothelioma have historically yielded modest survival ranging between 6 and 16 mo with median survival being approximately one year[21,22]. Operative therapies have largely centered around palliative cytoreduction. Rogoff et al[23] reported one of the earliest series with 6 of 12 patients undergoing debulking with a median survival being no greater than 13 mo for the entire cohort. More recent series have reported similar median survival periods of one year[13,24,25]; compared with operative biopsy alone, however, debulking offered a modest survival improvement of 7 mo[24]. The use of conventional systemic chemotherapy likewise has not greatly impacted the natural course of PM with response rates between 11% and 28%[26-28]. More recent novel cytotoxic agents like premetrexed have improved response rates to as high as 37% with median survival ranging between 7.6 and 12.1 mo with dual agent regimens; Simon et al[29] reported an improved survival period of 26.8 mo with gemcitabine combination therapy in 20 patients but regimen was accompanied with a 60% incidence of grade 3 or 4 neutropenia[27-29]. Monotherapy with intraperitoneal chemoinfusion has likewise not offered any significant benefit with reported outcomes of 9 to 12 mo[30,31].
Cytoreduction (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) has been more widely adopted over the last 15 years and has been campaigned as the standard of care for patients with operable disease[1]. Ma et al[32] was the first to report its technical efficacy in ten patients in 1997; he reported no treatment related mortality and symptomatic ascites palliation in 7 patients. Two years later, Park et al[33] reported on 18 patients undergoing CRS + HIPEC with a two year survival rates of 80% and progression free survival of 26 mo. In studies reporting at least 2 year outcome data, median survival has varied greatly ranging between 29.5 and 100 mo and five year overall survival between 30%-90% when reported (Table 1). The wide range of these findings reflects the heavily heterogeneous nature of differing study populations, eras of treatment, institutional follow-up protocols, chemotherapy regimens, operative techniques, and tumor biology. The most numerous reports have been coordinated or originated mainly from two principal centers: the National Cancer Institute of Milan (Milan, Italy) and the Washington Cancer Center (Washington, DC, United States). Larger series, however, have relied on appropriate collaborative approaches[20,34-45]. Yan et al[42] published the largest longitudinal series with 405 patients in a multi-institutional review between 1989 and 2009. Here, 92% of patients received HIPEC most commonly with cisplatin and doxorubicin and an additional 23% subsequently received early post-operative chemotherapy between postoperative day 1 and 5, most commonly with paclitaxel. Overall median survival was 53 mo and five year survival was 47%. Such multi-institutional registries have gone onto be utilized in assessing large-population based prognostic factors[43,46,47].
| Ref. | Year | Era | Sample No. | Age | Gender (female) | HIPEC Agents | Histological Subtype or Grade | Follow-up | Median survival | 1 yr | 2 yr | 3 yr | 5 yr | 7 yr | 10 yr |
| Park et al[33] | 1999 | 1993-1998 | 18 | 47 | 28% | CDDP | 9 E, 3 mixed 1 MMF 1 cystic 4 Unk | 19 | NR | 80 | |||||
| Loggie et al[112] | 2001 | 12 | 511 | 8% | MMC | Not stated | 45.2 | 34.2 | 60 | 60 | 50 | 33 | 33 | ||
| Feldman et al[48] | 2003 | 1993-2002 | 49 | 47 | 43% | CDDP | 26 E, 4 S 16 TP 1 adenomatoid | 28.3 | 92 | 86 | 77 | 59 | 59 | ||
| Costamagna et al[61] | 2003 | 1995-2003 | 24 | CDDP + DOXO, single agent MMC or DOXO | 40 | 78 | 70 | 70 | 52 | ||||||
| Brigand et al[6] | 2006 | 1989-2004 | 15 | 53.6 | 29% | CDDP + MMC | 12 E, 2 B 1 MC | 46.7 | 35.6 | 69.3 | 57.7 | 43.3 | 28.9 | ||
| Elias et al[60] | 2007 | 1996-2005 | 26 | 461 | 46% | oxaliplatin + irinotecan, single agent oxaliplatin or DOXO | 13 E, 1 B 11 TP, 1 MC | 55 | 100 | 88 | 83 | 68 | 63 | ||
| Gómez Portilla et al[113] | 2007 | 1998-2005 | 7 | 501 | 43% | CDDP + DOXO, single agent MMC or oxaliplatin | 5 E, 2 B | 11 | NR | 43 | 43 | 43 | 43 | ||
| Hesdorffer et al[55] | 2008 | 1997-2000 | 27 | 531 | 26% | CDDP + MMC | 23 E, 4 S | 70 | 67% | ||||||
| Passot et al[114] | 2008 | 1989-2006 | 22 | CDDP + MMC | 16 E, 3 B 3 MC | 47 | 36.9 | 69 | 62 | 52 | 31 | ||||
| Chua et al[115] | 2009 | 1997-2008 | 20 | 55.71 | 30% | CDDP + DOXO | 16 E, 1 B, 2 S 1 MC | 18.1 | 29.5 | 78.2 | 46.3 | ||||
| Blackham et al[49] | 2010 | 1993-2008 | 34 | 54.91 | 32% | CDDP or MMC | 29 E, 4 B 1 Unk | 72 | 40.8 | 61 | 56 | 17 | |||
| Kluger et al[54] | 2010 | 1997-2004 | 47 | 491 | 36% | CDDP + MMC | 43 E, 4 B | 54 | 54.9 | 80.9 | 61.7 | 48.9 | |||
| Cao et al[46] | 2012 | 1989-2009 | 294 | 50 | 46% | CDDP + DOXO2 | 259 E, 27 B/S 8 Unk | 25 | 67 | 83 | 62 | 52 | |||
| Alexander et al[47] | 2013 | 1992-2010 | 211 | 52 | 60% | CDDP or MMC | 113 High, 54 Low 44 Unk | 38.4 | 41 | 26 | |||||
| Schaub et al[59] | 2013 | 1994-2010 | 104 | 50.9 | 39% | CDDP | 90 E, 14B/S | 49.4 | 52 | 58 | 46 | ||||
| Wong et al[52] | 2013 | 2004-2012 | 26 | 64 | 38% | CDDP | 15 E, 3 B 1 MMF 3 WD, 1 cystic 3 Unk | 41.2 | |||||||
| NMDeraco et al[37] | 2003 | 1995-2002 | 19 | 491 | 53% | CDDP + MMC or DOXO | 13 E, 1 S, 1 mixed 2WD, 2MC | 27 | NR | 69 | |||||
| NMDeraco et al[39] | 2003 | 28 | 61% | 70 | |||||||||||
| NMDeraco et al[38] | 2003 | 61 | 511 | 49% | CDDP + MMC or DOXO | 20 | 54 | ||||||||
| NMDeraco et al[40] | 2006 | 1995-2005 | 49 | 521 | CDDP + DOXO or MMC | 43 E, 6 B | 20.31 | 57 | |||||||
| NMDeraco et al[50] | 2013 | 1995-2011 | 116 | 54.41 | 48% | CDDP + DOXO or MMC | 105 E, 11 B/S | 32.91 | 49 | ||||||
| NMBaratti et al[20] | 2007 | 1997-2005 | 60 | 53.5 | 60% | 43 E, 6 B 5 MC, 6 P | 23 | 53.7 | |||||||
| NMBaratti et al[36] | 2007 | 1995-2006 | 12 | 38 | 100% | CDDP + DOXO | 4 MC, 8 WD | 27 | NR | 90 | |||||
| NMBaratti et al[35] | 2010 | 1996-2008 | 83 | 54 | 55% | CDDP + MMC or DOXO | 72 E, 10 B, 1 S | 52 | 44 | 49.5 | 45.5 | ||||
| NMBaratti et al[34] | 2013 | 1996-2012 | 108 | 56.5 | 46% | CDDP + DOXO or MMC | 93 E, 14 B, 1 S | 48.8 | 63.2 | 52.4 | 44.6 | ||||
| WCYan et al[45] | 2006 | 100 | 501 | 40% | CDDP + DOXO | 86 E, 7 B/S 7 Unk | 48 | 52 | 78 | 55 | 46 | 39 | |||
| WCYan et al[44] | 2007 | 1989-2005 | 70 | 471 | 43% | CDDP + DOXO | 65 E, 5 B | 35 | 59 | 82 | 67 | 57 | 49 | ||
| WCYan et al[41] | 2007 | 1989-2005 | 62 | 471 | 45% | CDDP + DOXO | 57 E, 5 B | 37 | 79 | 84 | 58 | 50 | |||
| WCYan et al[42] | 2009 | 1989-2009 | 401 | 501 | 44% | CDDP + DOXO2 or MMC, single agent CDDP or MMC | 318 E, 48 B/S 35 Unk | 33 | 53 | 81 | 60 | 47 | |||
| WCYan et al[43] | 2011 | 1989-2009 | 294 | 501 | 46% | CDDP + DOXO2 | 259 E, 27 B/S 8 Unk | 24 | 67 | 83 | 62 | 52 |
Reports describing long term survival at 10 years are slowly emerging. Two studies listed survival estimates as secondary outcomes and ranged between 26% and 44.6%[35,47]. Baratti et al[34] reported on 10 year prognosis in 108 patients undergoing complete cytoreduction and HIPEC at two centers. Chemotherapy regimens included cisplatin and doxorubicin in the vast majority of patients and cisplatin or mytomycin-C in six. At five and ten years, overall estimates were 52.4% and 44.6%, respectively, and median survival was 63.2 mo.
The most optimistic, large series survival estimates were reported by Feldman et al[48] in 49 patients. More than half of patients had previously undergone debulking surgery, and thereafter, all subjects underwent HIPEC with cisplatin. Thirty five patients or 72% additionally received a single postoperative intraperitoneal (IP) dose of fluorouracil and paclitaxel. Median overall survival was 92 mo and 1, 3, and 5 year survival was 86%, 59%, and 59%, respectively. Worse survival was associated with deep tumor invasion, age greater than 60 years, absence of prior debulking, and residual disease greater than 1 cm. Of note, 36% of patients had lower grade histology which impacted survival on univariate analyses. Baratti et al[36], likewise, reported an exceptional 5 year survival estimate of 90% with median survival not being reached; however, this study sought to describe borderline malignant sub-types of PM in 12 patients.
No randomized clinical trials exist that assess which HIPEC chemotherapy regimen is superior. The majority of retrospective reports have described cisplatin mono- or dual therapy (Table 1). Blackham et al[49] investigated 19 patients receiving monoagent mitomycin and 15 receiving cisplatin in a retrospective review; he found patients administered cisplatin were more likely to be alive at 1, 2, and 3 years with a nearly 30 mo median survival advantage. Similarly, Alexander et al[47] identified the use of cisplatin over mitomycin-C to be associated with favorable survival; this however was noted only in optimally cytoreduced patients. The choice of agents seems largely driven by an institution’s experience more so than empiric evidence.
The role of peri-operative systemic chemotherapy in addition to HIPEC with CRS was examined retrospectively by Deraco et al[50] in 90 patients. Sixty of these patients received preoperative chemotherapy most commonly with a platinum-based agent and premetrexed or gemcitabine; 12 patients underwent triple agent therapy and two received more than three drugs. An additional 30 patients naïve to systemic therapy received post-operative treatment with platinum with premetrexed or gemcitabine. These cohorts were compared to 26 patients who underwent a logoregional approach only. No significant difference was observed in overall survival among groups with the survival estimate being 49% at 5 years for the entire series. A trend towards improved progression free survival, however, was observed in those receiving preoperative treatment, and overall 3 year survival favored those treated with preoperative pemetrexed and platinum chemotherapy (63% vs 42%-48%, non-significant). No differences in prognostic factors were identified among groups and the epithelioid histological was most common subtype. Yan et al[42] similarly reported on 22 patients receiving pemetrexed dual agent therapy after cytoreductive surgery and demonstrated no significant influence on survival. To date, combination regional and systemic therapies for PM remain largely unexplored.
The extent of cytoreduction has repeatedly shown to impact survival[40,51,52]; a handful of studies have gone onto better define the role of aggressive cytoreduction. The underlying principle of cytoreduction is to remove all the macroscopic disease and use HIPEC to address any remaining microscopic disease[1]. Baratti et al[53] attempted to address the benefit of patients undergoing resection of peritoneum free of gross disease in addition to macroscopic disease. In a case-control study, 30 patients undergoing selective resection of macroscopic disease were compared to a cohort of 30 individuals undergoing “complete” parietal peritonectomy, which included abdominal regions uninvolved by disease. The five year overall survival was significantly greater at 63.9% vs 40% in the “complete” resection group. The median overall survival was not reached in the “complete” group despite a follow-up of 50.3 mo and was 29.6 mo in the selective resection group. Progression free survival was likewise significant being 54.3% vs 24.9% in favor of more aggressive peritonectomy. Interestingly, “complete” resection carried no significant increase in operative risk and was associated with a shorter length of stay by 8 d. A subsequent pathologic review revealed peritoneal disease involvement in 54% of samples deemed grossly negative at exploration which may warrant more aggressive cytoreduction approach.
More recently, previously abandoned and multi-stage modalities have been re-explored with the use of CRS and HIPEC. Wong et al[52] addressed the outcomes of repeated CRS with HIPEC. Twenty six of 29 patients underwent debulking with cisplatin-based HIPEC. Eight or 31% then went on to have one or more repeated HIPEC procedures. The median overall survival for the re-operation group was far superior at 80 mo compared to 27.2 mo in the single treatment cohort. The median time to the second operation was 15.6 mo and most (77%) received early postoperative chemotherapy with Taxol and 5-fluorouracil. Both groups otherwise had similar completeness of cytoreduction scores, demographics, and similar overall number of complications. Kluger et al[54] reported on two-stage operative cytoreduction with intraperitoneal chemotherapy in 47 patients. Subjects initially underwent partial cytoreduction with peri-operative intraperitoneal therapy with single or dual regimens of cisplatin, gemcitabine, doxorubicin, or gamma interferon. A second laparotomy with CRS and HIPEC was performed in 35 using cisplatin and mitomycin C; median survival was 54.9 mo with 1, 3, and 5 year overall survival being 81%, 62% and 49%, respectively. Hesdorffer et al[55] reported on multi-modality treatment in 27 patients who underwent operative debulking with post-operative IP therapy followed by HIPEC with mitomycin and cisplatin and then followed by whole abdominal radiation between 3000 and 3080 cGy. Overall median survival was 70 mo and three year survival was 67%. The retrospective nature of these reviews limits drawing any firm conclusions, but a multi-modality approach may offer the most aggressive treatment for patients with PM.
Diagnostic laparoscopy with biopsy has been previously described as a safe alternative in obtaining a histological diagnosis[13,56]. Its role in assessing resectability before CRS with HIPEC in PM was explored in 33 patients. Patients with potentially resectable disease on pre-operative imaging underwent exploration. Ninety one percent of patients were deemed likely to obtain complete cytoreduction; of these, only one patient was not on subsequent laparotomy, yielding an overall specificity of 75% and accuracy of 97%[57].
More than half of the studies reporting on prognostic factors have reported completeness of cytoreduction to be associated with improved survival on multivariate analyses[35,38,40,42,43,45,50-53]. Nodal status, histological subtype, nuclear grade, and mitotic count have also been cited[34,35,40,42,43,45,47,51-54] (Table 2). Concordant findings were reported in the large multi-institutional series by Yan et al[42]. Interestingly, 29 patients did not receive HIPEC; a subsequent multivariate sub-analysis demonstrated that HIPEC correlated with improved survival. Baratti et al[53] similarly identified another surgical factor, “complete” peritonectomy, as positive influencing survival.
| Ref. | Year | Sample No. | Prognostic factors overall survival (multivariate only) |
| Deraco et al[38] | 2003 | 61 | Completeness of cytoreduction |
| Feldman et al[48] | 2003 | 49 | 1No prior debulking, deep invasion, age > 60, residual disease > 1 cm |
| Nonaka et al[51] | 2005 | 35 | Completeness of cytoreduction, low mitotic count, lower nuclear grade |
| Deraco et al[40] | 2006 | 49 | Completeness of cytoreduction, low mitotic count/50 HPF |
| Yan et al[45] | 2006 | 100 | No lymph node metastasis, female gender, epithelial type, adequate cytoreduction |
| Baratti et al[20] | 2007 | 60 | 1High-grade histology, WHO performance status > 0, Inadequate cytoreduction |
| Yan et al[41] | 2007 | 62 | Mesothelioma nuclear size |
| Yan et al[42] | 2009 | 401 | Epithelial subtype, absence of lymph node metastasis, completeness of cytoreduction 0/1, HIPEC |
| Baratti et al[35] | 2010 | 83 | Pathologically negative lymph nodes, epithelial subtype, mitotic count ≤ 5/50 HPF, Completeness of cytoreduction |
| Kluger et al[54] | 2010 | 47 | 1Biphasic histological subtype |
| Yan et al[43] | 2011 | 294 | 1Biphasic/sarcomatoid subtype, completeness of cytoreduction score of 2/3, proposed TNM Stage II or III |
| Cao et al[46] | 2012 | 294 | Female gender, TNM staging |
| Baratti et al[53] | 2012 | 60 | Complete parietal peritonectomy, complete cytoreduction, negative lymph nodes, Epithelial histology, low MIB-1 index |
| Alexander et al[47] | 2013 | 211 | Age < 60 yr, R0-1 vs R2-3, low histologic grade, use of cisplatin vs mitomycin-C |
| Baratti et al[34] | 2013 | 108 | Epithelial histology, histologically negative lymph nodes, Ki-67 < 10% |
| Deraco et al[50] | 2013 | 116 | Histological subtype, completeness of cytoreduction, absence of morbidity 3-5 grade |
| Schaub et al[59] | 2013 | 104 | Histological subtype, pre-CRS PCI, preoperative serum CA-125 |
| Wong et al[52] | 2013 | 29 | Lower peritoneal carcinoma index, completeness of cytoreduction |
| Pillai et al[58] | 2013 | 33 | Presence of nuclear estrogen receptor beta |
Female gender was shown by Cao et al[46] to be among patient factors to positively influence survival; the female cohort accounted for 46% in the study population and was more likely to have lower peritoneal cancer indices and earlier stage compared to males[41,46]. Interestingly, the presence of the nuclear estrogen receptor beta was shown to be an independent predictor of survival in peritoneal disease[58]. Schaub et al[59] found pre-operative CA-125 to influence survival; a prognostic nomogram was proposed by incorporating this marker along with peritoneal cancer index and histological subtype as clinical assessment tool for 3 and 5 year survival; positive predictive values have been reported as 73.1% and 73.9% respectively.
Overall morbidity rates have varied widely between 14% and 71% (Table 3). Larger series with at least 50 subjects have reported 28%-41% incidence of grade 3 or greater complications[35,42,44,45,50,53]. When reported, peri-operative mortality has ranged between 1% and 11% and re-operation rates up to 20% with the most common indication being hemorrhage[6,34,37,42,44,46,47,52,60-62]. Complications related to fistula formation, perforation, dehiscence, abscess formation are significant and in large series have been reported in up to 18% of cases[42]. Cardiopulmonary complications are the second most frequently encountered followed by those related to infection. It remains difficult to distinguish those complications stemming from operative intervention and those related to chemotherapy regimens. In terms of long-term survival, Deraco demonstrated worse survival with patients with grade 3 to 5 complications[50]. Length of stay has been investigated in a handful of reports and has ranged from 9 to 41.5 d; Wong et al[52] reported on a median stay of only 8 d for patients undergoing repeat HIPEC. Surprisingly, morbidity rates only have a weak association with the duration of inpatient admission; other factors such as administration of early post-operative chemotherapy may be involved but are not reported.
| Ref. | Year | Sample No. | Complication rate | Peri-operative mortality | Re-operation | Abdominal | Cardiac/pulmonary | Sepsis | Wound Infection | Other infection | Renal | Vascular | Hematologic | Other | LOS |
| Park et al[33] | 1999 | 18 | 24% | 1 | 1 | 1 | 1 | 2 | 1 | 1 | |||||
| Feldman et al[48] | 2003 | 49 | 25% | 0 | 2 | 4 | 5 | 2 | 3 | 1 | 1 | 2 | |||
| Costamagna et al[61] | 2003 | 24 | 26% | 11 | |||||||||||
| Brigand et al[6] | 2006 | 15 | 40% | 0 | 0 | 0 | 0 | 0 | 16.3 | ||||||
| Elias et al[60] | 2007 | 26 | 54% | 4 | 4 | 1 | 1 | 4 | 1 | 3 | 2 | 2 | 281 | ||
| Gomez et al[113] | 2007 | 7 | 71% | 41.51 | |||||||||||
| Hesdorffer et al[55] | 2008 | 27 | 30% | 0 | 2 | 1 | 1 | 1 | 3 | ||||||
| Passot et al[114] | 2008 | 22 | 47% | 0 | |||||||||||
| Chua et al[115] | 2009 | 20 | 65% | 1 | 2 | 5 | 2 | 1 | 1 | 3 | 16.51 | ||||
| Yano et al[62] | 2009 | 17 | 41% | 5.8 | 2 | 2 | 1 | 3 | |||||||
| Kluger et al[54] | 2010 | 47 | 34% | 2 | 1 | 3 | 9 | 2 | 2 | 9 | 2 | 1 | 1 | 161 | |
| Cao et al[46] | 2012 | 294 | 33% | 2 | 50 | 38 | 26 | 15 | 231 | ||||||
| Alexander et al[47] | 2013 | 211 | 30% | 2.3 | 20 | 20 | 25 | 9 | 6 | 8 | 11 | 11 | |||
| Wong et al[52] | 2013 | 29 | 65% | 4 | 1 | 4 | 1 | 6 | 2 | 10 | 4 | 9 | |||
| Deraco et al[37] | 2003 | 19 | 25% | 0 | 3 | 1 | 1 | 3 | 321 | ||||||
| Deraco et al[39] | 2003 | 28 | 14% | 0 | |||||||||||
| Deraco et al[38] | 2003 | 61 | 23% | 0 | 7 | ||||||||||
| Deraco et al[40] | 2006 | 49 | 15%G3 | 9 | 2 | 4 | 5 | 4 | 3 | 5 | 241 | ||||
| Deraco et al[50] | 2013 | 116 | 41%G3 | 2.6 | 25 | ||||||||||
| Baratti et al[36] | 2007 | 12 | 8%G3 | 0 | 1 | 1 | |||||||||
| Baratti et al[116] | 2008 | 5 | 20%G3 | 0 | 1 | 1 | |||||||||
| Baratti et al[35] | 2010 | 83 | 28%G3 | 2.4 | |||||||||||
| Baratti et al[117] | 2010 | 12 | 8%G3 | 0 | 18 | ||||||||||
| Baratti et al[53] | 2012 | 60 | 28%G3 | 0 | 7 | 8 | 4 | 3 | 1 | 7 | 2 | 5 | 1 | ||
| Baratti et al[34] | 2013 | 108 | 39% | 1.9 | 14 | 10 | 6 | 10 | 7 | ||||||
| Yan et al[45] | 2006 | 100 | 36%G3 | 5 | 6 | 8 | 7 | 11 | 5 | 8 | 10 | 221 | |||
| Yan [44] | 2007 | 70 | 41%G3 | 3 | 4 | 3 | 4 | 3 | 2 | 4 | 5 | 3 | 231 | ||
| Yan et al[42] | 2009 | 401 | 46%G3 | 2 | 74 | 57 | 39 | 25 | 221 |
A variety of molecular targets have been identified in PM and of these, a handful of respective therapeutic agents have been investigated. Foster et al[63] discovered mutations in the epidermal growth factor receptor (EGFR) in a subset of 29 patients, which were associated with a higher rate of optimal cytoreduction and a trend towards improved 3 year overall and progression free survival. Erlotinib, an EGFR inhibitor, was then investigated using COS-7 cell lines transfected with mutant EGFR at different drug concentrations along with EGF; based on subsequently decreased EGFR phosphorylation, it was postulated that erlotinib may warrant further exploration. Kalra et al[64], however, questioned any wide spread role of EGFR targeted therapies because none of 33 peritoneal mesothelioma tumors he interrogated expressed EGFR sensitizing mutations.
Varghese et al[65] identified up-regulation in genes related to the phosphatidylinositol-3 kinase (PI3K) and the mammalian target of rapamycin (mTOR) signaling pathways to be associated with poorer survival among 41 patients undergoing CRS and HIPEC. Under-expression was associated with an 80% 3 year survival with a median period of 69.5 mo compared with 47.4 mo for the entire cohort and 24 mo for over-expressers. Using an in vitro model, cells were treated with a dual PI3K and mTOR inhibitor, NVP-BEZ235, demonstrated significant suppression of cell proliferation[65].
Mesothelin, glycosylphosphatidylinositol-anchored glycoprotein, has also been recognized to be highly expressed in malignant mesothelioma along with pancreatic, ovarian, and some lung cancers. Three agents targeting mesothelin have been tested to date; SS1P, a recombinant immunotoxin targeting mesothelin; MORAb-009, a chimeric anti-mesothelin monoclonal antibody; and CRS-207, a live-attenuated Listeria monocytogenes vector encoding human mesothelin[66]. Of these, SS1P has undergone phase I testing in 24 patients including five with peritoneal mesothelioma and has shown short term resolution of ascites in one patient[67,68]. The second trial demonstrated a partial response with SS1P in two of 12 patients with PM[67,68]. Hassan et al[69] reported on a regimen of SS1P, pentostatin, and cyclophosphamide in eleven patients with mesothelioma; two patients with peritoneal disease had a significant tumor reduction up to 8 and 14 mo, respectively. More recent studies have reported on newer mesothelin-targeted agents including the immunocytokine IL12, which has shown comparable anti-tumor activity to SS1P in a murine model of PM[70].
Molecular targets on the horizon include MUC1, a glycoprotein associated with various cancers including breast, colon, and pancreatic adenocarcinoma; recently, Pillai demonstrated that MUC1 was expressed in 90% of patients with PM and may have some prognostic value in predicting poorer survival[71]. Bromelain, a complex of proteolytic enzymes, has been postulated to target glycoproteins including MUC1, and initial experiments have demonstrated that chemo-resistant peritoneal mesothelioma cells lines have increased chemotherapy sensitivity with bromelain combination therapy[72]. Some skepticism exists as no studies have directly examined the effect of this agent on MUC1 in peritoneal disease.
Sphingosine kinase 1 (SphK1) is the lipid kinases that phosphorylate sphingosine to generate sphingosine-1-phosphate (S1P), a lipid mediator. S1P is an important bioactive lipid that has been implicated in multiple physiologic and pathologic processes such as, inflammation, atherosclerosis, asthma, osteoporosis, diabetes, obesity, and particularly cancer, due to its role in cell survival, proliferation, migration, angiogenesis, and lymphocyte trafficking[73,74].
The molecular functions of S1P can be divided into its intracellular action and extracellular signaling, which is coined “inside-out” signaling[73,74]. Intracellular S1P can directly regulate its target proteins, which are histone deacetylases (HDACs) and the E3 ubiquitin ligase tumor-necrosis factor (TNF) receptor-associated factor 2 (TRAF2)[75,76]. Through the regulation on these proteins, S1P involves epigenetic regulation of gene expression of NF-κB signaling, which play key roles in cancer biology. As for the inside-out signaling of S1P, transporters such as ABC transporters and Spns2 have been identified[77,78]. These transporters allow S1P to be exported outside the cell and act as a ligand on membranous five S1P specific G-protein coupled receptors (S1PR1-5), which activate multiple downstream signaling pathways regulating cell differentiation, migration, and survival in an autocrine, paracrine, and/or endocrine manner[79].
Owing to the role of S1P in cancer cells, studies investigating SphK1 as an oncogene have steadily increased[80]. It has been known that S1P possesses a strong angiogenic property[81]. Considering a critical role of lymphangiogenesis in cancer progression[82], our group reported on a new aspect of the SphK1/S1P axis by its involvement in breast cancer-induced lymphangiogenesis which precedes breast cancer metastasis[83,84]. Recently, we have also further demonstrated the indispensable role of the SphK1/S1P axis in colitis and colitis-associated cancer[85-87] .
The role of the SphK1/S1P axis in mesothelioma has not been reported only until recently. Kalari et al[88] demonstrated elevations in SphK1 expression in both the epithelial and sarcomatoid subtypes of human pleural mesothelioma compared with non-tumor specimens. They further delineated the function of SphK1 in vitro and in vivo. Examining mesothelioma cell lines, SphK1 mRNA and protein expression were higher in malignant cells, and this over-expression correlated with cellular proliferation. The possible mechanism was S1P regulation on expression of cell cycle-related genes via histone acetylation. The SphK2 isotype was not implicated in tumorigenesis.
Using the SphK1 inhibitor, SphK-I2, or gene silencing, S1P production and cell proliferation were likewise reduced. The authors additionally conducted an alternative in vivo model in which they exposed the peritoneal lining of mice to mesothelioma inducing agents, specifically long multiwalled carbon nanotubes (MWNT). Exposure to MWNTs have been reported to cause development of granulomas in p53-knockout mice[89]. Compared to wild type mice, the Sphk1 knockout mice demonstrated significantly less MWNT-induced granulomatous inflammation. This result suggested the in vivo role of SphK1 as promoting mesothelioma development.
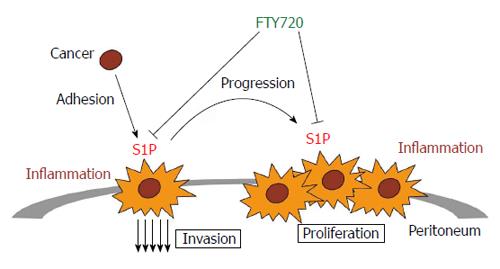
Recently, studies investigating agents targeting S1P signaling have been tested in various settings[90]. Among these, FTY720 has shown some promise; FTY720 (Fingolimod; trade name Gilenya, Novartis) is a FDA-approved drug for treating relapsing forms of multiple sclerosis[91]. It has been shown FTY720 acts as a pro-drug which is mainly phosphorylated in vivo by SphK2[92-94]. The phospho-FTY720 mimics S1P action by binding to S1PR1 which is then internalized and degraded[95,96]. S1PR1 signaling, itself, is important for lymphocyte egress from thymus and secondary lymphoid organs to the periphery[97,98]. The down-regulation of S1PR1, therefore, through the known action of FTY720, is considered as immunomodulatory by inducing lymphopenia without generalized immunosuppression[96,99]. In addition to the immunosuppressant property, several reports about FTY720 as an anti-cancer drug in various malignancies have rapidly accumulated[100,101]. We recently reported, in a murine colitis-associated colon cancer model, the administration of FTY720 dramatically reduced tumor size, multiplicity, and tumor load via the reduction of SphK1 and S1PR1 expression[85,86]. Others have gone onto also characterize FTY720 as a SphK1 inhibitor in multiple cancer cell lines[102-104]. In hematopoietic malignancies or lung cancer, FTY720 acts as an activator of tumor suppressor protein phosphatase 2A (PP2A) and shows promising preclinical activity[105-108]. In hepatocellular carcinoma, FTY720 was found to decrease recurrence after liver transplantation via down-regulation of S1PR1[109]. FTY720 was additionally suggested in combinational therapy with sunitinib for breast cancer, with milatuzumab for lymphoma, and radiotherapy for prostate cancer[103,110,111]. Taken together, targeting S1P signaling by FTY720 might be a potential strategy for pharmoco-therapeutics for peritoneal mesothelioma (Figure 1).
Peritoneal mesothelioma remains a rare, infrequent disease which historically has been associated with a poor prognosis. Demonstrable improvements in survival have been made with the wider employment of cytoreduction and HIPEC and a generally more aggressively-focused treatment regimen. Yet despite these advances, significant morbidity still persists and a few options exist for those not amenable to operative intervention. Novel molecular targets such as SphK1 have only recently been associated with PM and represent a potentially promising venue for drug therapy in the future.
P- Reviewer: Franko J, Levine EA, Morris DL S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Blackham AU, Levine EA. Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Malignant Peritoneal Mesothelioma. European J Clin Med Oncol. 2012;4:25-32. [PubMed] |
| 2. | Kindler HL. Peritoneal Mesothelioma: The Site of Origin Matters. American Society of Clinical Oncology EDUCATIONAL BOOK. Alexandria, VA: ASCO 2013; 182-187. |
| 3. | Boffetta P. Epidemiology of peritoneal mesothelioma: a review. Ann Oncol. 2007;18:985-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 184] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Rodríguez D, Cheung MC, Housri N, Koniaris LG. Malignant abdominal mesothelioma: defining the role of surgery. J Surg Oncol. 2009;99:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Moolgavkar SH, Meza R, Turim J. Pleural and peritoneal mesotheliomas in SEER: age effects and temporal trends, 1973-2005. Cancer Causes Control. 2009;20:935-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Brigand C, Monneuse O, Mohamed F, Sayag-Beaujard AC, Isaac S, Gilly FN, Glehen O. Peritoneal mesothelioma treated by cytoreductive surgery and intraperitoneal hyperthermic chemotherapy: results of a prospective study. Ann Surg Oncol. 2006;13:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Parazzini F, Ricci E, Cipriani S, Chiaffarino F, Bortolus R, Chiantera V, Bulfoni G. Temporal trends and determinants of peripartum hysterectomy in Lombardy, Northern Italy, 1996-2010. Arch Gynecol Obstet. 2013;287:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Welch LS, Acherman YI, Haile E, Sokas RK, Sugarbaker PH. Asbestos and peritoneal mesothelioma among college-educated men. Int J Occup Environ Health. 2005;11:254-258. [PubMed] |
| 9. | Yan TD, Welch L, Black D, Sugarbaker PH. A systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for diffuse malignancy peritoneal mesothelioma. Ann Oncol. 2007;18:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 240] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 10. | Berry G, Reid A, Aboagye-Sarfo P, de Klerk NH, Olsen NJ, Merler E, Franklin P, Musk AW. Malignant mesotheliomas in former miners and millers of crocidolite at Wittenoom (Western Australia) after more than 50 years follow-up. Br J Cancer. 2012;106:1016-1020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Hassan R, Alexander R, Antman K, Boffetta P, Churg A, Coit D, Hausner P, Kennedy R, Kindler H, Metintas M. Current treatment options and biology of peritoneal mesothelioma: meeting summary of the first NIH peritoneal mesothelioma conference. Ann Oncol. 2006;17:1615-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Chua TC, Chong CH, Morris DL. Peritoneal mesothelioma: current status and future directions. Surg Oncol Clin N Am. 2012;21:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Manzini Vde P, Recchia L, Cafferata M, Porta C, Siena S, Giannetta L, Morelli F, Oniga F, Bearz A, Torri V. Malignant peritoneal mesothelioma: a multicenter study on 81 cases. Ann Oncol. 2010;21:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Ribak J, Lilis R, Suzuki Y, Penner L, Selikoff IJ. Malignant mesothelioma in a cohort of asbestos insulation workers: clinical presentation, diagnosis, and causes of death. Br J Ind Med. 1988;45:182-187. [PubMed] |
| 15. | Yan TD, Haveric N, Carmignani CP, Chang D, Sugarbaker PH. Abdominal computed tomography scans in the selection of patients with malignant peritoneal mesothelioma for comprehensive treatment with cytoreductive surgery and perioperative intraperitoneal chemotherapy. Cancer. 2005;103:839-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Low RN, Sebrechts CP, Barone RM, Muller W. Diffusion-weighted MRI of peritoneal tumors: comparison with conventional MRI and surgical and histopathologic findings--a feasibility study. AJR Am J Roentgenol. 2009;193:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Cao Q, Lu M, Heath J, Hausner PF, Alexander HR, Dilsizian V, Chen W. 18F-FDG PET/CT in a recurrent diffuse malignant peritoneal mesothelioma. Clin Nucl Med. 2012;37:492-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Turner KM, Varghese S, Alexander HR. Surgery for peritoneal mesothelioma. Curr Treat Options Oncol. 2011;12:189-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Baratti D, Kusamura S, Deraco M. Circulating CA125 and diffuse malignant peritoneal mesothelioma. Eur J Surg Oncol. 2009;35:1198-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Baratti D, Kusamura S, Martinetti A, Seregni E, Oliva DG, Laterza B, Deraco M. Circulating CA125 in patients with peritoneal mesothelioma treated with cytoreductive surgery and intraperitoneal hyperthermic perfusion. Ann Surg Oncol. 2007;14:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Baratti D, Kusamura S, Deraco M. Diffuse malignant peritoneal mesothelioma: systematic review of clinical management and biological research. J Surg Oncol. 2011;103:822-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Sugarbaker PH, Yan TD, Stuart OA, Yoo D. Comprehensive management of diffuse malignant peritoneal mesothelioma. Eur J Surg Oncol. 2006;32:686-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Rogoff EE, Hilaris BS, Huvos AG. Long-term survival in patients with malignant peritoneal mesothelioma treated with irradiation. Cancer. 1973;32:656-664. [PubMed] |
| 24. | Eltabbakh GH, Piver MS, Hempling RE, Recio FO, Intengen ME. Clinical picture, response to therapy, and survival of women with diffuse malignant peritoneal mesothelioma. J Surg Oncol. 1999;70:6-12. [PubMed] |
| 25. | van Gelder T, Hoogsteden HC, Versnel MA, de Beer PH, Vandenbroucke JP, Planteydt HT. Malignant peritoneal mesothelioma: a series of 19 cases. Digestion. 1989;43:222-227. [PubMed] |
| 26. | Chahinian AP, Pajak TF, Holland JF, Norton L, Ambinder RM, Mandel EM. Diffuse malignant mesothelioma. Prospective evaluation of 69 patients. Ann Intern Med. 1982;96:746-755. [PubMed] |
| 27. | Garcia-Carbonero R, Paz-Ares L. Systemic chemotherapy in the management of malignant peritoneal mesothelioma. Eur J Surg Oncol. 2006;32:676-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Carteni G, Manegold C, Garcia GM, Siena S, Zielinski CC, Amadori D, Liu Y, Blatter J, Visseren-Grul C, Stahel R. Malignant peritoneal mesothelioma-Results from the International Expanded Access Program using pemetrexed alone or in combination with a platinum agent. Lung Cancer. 2009;64:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Simon GR, Verschraegen CF, Jänne PA, Langer CJ, Dowlati A, Gadgeel SM, Kelly K, Kalemkerian GP, Traynor AM, Peng G. Pemetrexed plus gemcitabine as first-line chemotherapy for patients with peritoneal mesothelioma: final report of a phase II trial. J Clin Oncol. 2008;26:3567-3572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Markman M, Kelsen D. Efficacy of cisplatin-based intraperitoneal chemotherapy as treatment of malignant peritoneal mesothelioma. J Cancer Res Clin Oncol. 1992;118:547-550. [PubMed] |
| 31. | Kirmani SCS, Mowry J. Intracavitary cisplatin for malignant mesothelioma: an update. Proc Am Clin Oncol. 1988;7:Abstract 1057. |
| 32. | Ma GY, Bartlett DL, Reed E, Figg WD, Lush RM, Lee KB, Libutti SK, Alexander HR. Continuous hyperthermic peritoneal perfusion with cisplatin for the treatment of peritoneal mesothelioma. Cancer J Sci Am. 1997;3:174-179. [PubMed] |
| 33. | Park BJ, Alexander HR, Libutti SK, Wu P, Royalty D, Kranda KC, Bartlett DL. Treatment of primary peritoneal mesothelioma by continuous hyperthermic peritoneal perfusion (CHPP). Ann Surg Oncol. 1999;6:582-590. [PubMed] |
| 34. | Baratti D, Kusamura S, Cabras AD, Bertulli R, Hutanu I, Deraco M. Diffuse malignant peritoneal mesothelioma: long-term survival with complete cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy (HIPEC). Eur J Cancer. 2013;49:3140-3148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 35. | Baratti D, Kusamura S, Cabras AD, Laterza B, Balestra MR, Deraco M. Lymph node metastases in diffuse malignant peritoneal mesothelioma. Ann Surg Oncol. 2010;17:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Baratti D, Kusamura S, Nonaka D, Oliva GD, Laterza B, Deraco M. Multicystic and well-differentiated papillary peritoneal mesothelioma treated by surgical cytoreduction and hyperthermic intra-peritoneal chemotherapy (HIPEC). Ann Surg Oncol. 2007;14:2790-2797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Deraco M, Casali P, Inglese MG, Baratti D, Pennacchioli E, Bertulli R, Kusamura S. Peritoneal mesothelioma treated by induction chemotherapy, cytoreductive surgery, and intraperitoneal hyperthermic perfusion. J Surg Oncol. 2003;83:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Deraco M, De Simone M, Rossi CR, Cavaliere F, Difilippo F, Scuderi S, Pilatti P, Kusamura S. An Italian Multicentric Phase II study on peritonectomy and intra peritoneal hyperthermic perfusion (IPHP) to treat patients with peritoneal mesothelioma. J Exp Clin Cancer Res. 2003;22:41-45. [PubMed] |
| 39. | Deraco M, Kusamura S, Baratti D, Casali P, Zaffaroni N. [Peritoneal mesothelioma: results of a complicated and aggressive procedure incorporating peritonectomy and intraperitoneal hyperthermic chemotherapy, and prospects derived from bench-to-bedside research]. Tumori. 2003;89:56-57. [PubMed] |
| 40. | Deraco M, Nonaka D, Baratti D, Casali P, Rosai J, Younan R, Salvatore A, Cabras Ad AD, Kusamura S. Prognostic analysis of clinicopathologic factors in 49 patients with diffuse malignant peritoneal mesothelioma treated with cytoreductive surgery and intraperitoneal hyperthermic perfusion. Ann Surg Oncol. 2006;13:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 41. | Yan TD, Brun EA, Cerruto CA, Haveric N, Chang D, Sugarbaker PH. Prognostic indicators for patients undergoing cytoreductive surgery and perioperative intraperitoneal chemotherapy for diffuse malignant peritoneal mesothelioma. Ann Surg Oncol. 2007;14:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, Gilly FN, Levine EA, Shen P, Mohamed F. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol. 2009;27:6237-6242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 488] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 43. | Yan TD, Deraco M, Elias D, Glehen O, Levine EA, Moran BJ, Morris DL, Chua TC, Piso P, Sugarbaker PH. A novel tumor-node-metastasis (TNM) staging system of diffuse malignant peritoneal mesothelioma using outcome analysis of a multi-institutional database*. Cancer. 2011;117:1855-1863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 44. | Yan TD, Edwards G, Alderman R, Marquardt CE, Sugarbaker PH. Morbidity and mortality assessment of cytoreductive surgery and perioperative intraperitoneal chemotherapy for diffuse malignant peritoneal mesothelioma--a prospective study of 70 consecutive cases. Ann Surg Oncol. 2007;14:515-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Yan TD, Yoo D, Sugarbaker PH. Significance of lymph node metastasis in patients with diffuse malignant peritoneal mesothelioma. Eur J Surg Oncol. 2006;32:948-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 46. | Cao C, Yan TD, Deraco M, Elias D, Glehen O, Levine EA, Moran BJ, Morris DL, Chua TC, Piso P. Importance of gender in diffuse malignant peritoneal mesothelioma. Ann Oncol. 2012;23:1494-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Alexander HR, Bartlett DL, Pingpank JF, Libutti SK, Royal R, Hughes MS, Holtzman M, Hanna N, Turner K, Beresneva T. Treatment factors associated with long-term survival after cytoreductive surgery and regional chemotherapy for patients with malignant peritoneal mesothelioma. Surgery. 2013;153:779-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 48. | Feldman AL, Libutti SK, Pingpank JF, Bartlett DL, Beresnev TH, Mavroukakis SM, Steinberg SM, Liewehr DJ, Kleiner DE, Alexander HR. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol. 2003;21:4560-4567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 252] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 49. | Blackham AU, Shen P, Stewart JH, Russell GB, Levine EA. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for malignant peritoneal mesothelioma: mitomycin versus cisplatin. Ann Surg Oncol. 2010;17:2720-2727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | Deraco M, Baratti D, Hutanu I, Bertuli R, Kusamura S. The role of perioperative systemic chemotherapy in diffuse malignant peritoneal mesothelioma patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2013;20:1093-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 51. | Nonaka D, Kusamura S, Baratti D, Casali P, Cabras AD, Younan R, Rosai J, Deraco M. Diffuse malignant mesothelioma of the peritoneum: a clinicopathological study of 35 patients treated locoregionally at a single institution. Cancer. 2005;104:2181-2188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Wong J, Koch AL, Deneve JL, Fulp W, Tanvetyanon T, Dessureault S. Repeat cytoreductive surgery and heated intraperitoneal chemotherapy may offer survival benefit for intraperitoneal mesothelioma: a single institution experience. Ann Surg Oncol. 2014;21:1480-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Baratti D, Kusamura S, Cabras AD, Deraco M. Cytoreductive surgery with selective versus complete parietal peritonectomy followed by hyperthermic intraperitoneal chemotherapy in patients with diffuse malignant peritoneal mesothelioma: a controlled study. Ann Surg Oncol. 2012;19:1416-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 54. | Kluger MD, Taub RN, Hesdorffer M, Jin Z, Chabot JA. Two-stage operative cytoreduction and intraperitoneal chemotherapy for diffuse malignant peritoneal mesothelioma: Operative morbidity and mortality in phase I and II trials. Eur J Surg Oncol. 2010;36:997-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Hesdorffer ME, Chabot JA, Keohan ML, Fountain K, Talbot S, Gabay M, Valentin C, Lee SM, Taub RN. Combined resection, intraperitoneal chemotherapy, and whole abdominal radiation for the treatment of malignant peritoneal mesothelioma. Am J Clin Oncol. 2008;31:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Piccigallo E, Jeffers LJ, Reddy KR, Caldironi MW, Parenti A, Schiff ER. Malignant peritoneal mesothelioma. A clinical and laparoscopic study of ten cases. Dig Dis Sci. 1988;33:633-639. [PubMed] |
| 57. | Laterza B, Kusamura S, Baratti D, Oliva GD, Deraco M. Role of explorative laparoscopy to evaluate optimal candidates for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal mesothelioma. In Vivo. 2009;23:187-190. [PubMed] |
| 58. | Pillai K, Pourgholami MH, Chua TC, Morris DL. Oestrogen receptors are prognostic factors in malignant peritoneal mesothelioma. J Cancer Res Clin Oncol. 2013;139:987-994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Schaub NP, Alimchandani M, Quezado M, Kalina P, Eberhardt JS, Hughes MS, Beresnev T, Hassan R, Bartlett DL, Libutti SK, Pingpank JF, Royal RE, Kammula US, Pandalai P, Phan GQ, Stojadinovic A, Rudloff U, Alexander HR, Avital I. A novel nomogram for peritoneal mesothelioma predicts survival. Ann Surg Oncol. 2013;20:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 60. | Elias D, Bedard V, Bouzid T, Duvillard P, Kohneh-Sharhi N, Raynard B, Goere D. Malignant peritoneal mesothelioma: treatment with maximal cytoreductive surgery plus intraperitoneal chemotherapy. Gastroenterol Clin Biol. 2007;31:784-788. [PubMed] |
| 61. | Costamagna D, Scuderi S, Vaira M, Barone R, De Simone M. [Treatment of peritoneal mesothelioma using cytoreduction and intraperitoneal hyperthermic chemotherapy]. Tumori. 2003;89:40-42. [PubMed] |
| 62. | Yano H, Moran BJ, Cecil TD, Murphy EM. Cytoreductive surgery and intraperitoneal chemotherapy for peritoneal mesothelioma. Eur J Surg Oncol. 2009;35:980-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Foster JM, Radhakrishna U, Govindarajan V, Carreau JH, Gatalica Z, Sharma P, Nath SK, Loggie BW. Clinical implications of novel activating EGFR mutations in malignant peritoneal mesothelioma. World J Surg Oncol. 2010;8:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Kalra N, Ashai A, Xi L, Zhang J, Avital I, Raffeld M, Hassan R. Patients with peritoneal mesothelioma lack epidermal growth factor receptor tyrosine kinase mutations that would make them sensitive to tyrosine kinase inhibitors. Oncol Rep. 2012;27:1794-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 65. | Varghese S, Chen Z, Bartlett DL, Pingpank JF, Libutti SK, Steinberg SM, Wunderlich J, Alexander HR. Activation of the phosphoinositide-3-kinase and mammalian target of rapamycin signaling pathways are associated with shortened survival in patients with malignant peritoneal mesothelioma. Cancer. 2011;117:361-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 66. | Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer. 2008;44:46-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 261] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 67. | Kreitman RJ, Hassan R, Fitzgerald DJ, Pastan I. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res. 2009;15:5274-5279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 68. | Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC, Pastan I. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144-5149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 311] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 69. | Hassan R, Miller AC, Sharon E, Thomas A, Reynolds JC, Ling A, Kreitman RJ, Miettinen MM, Steinberg SM, Fowler DH. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med. 2013;5:208ra147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 70. | Kim H, Gao W, Ho M. Novel immunocytokine IL12-SS1 (Fv) inhibits mesothelioma tumor growth in nude mice. PLoS One. 2013;8:e81919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Pillai K, Pourgholami MH, Chua TC, Morris DL. MUC1 has prognostic significance in malignant peritoneal mesothelioma. Int J Biol Markers. 2013;28:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 72. | Pillai K, Akhter J, Chua TC, Morris DL. Anticancer property of bromelain with therapeutic potential in malignant peritoneal mesothelioma. Cancer Invest. 2013;31:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 73. | Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 661] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 74. | Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 577] [Cited by in RCA: 572] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 75. | Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 637] [Cited by in RCA: 623] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 76. | Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254-1257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 817] [Cited by in RCA: 810] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 77. | Takabe K, Kim RH, Allegood JC, Mitra P, Ramachandran S, Nagahashi M, Harikumar KB, Hait NC, Milstien S, Spiegel S. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J Biol Chem. 2010;285:10477-10486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 78. | Nagahashi M, Kim EY, Yamada A, Ramachandran S, Allegood JC, Hait NC, Maceyka M, Milstien S, Takabe K, Spiegel S. Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J. 2013;27:1001-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 79. | Kim RH, Takabe K, Milstien S, Spiegel S. Export and functions of sphingosine-1-phosphate. Biochim Biophys Acta. 2009;1791:692-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 80. | Shida D, Takabe K, Kapitonov D, Milstien S, Spiegel S. Targeting SphK1 as a new strategy against cancer. Curr Drug Targets. 2008;9:662-673. [PubMed] |
| 81. | Takabe K, Yamada A, Rashid OM, Adams BJ, Huang WC, Aoyagi T, Nagahashi M. Twofer anti-vascular therapy targeting sphingosine-1-phosphate for breast cancer. Gland Surg. 2012;1:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 82. | Nagahashi M, Ramachandran S, Rashid OM, Takabe K. Lymphangiogenesis: a new player in cancer progression. World J Gastroenterol. 2010;16:4003-4012. [PubMed] |
| 83. | Aoyagi T, Nagahashi M, Yamada A, Takabe K. The role of sphingosine-1-phosphate in breast cancer tumor-induced lymphangiogenesis. Lymphat Res Biol. 2012;10:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 84. | Nagahashi M, Ramachandran S, Kim EY, Allegood JC, Rashid OM, Yamada A, Zhao R, Milstien S, Zhou H, Spiegel S. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 2012;72:726-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 261] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 85. | Liang J, Nagahashi M, Kim EY, Harikumar KB, Yamada A, Huang WC, Hait NC, Allegood JC, Price MM, Avni D. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell. 2013;23:107-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 472] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 86. | Nagahashi M, Hait NC, Maceyka M, Avni D, Takabe K, Milstien S, Spiegel S. Sphingosine-1-phosphate in chronic intestinal inflammation and cancer. Adv Biol Regul. 2014;54:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 87. | Huang WC, Nagahashi M, Terracina KP, Takabe K. Emerging Role of Sphingosine-1-phosphate in Inflammation, Cancer, and Lymphangiogenesis. Biomolecules. 2013;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 88. | Kalari S, Moolky N, Pendyala S, Berdyshev EV, Rolle C, Kanteti R, Kanteti A, Ma W, He D, Husain AN. Sphingosine kinase 1 is required for mesothelioma cell proliferation: role of histone acetylation. PLoS One. 2012;7:e45330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 89. | Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, Stone V, Brown S, Macnee W, Donaldson K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1975] [Cited by in RCA: 1443] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 90. | Selvam SP, Ogretmen B. Sphingosine kinase/sphingosine 1-phosphate signaling in cancer therapeutics and drug resistance. Handb Exp Pharmacol. 2013;3-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 91. | Chun J, Brinkmann V. A mechanistically novel, first oral therapy for multiple sclerosis: the development of fingolimod (FTY720, Gilenya). Discov Med. 2011;12:213-228. [PubMed] |
| 92. | Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, Hajdu R, Rosenbach M, Keohane CA, Mandala S. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487-52492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 380] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 93. | Kharel Y, Lee S, Snyder AH, Sheasley-O’neill SL, Morris MA, Setiady Y, Zhu R, Zigler MA, Burcin TL, Ley K. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J Biol Chem. 2005;280:36865-36872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 175] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 94. | Zemann B, Kinzel B, Müller M, Reuschel R, Mechtcheriakova D, Urtz N, Bornancin F, Baumruker T, Billich A. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood. 2006;107:1454-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 221] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 95. | Gräler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 2004;18:551-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 443] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 96. | Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004;4:1019-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 389] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 97. | Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1913] [Cited by in RCA: 2055] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 98. | Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 670] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 99. | Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453-21457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1194] [Cited by in RCA: 1232] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 100. | Pitman MR, Woodcock JM, Lopez AF, Pitson SM. Molecular targets of FTY720 (fingolimod). Curr Mol Med. 2012;12:1207-1219. [PubMed] |
| 101. | Zhang L, Wang HD, Ji XJ, Cong ZX, Zhu JH, Zhou Y. FTY720 for cancer therapy (Review). Oncol Rep. 2013;30:2571-2578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 102. | Lim KG, Tonelli F, Li Z, Lu X, Bittman R, Pyne S, Pyne NJ. FTY720 analogues as sphingosine kinase 1 inhibitors: enzyme inhibition kinetics, allosterism, proteasomal degradation, and actin rearrangement in MCF-7 breast cancer cells. J Biol Chem. 2011;286:18633-18640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 103. | Pchejetski D, Bohler T, Brizuela L, Sauer L, Doumerc N, Golzio M, Salunkhe V, Teissié J, Malavaud B, Waxman J. FTY720 (fingolimod) sensitizes prostate cancer cells to radiotherapy by inhibition of sphingosine kinase-1. Cancer Res. 2010;70:8651-8661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 104. | Tonelli F, Lim KG, Loveridge C, Long J, Pitson SM, Tigyi G, Bittman R, Pyne S, Pyne NJ. FTY720 and (S)-FTY720 vinylphosphonate inhibit sphingosine kinase 1 and promote its proteasomal degradation in human pulmonary artery smooth muscle, breast cancer and androgen-independent prostate cancer cells. Cell Signal. 2010;22:1536-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 105. | Oaks JJ, Santhanam R, Walker CJ, Roof S, Harb JG, Ferenchak G, Eisfeld AK, Van Brocklyn JR, Briesewitz R, Saddoughi SA. Antagonistic activities of the immunomodulator and PP2A-activating drug FTY720 (Fingolimod, Gilenya) in Jak2-driven hematologic malignancies. Blood. 2013;122:1923-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 106. | Saddoughi SA, Gencer S, Peterson YK, Ward KE, Mukhopadhyay A, Oaks J, Bielawski J, Szulc ZM, Thomas RJ, Selvam SP. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO Mol Med. 2013;5:105-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 107. | Liu Q, Zhao X, Frissora F, Ma Y, Santhanam R, Jarjoura D, Lehman A, Perrotti D, Chen CS, Dalton JT. FTY720 demonstrates promising preclinical activity for chronic lymphocytic leukemia and lymphoblastic leukemia/lymphoma. Blood. 2008;111:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 108. | Yang Y, Huang Q, Lu Y, Li X, Huang S. Reactivating PP2A by FTY720 as a novel therapy for AML with C-KIT tyrosine kinase domain mutation. J Cell Biochem. 2012;113:1314-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 109. | Ushitora Y, Tashiro H, Ogawa T, Tanimoto Y, Kuroda S, Kobayashi T, Miyata Y, Itamoto T, Asahara T, Ohdan H. Suppression of hepatocellular carcinoma recurrence after rat liver transplantation by FTY720, a sphingosine-1-phosphate analog. Transplantation. 2009;88:980-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 110. | Mousseau Y, Mollard S, Faucher-Durand K, Richard L, Nizou A, Cook-Moreau J, Baaj Y, Qiu H, Plainard X, Fourcade L. Fingolimod potentiates the effects of sunitinib malate in a rat breast cancer model. Breast Cancer Res Treat. 2012;134:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 111. | Alinari L, Mahoney E, Patton J, Zhang X, Huynh L, Earl CT, Mani R, Mao Y, Yu B, Quinion C. FTY720 increases CD74 expression and sensitizes mantle cell lymphoma cells to milatuzumab-mediated cell death. Blood. 2011;118:6893-6903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 112. | Loggie BW, Fleming RA, McQuellon RP, Russell GB, Geisinger KR, Levine EA. Prospective trial for the treatment of malignant peritoneal mesothelioma. Am Surg. 2001;67:999-1003. [PubMed] |
| 113. | Gómez Portilla A, Cendoya I, Muriel J, Olabarria I, Guede N, Moraza N, Fernández E, Martínez de Lecea C, Magrach L, Martín E. [Malignant peritoneal mesothelioma. Our experienced with triple combined therapy: cytoreduction, intraperitoneal perioperative chemotherapy and hyperthermia]. Cir Esp. 2007;81:82-86. [PubMed] |
| 114. | Passot G, Cotte E, Brigand C, Beaujard AC, Isaac S, Gilly FN, Glehen O. [Peritoneal mesothelioma: treatment with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy]. J Chir (Paris). 2008;145:447-453. [PubMed] |
| 115. | Chua TC, Yan TD, Morris DL. Outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal mesothelioma: the Australian experience. J Surg Oncol. 2009;99:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 116. | Baratti D, Kusamura S, Sironi A, Cabras A, Fumagalli L, Laterza B, Deraco M. Multicystic peritoneal mesothelioma treated by surgical cytoreduction and hyperthermic intra-peritoneal chemotherapy (HIPEC). In Vivo. 2008;22:153-157. [PubMed] |
| 117. | Baratti D, Vaira M, Kusamura S, D’Amico S, Balestra MR, Cioppa T, Mingrone E, De Simone M, Deraco M. Multicystic peritoneal mesothelioma: outcomes and patho-biological features in a multi-institutional series treated by cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Eur J Surg Oncol. 2010;36:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |