Published online Sep 7, 2014. doi: 10.3748/wjg.v20.i33.11618
Revised: February 28, 2014
Accepted: April 15, 2014
Published online: September 7, 2014
Processing time: 306 Days and 22.7 Hours
Hepatitis B virus (HBV) infection is one of the major causes of liver diseases, affecting more than 350 million people worldwide. The interferon (IFN)-mediated innate immune responses could restrict HBV replication at the different steps of viral life cycle. Indeed, IFN-α has been successfully used for treatment of patients with chronic hepatitis B. However, the role of the innate immune response in HBV replication and the mechanism of the anti-HBV effect of IFN-α are not completely explored. In this review, we summarized the currently available knowledge about the IFN-mediated anti-HBV effect in the HBV life cycle and the possible effectors downstream the IFN signaling pathway. The antiviral effect of Toll-like receptors (TLRs) in HBV replication is briefly discussed. The strategies exploited by HBV to evade the IFN- and TLR-mediated antiviral actions are summarized.
Core tip: Hepatitis B virus (HBV) infection is one of the major causes of liver diseases affecting more than 350 million people worldwide. Interferon (IFN)- and Toll-like receptors (TLR)-mediated innate immune responses could restrict HBV replication at the different steps of viral life cycle. Though a great number of publications in this field appeared during the last years, there is no review to discuss the progress. Here, we summarized the currently available knowledge about the anti-HBV effect of IFNs and TLRs and the possible effectors downstream the IFN signaling pathway. This review provides an overview for scientists working on HBV and related fields.
- Citation: Pei RJ, Chen XW, Lu MJ. Control of hepatitis B virus replication by interferons and Toll-like receptor signaling pathways. World J Gastroenterol 2014; 20(33): 11618-11629
- URL: https://www.wjgnet.com/1007-9327/full/v20/i33/11618.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i33.11618
The hepatitis B virus (HBV) is a member of the Hepadnaviridae family and an enveloped virus with a partially double stranded DNA genome[1]. Primary HBV infection in about 90% of infants infected at birth, 20%-50% of children, and 5% of adults will progress to chronic course of infection[2]. Chronic HBV infection is a global public health problem, affecting more than 350 million people worldwide[3]. The risk of developing severe liver diseases such as cirrhosis and hepatocellular carcinoma is increased in patients with chronic hepatitis B[3]. Interferons (IFNs) and nucleoside analogues are the commonly used drugs for anti-HBV treatment.
The innate immunity is the first line of active host defense against viral infection. The recognition of viral pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs), RIG-I like receptors (RLRs), NOD-like receptors (NLRs), and others triggers signals to activate intracellular pathways and leads to the production of antiviral and immune regulatory effector molecules. However, HBV was believed to be a stealth virus since it did not trigger or only triggered a limited innate response in infected Chimpanzees and patients during the acute phase of infection[4-6]. The HBV-specific T cell response is thought to be essential for the control of HBV infection. Besides the classical way to remove infected hepatocytes by cytolytic mechanisms, HBV may also be cleared via noncytopathic mechanisms involving antiviral cytokines such as IFN-γ and TNF-α[7-9]. Though HBV does not trigger type I IFN production in hepatocytes and in the infected liver, IFN-α and -β are shown to be able to suppress HBV replication in vitro and in HBV transgenic mouse models. Recombinant IFN-α has been approved and successfully used as a standard treatment for chronic HBV infection. Here, we summarize the available data about IFN-mediated anti-HBV actions and the control of HBV by TLR activation.
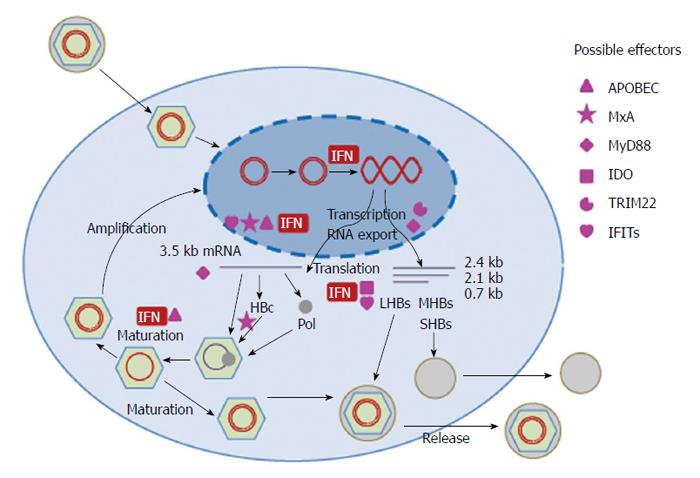
The full HBV infection cycle could only be studied in primary human and Tupaia hepatocyte cultures before the recent discovery of the HBV receptor[10]. The viral entry is supposed to be a receptor mediated endocytosis process or a membrane fusion process[11]. Two elements of the envelop protein are required for HBV infection, the receptor binding site located within the N-terminal pre-S1 domain and the infectivity determinant in the surface-exposed antigenic loop[12-14]. Recently, sodium taurocholate cotransporting polypeptide (NTCP) was identified as a functional receptor mediating HBV entry[15]. After the entry, the endosomal vesicles play an important role in uncoating and release of viral capsids containing HBV genome. The transport of viral particles to the late endosomes is required in the early step of HBV infection[16]. After uncoating, HBV capsid is actively transported to the nucleus in a microtubule-dependent manner[17]. It is proposed that HBV capsid is disassembled in the nuclear basket to release HBV genome into the nucleus[18,19]. In the nucleus, HBV genome, the partially double-stranded viral relaxed circular DNA (rcDNA), is repaired by both viral and cellular enzymes and converted to covalently closed circular DNA (cccDNA). The cccDNA is in complex with cellular proteins like histones and other regulatory proteins and organized as a viral minichromosome[20]. The episomal cccDNA then serves as template for viral transcripts, including the 3.5 kb (pregenomic (pg) RNA), 2.4 kb, 2.1 kb, and 0.7 kb mRNAs. The transcription is under the control of four promoters (the core, pre-S1, pre-S2/S, and X promoters) and two enhancers (EnI and EnII). The HBV mRNAs encode different proteins: the 3.5 kb mRNA for HBV polymerase (pol), HBcAg and HBeAg, the 2.4 kb and 2.1 kb mRNAs for the large, middle, and small surface proteins (L, M and S HBsAg), and the 0.7 kb mRNA for HBx protein[21]. HBcAg encapsidates the pgRNA and pol protein and forms the immature nulceocapsid. Within the capsid, the pgRNA is first reverse transcribed to minus strand DNA; meanwhile, the pgRNA is degraded by a ribonuclease H which is a domain of HBV pol protein. The plus strand of HBV DNA is only partly synthesized using the minus strand as the template to form the relaxed circle DNA form of HBV genome. The mature HBV capsids may be either recycled to the nucleus for amplification of the cccDNA pool or be enveloped in HBsAg to form infectious virions and released from hepatocytes[22,23].
IFNs act through binding to the cellular receptors and are able to induce a great numbers of genes termed as IFN-stimulated genes (ISGs)[24]. The ISGs have diverse functions and could inhibit HBV replication at different steps as discussed in detail below. Due to the lack of efficient in vitro infection system for HBV, the antiviral effect of IFNs on the entry and uncoating steps of HBV life cycle has not been studied so far. IFN-mediated antiviral functions against HBV were mainly examined in human hepatoma cells transiently or stably transfected with replication-competent HBV genomes and HBV transgenic mouse models. In addition, IFNs have immunomodulatory functions which will not be discussed in the present review[25,26] (Figure 1 and Table 1).
| cccDNA minichromosome | Promoter and enhancer | Posttranscriptional control | Nucleocapsid formation | Others | |
| IFNs | Modifying the composition of HBV minichromosome[27,29] Suppressing cccDNA transcription[27,29] accelerating cccDNA decay[29] | Suppressing HBV EnI and EnII activity[35-37] | Stimulating HBV RNA degradation[42,43] | Inhibiting nucleocapsid formation[48] Accelerating nucleocapsid degradation[49] | |
| APOBEC | Blocking nucleocapsid maturation[65] | Suppressing HBV S promoter activity[57] | Editing HBV genome[70] | ||
| MxA | Inhibiting the nuclear export of HBV RNAs[77] | Blocking HBV nucleocapsid formation[78] | |||
| MyD88 | Accelerating the decay of HBV pgRNA[83] Inhibiting the nuclear export of the HBV pre-S/S RNAs[83] | ||||
| IDO | Inhibiting translation through tryptophan depletion[88] | ||||
| TRIM22 | Inhibiting HBV core promoter activity[92] | ||||
| IFITs | Inhibiting HBV S promoter activity[96] | Inhibiting HBV replication at posttranscriptional steps[96] |
HBV cccDNA can persist in the liver of infected patients and serves as the template to initiate HBV replication. Thus, cccDNA is an important factor for HBV persistence and the recurrence of HBV infection in resolved patients. To study the role of IFN-α in HBV cccDNA transcription, Belloni et al[27] utilized a transfection system using the monomeric linear full length HBV genome to show that IFN-α suppresses HBV cccDNA transcription. The HBV interferon-sensitive response element (ISRE) segment mediates STAT1 and STAT2 recruitment on the cccDNA and transcriptional repression by IFN-α. The recruitment of HDAC2, Sirt1, Ezh2, and YY1 to HBV cccDNA was increased by IFN-α treatment, indicating the epigenetic control of HBV cccDNA minichromosome. However, future studies are needed to determine whether and how IFN-α influences the stability of HBV cccDNA and thereby controls HBV replication, as indicated in the clinical studies[28]. The acetylation and methylation status of HBV bound histones upon IFN-α treatment needs to be further analyzed. More details about the control of HBV minichromosome by IFNs will be uncovered once the compositions of the HBV cccDNA minichromosome are better defined.
In a recently published study, the antiviral functions of IFN-α on duck HBV (DHBV) cccDNA were analyzed in detail[29]. Base on a chicken hepatoma cell line with tet-inducible DHBV replication, experimental conditions were established where cccDNA was the sole source for pgRNA transcription and DNA replication. Based on this cell culture system, Guo et al[30] demonstrated that IFN-α suppresses cccDNA transcription, which is associated with the reduction of acetylated histone H3 lysines 9 (H3K9) and 27 (H3K27) in cccDNA minichromosome. Their experimental data suggest that IFN-α may induce accelerated cccDNA decay. Although the DHBV cccDNA metabolism and transcription regulation may differ from that of mammalian hepadnaviruses, the study on DHBV cccDNA still provided important clues for HBV cccDNA biology and the antiviral mechanism of IFN-α. Nonetheless, the stability of HBV cccDNA is different in vivo and in vitro, as hepadnaviral cccDNA decayed within days in cell culture while probably significantly longer in the liver[30-33]. Thus, the mechanism regulating the cccDNA stability should be examined in an in vivo model in the future.
Two enhancer elements En I and En II were identified in the HBV genome and were found to be critical for HBV gene expression and replication[34]. By transient transfection of human hepatoma cells with reporter plasmids under the control of HBV regulatory sequences, several groups have confirmed that type I IFN reduces the activity of En I and En II[35,36]. Nakao et al[37] identified an ISRE-like sequence in the En I region and experimentally confirmed that this region could interact with the protein complex containing p48 (ISGF-3γ) and mediate the suppression of En I activity by IFN-α. However, the antiviral effect of both IFN-α and p48 was similar in the complete HBV genomes with the wild-type or a mutated ISRE sequence, indicating that the ISRE within the En I region is not required for the antiviral effect of IFN-α[38,39]. Despite the lack of functions of the ISRE in the En I region in the cell culture, it is not excluded that the interaction between ISGF3 and IFN responsive factors with the ISRE in En I could modulate HBV replication in vivo. By deletion/mutation analysis, two segments nt 1703-1727 and 1746-1770 within the En II sequence were identified to be responsible for the suppressive effects of IFN-α[36]. Evidence shows that IFN-α suppresses the EN II activity in a PKC-dependent way. However, the exact function of these segments in the context of replication-competent HBV genomes remains to be uncovered.
A large body of publications indicated that IFNs control the HBV replication at the posttranscriptional level. Chisari’s group demonstrated that the secretion of IFN-γ and TNF-α by cytotoxic T lymphocytes (CTLs) could inhibit HBV replication by a posttranscriptional mechanism[8,40]. This could explain the noncytopathic inhibition of HBV replication by CTLs and the reduction of HBV replication in HBV transgenic mice during lymphocytic choriomeningitis virus (LCMV) and murine cytomegalovirus (MCMV) infections[7,41]. Three cellular proteins (p45, p39, and p26) were identified to bind HBV RNAs in association with IFN-γ and TNF-α-induced down-regulation of HBV RNAs[42]. These three proteins were the full length or cleaved products of La protein, a well-described RNA-binding protein, whose membrane expression could be induced by IFN-γ and TNF-α. La protein could bind to the segment nt 1275-1291 of HBV RNA sequence and may contribute to HBV RNA stability[43]. The association between La protein and HBV RNA was further demonstrated by co-precipitation of HBV RNA with human La protein in human hepatoma cells. The modulation of HBV RNA stability by La protein was implied as the half life time of HBV RNA with mutations in the La binding site was reduced[44]. These findings provided evidence for the posttranscriptional control of HBV by IFN-γ and TNF-α through the disruption of La protein that acts as an HBV RNA stabilizing factor. More factors that either stabilize or degrade HBV RNA might also be involved in this process. In fact, the interaction between La protein and HBV RNA is likely modulated by accessory factors in a phosphorylation-dependent manner[44]. Furthermore, the endonucleolytic activity that cleaves HBV RNA near the La protein binding site was upregulated in the liver by CTL injection or MCMV infection.
The HBV replication in the liver of HBV transgenic mice could be abolished by poly(I:C) injection in an IFN-dependent manner[45]. Further analysis showed that the pgRNA containing capsid in the liver tissue of HBV transgenic mice was eliminated by poly(I:C) injection, either by inhibition of the assembly or acceleration of the degradation[46]. A similar conclusion was reached by experiments with immortalized HBV-transgenic hepatocytes in vitro[47]. Later on, IFNs were shown to be responsible to reduced HBV pgRNA containing capsids by the inhibition of HBV capsid assembly[48]. The inhibition of the capsid formation by IFNs might occur at one or several steps in the process of the virion assembly, such as the core protein dimerization, the interaction between polymerase and HBV pgRNA, the encapsidation of polymerase-pgRNA, and the icosahedral capsid formation.
Another report showed that HBV DNA containing capsid was affected by IFN treatment. Xu et al[49] explored a cell system derived from immortalized mouse hepatocyte (AML12) with tetracycline (Tet)-inducible transcription of HBV pgRNA and viral DNA replication to examine the antiviral effect of IFN-α and -γ. Compared with Bay-4109 and AT-61, two drugs inhibiting HBV capsid assembly or preventing the incorporation of pgRNA into nucleocapsids, IFN-α and -γ accelerate the decay of HBV DNA containing nucleocapsids in a proteasome-dependent manner. However, the influence of IFNs on the formation of HBV pgRNA containing capsids as described above was not confirmed. This discrepancy probably caused by the different experimental systems and strategies used by the two groups to determine the influence of IFNs on HBV pgRNA containing capsids. Xu et al[49] treated cells with IFN-α and -γ after the establishment of HBV replication, while Wieland et al[48] treated cells with IFN-β and -γ prior to the initiation of HBV replication. However, IFNs may interfere with the different steps of HBV capsid formation by activation of the downstream effectors. The ISGs like APOBEC3G and MxA may be involved in the inhibition of HBV nucleocapsid formation as discussed below.
Upon binding to the receptors, IFNs activate a variety of IFN-inducible genes through the Janus tyrosine kinase-STAT (JAK-STAT) pathway, which mediate the antiviral effects against HBV[50,51]. Some ISGs are able to trigger common intracellular antiviral pathways. However, the absence of three major antiviral factors IRF1, PKR, and RNase L did not impair the anti-HBV effect of IFN-α/β or -γ[52]. In contrast, the cellular proteasome activity was required for IFN-mediated inhibition of HBV replication[53]. The inducible nitric oxide synthase was also required for the anti-HBV effect of IFN-γ in HBV transgenic mice[54]. To identify the genes linked to the anti-HBV effect of IFN-α/β and -γ, hepatic gene expression profiles were compared in HBV transgenic mice before and after the IFN treatment. Twenty-nine genes were identified and supposed to be associated with the IFN-induced inhibition of HBV replication[55]. However, the role of these identified ISGs is still not fully understood.
Other studies focused on ISGs which were reported to possess antiviral activities. We summarize the available information about those ISGs published so far.
Apolipoprotein B mRNA editing enzyme, catalytic polypeptide (APOBEC) is a family of cytidine deaminases that edit DNA and/or RNA sequences by deaminating a cytidine base and thereby generating a uridine base. At least 11 members of this family were found in humans, including activation-induced cytidine deaminase (AID) and APOBECs 1, 2, 3A, 3B, 3C, 3DE, 3F, 3G, 3H, and 4. APOBECs are IFN-inducible and play an important role in the innate immune response against many viruses. The antiviral activity of the APOBEC family was first found in HIV infection, and then extended to other viruses including HTLV, HCV, HBV, HPV, HSV-1, and EBV (reviewed in[56]). The control of HBV by members of the APOBEC family, such as AID, APOBEC 1, 3B, 3C, 3F, 3G, and 3H[57-62], could be editing-dependent and independent[63]. In the first report extending the antiviral spectrum of APOBEC3G (A3G) to HBV, A3G was supposed to block HBV DNA accumulation in a way independent on its catalytic activity[64]. Nguyen et al[65] further confirmed the deamination-independent inhibition of HBV DNA synthesis by A3G and provided evidence that A3G inhibits the very early steps in viral reverse transcription and blocks DNA strand elongation within the nucleocapsid. Thus, A3G is thought to be incorporated into HBV nucleocapsids. It could be demonstrated that the incorporation of A3G into replication-competent HBV nucleocapsids is specifically dependent on both the viral RT and the packaging signal ε[66]. Thus, A3G likely exerts its anti-HBV activity within HBV nucleocapsids and blocks HBV DNA synthesis and the maturation of viral particles. Another member of the family, APOBEC3B (A3B), was reported to inhibit the binding of hnRNP K (ribonucleoprotein K), a positive regulator of HBV gene expression, to the EnhII and to directly suppress HBV-S gene promoter activity[57]. Though the deaminase activity of APOBECs may not represent the main mode of the anti-HBV action since the frequency of edited genomes in cell culture supernatants or patient sera is low[64,67-69], it is unknown whether the majority of edited HBV genomes are able to be secreted. Recent data suggested APOBEC3G (A3G) as the dominant deaminase restricting HBV in vivo with up to 35% of HBV genomes being edited[70]. All APOBEC family members except A3DE were able to edit the HBV genome in vitro at levels from 10-2 to10-5 based on the assessment by the 3DPCR technology[71], implying the potential significance of the deaminase activity of the APOBEC family in HBV restriction.
Most of the mutations in vivo caused by A3G editing were deleterious, however, a small fraction of genomes could survive and might promote viral evolution and eventually viral evasion of host immune responses. A3G editing might be responsible for the G1896A and G1897A mutations in the HBV precore region which result in the loss of HBsAg synthesis, and for the HBsAg mutations G145E and G145R which are the well-known vaccine escape mutations[70]. It was also suggested that a subset of A3G-edited genomes can be repaired in vivo. Kitamura et al[72] pointed out that uracil DNA glycosylase could initiate base excision repair of cccDNA and counteract A3G-induced hypermutation of HBV genomes. Though the APOBEC family plays a role in the control of HBV replication, its relative contribution in the IFN-mediated anti-HBV action is still a subject of debate[61,73,74].
MxA is an interferon-induced dynamin-like GTPase, which has antiviral activity against a wide range of RNA viruses and some DNA viruses including HBV. Though the interaction with viral nucleoprotein is the most likely common mechanism of the antiviral function of MxA[75], its anti-HBV effect might be achieved through more than one pathway. The anti-HBV effect of MxA was firstly demonstrated by Dremsdorf’s group by using a Huh7 cell line stably expressing MxA and HBV/MxA transgenic female mice lacking a functional IFN-α/β receptor[76,77]. The MxA expression led to the inhibition of HBV replication and gene expression. MxA inhibits the nuclear export of viral RNAs through the HBV PRE sequence in Huh7 cells but not in the transgenic mouse model, indicating that MxA exerts the antiviral effect through different mechanisms in vitro and in vivo[76,77]. The anti-HBV effect of MxA was also verified in HepG2.2.15 cells[78]. Using coimmunoprecipitation and the fluorescence resonance energy transfer (FRET) technique, the authors provide evidence for the direct interaction between MxA and HBcAg. The interaction with MxA causes the immobilization of HBcAg in the perinuclear structures and subsequently the loss of capsid assembly[78]. Thus, MxA is another candidate molecule blocking HBV nucleocapsid formation.
On the other side, HBV developed specific mechanisms to counteract the antiviral activity of MxA. The induction of MxA by IFN-α was impaired in PBMCs isolated from chronic hepatitis B patients, compared with healthy donors[79]. Rosmorduc et al[80] showed that HBcAg inhibits the induction of MxA expression. Further investigation confirmed these results and identified the interaction of precore/core proteins with MxA promoter, which was responsible for the downregulation of IFN-induced MxA expression[81].
Myeloid differentiation primary response protein 88 (MyD88) is an important molecule in the signaling cascade of the innate immune response mediated by Toll-like receptors (TLRs). Besides, it could be induced by IFNs and is involved in the anti-HBV effect of IFNs. HBV replication is reduced in MyD88-expressing cells and MyD88 overexpression inhibits HBV replication in hepatoma cells HepG2.2.15 and the mouse model[82,83]. MyD88 accelerates the decay of HBV pgRNA and inhibits the nuclear export of the HBV pre-S/S RNAs via the posttranscriptional regulatory element[83]. Though the inhibition of HBV replication by MyD88 is evident, the induction of MyD88 upon IFN stimulation is only moderate and not comparable with other ISGs such as MxA. Therefore, its role in the IFN-mediated anti-HBV effect might be limited. On the other side, HBV polymerase blocks the IFN-induced MyD88 expression by preventing nuclear translocation of Stat1 and thereby reducing the activity of the MyD88 promoter[84].
Indoleamine 2,3-dioxygenase (IDO) is an essential enzyme for tryptophan catabolism, which could cause the tryptophan depletion and suppress adaptive immune response[85]. IDO expression was increased in hepatocytes of HBV transgenic mice after adoptive transfer of HBV-specific CTLs, indicating that HBV infection facilitates the induction of IDO in response to proinflammatory cytokines, particularly IFN-γ[86]. Furthermore, IDO was reported to play a role in the immune tolerance in patients with chronic hepatitis B[87]. Recently, Mao et al[88] examined the role of IDO in HBV replication and found that IDO overexpression in HepG2 cells could suppress HBV replication through tryptophan depletion and is likely to be a major factor mediating the anti-HBV activity of IFN-γ since supplementation of tryptophan restores HBV replication inhibited by IFN-γ. Since the conclusion was drawn on the basis of experiments in HepG2 cells, experimental data from in vivo models are needed to confirm the role of IDO in IFN-γ mediated anti-HBV effect.
TRIM22 belongs to the tripartite motif (TRIM) family, is inducible by IFNs and has been reported to possess anti-viral activity against HIV, encephalomyocarditis virus (EMCV), HBV, and influenza A virus (IAV)[89-92]. The antiviral effect of TRIM22 had been demonstrated in HepG2 cells as well as in the mouse model and was supposed to inhibit HBV replication through the inhibition of HBV core promoter activity[92]. Previously, Mao et al[88] did not observe anti-HBV effect of TRIM22 during the screening of ISGs for their ability to inhibit HBV replication. The discrepancy between these studies is currently not known and needs further clarification.
The IFN-induced proteins with tetratricopeptide repeats 1 and 2 (IFIT1 and IFIT2) are related genes which can be strongly induced by type I IFN. IFIT1 and IFIT2 suppress cellular translation by binding to the translation initiator eIF3 subunits[93,94] and were shown to block viral replication by the sequestration of ppp-RNA (5’ triphosphate RNA)[95]. IFIT1 and IFIT2 were identified in a siRNA screening approach to block HBV replication. It was supposed that the baseline expression of IFIT1 and IFIT2 restricts HBV replication at both the transcriptional and posttranscriptional steps[96]. The majority of ISGs with anti-HBV effects were identified by overexpression strategy. We found a drastic enhancing effect of siRNAs targeting IFIT1 and IFIT2 on the HBV replication, though IFIT1 and IFIT2 were only expressed at a relatively low level without IFN-α stimulation. Thus, the baseline expression of ISGs might already contribute to the restriction of HBV replication. This fact probably explains the discrepancy of the anti-HBV effect of the same molecule in different cell lines and in the experiments of different groups. An increasing number of ISGs are identified as restriction factors for HBV replication. However, their relative contribution to the IFN-mediated anti-HBV action remains to be determined. Likely, the anti-HBV function of IFNs is a sum of different ISG functions at different stages of HBV life cycle.
Though HBV itself does only trigger innate immune responses to a limited extent, the activation of TLR signaling inhibits HBV replication and is considered a novel therapeutic strategy for the treatment of chronic HBV infection (reviewed in[97]). The ligands specific for TLR2, TLR3, TLR4, TLR5, TLR7, and TLR9 had been examined for their ability to control HBV replication in HBV transgenic mice[98]. All the ligands except for TLR2 could inhibit HBV replication in the liver of HBV transgenic mice in an IFN-dependent manner. Recently, GS-9620, an agonist of TLR7, was administered in chimpanzees with chronic HBV infection and effectively decreased HBV DNA loads in the serum and liver[99].
The antiviral effect of TLR ligands might be mediated by the activation of TLR signaling either in hepatocytes, nonparenchymal liver cells, or extra-hepatic macrophages and dendritic cells, or by the modulation of the adaptive immune response. In hepatoma cells (HepG2), ligands of TLR2, TLR3, TLR4, TLR7, and TLR9 were reported to suppress HBV replication[100]. In the same line, the overexpression of adaptor protein TRIF and IPS, as well as MyD88 mentioned above, could inhibit HBV replication[98,101]. Other groups also showed that stimulation of human hepatoma cells with TLR2 ligands inhibits the HBV DNA replication and nucleocapsid formation[102,103]. For the role of nonparenchymal liver cells in the anti-HBV effect of the TLR ligands, Wu et al[104] showed that the local innate immune system of the liver such as Kupffer cells (KCs) and sinusoidal endothelial cells (LSECs) is the mediator of anti-HBV activity of TLR agonists. Guo’s group[105] reported inhibition of HBV replication in a murine hepatocyte cell line (AML12HBV10) by conditioned media from a murine macrophage cell line treated with ligands to TLR1/2, TLR3, TLR4, TLR5, TLR7, or TLR9, indicating a potential role of extra-hepatic macrophages in TLR ligand-triggered anti-HBV effects. Lately, we demonstrated that pretreatment of LSECs with TLR1/2 ligand stimulates the maturation of antigen-presenting LSECs and enable them to activate virus-specific CD8+ T cells[106]. TLR ligands do not only activate the innate immune response but are also involved in modulating the adaptive immune response. Besides the innate and adaptive immune responses, TLR stimulation also modulates the expression of various microRNAs (miRNAs)[107,108]. Since miRNAs were shown to control HBV replication either through direct binding or through indirect mechanisms[109-111], the control of HBV replication by TLR ligands might also be partly achieved through miRNAs (Table 2).
| HBV transgenic mice[98] | Chimpazee[99] | Hepatoma cell line[100] | Nonparenchymal liver cells[104] | Macrophages[105] | |
| TLR1/2 | PGN and Pam3Cys, (-) | LTA , Pam2CSK4[102,103], Pam3CSK4[103], (+) | Pam3CSK4, (-) | Unpublished data, (+) | |
| TLR3 | Poly(I:C), (+) | Poly (I:C), (+) | Poly (I:C), (+) | Unpublished data, (+) | |
| TLR4 | LPS, (+) | LPS, (+) | LPS, (+) | Unpublished data, (+) | |
| TLR5 | Flagellin,(+) | Flagellin, (-) | Unpublished data, (+) | ||
| TLR7 | R848, (+) | R848, (+) | Single-stranded RNA 40, (-) | Unpublished data, (+) | |
| TLR9 | CpG oligodeoxynucleotides, (+) | GS-9620, (+) | cpG DNA, (+) | CpG oligonucleotides, (-) | Unpublished data, (+) |
HBV was thought to be a stealth virus; nonetheless, the lack of PRR activation in the liver could be due to the inhibition of PRR and IFN signaling by HBV. Clinical evidence suggests that the expression of TLR2, TLR3, TLR4, TLR7, and TLR9 in PBMCs was reduced in chronically HBV infected patients[112-116]. The reduction of TLR expression might be induced by HBV infection or a result of the exhaustion of TLR system under continuous activation. TLR signaling could be blocked by HBV components through different mechanisms. First, the activation of TLR signaling might be blocked by HBV infection since the activity of TBK1/IKKε, a key molecule downstream of the TLR signaling pathway, was inhibited by HBV pol through disrupting the interaction between IKKε and DDX3[117]. Further, recent findings suggest that the activation of TLR signaling in nonparenchymal liver cells, extra-hepatic macrophages, and dendritic cells is inhibited by HBV. Wu et al[118] found that pretreatment of hepatocytes and nonparenchymal liver cells with HBsAg, HBeAg, or HBV virions significantly reduced the TLR-induced antiviral activity, and this was correlated with the suppressed IRF3, NF-κB and ERL1/2 activation in pretreated cells. The response of pDC to TLR9 ligand was reduced by HBV through the blockage of the MyD88-IRAK4 axis[113], or through the HBsAg-mediated upregulation of SOCS-1 (suppressor of cytokine signaling-1) expression and BDCA-2 (dendritic cell lectin B) ligation[119]. Wang et al[120] reported that HBsAg inhibits TLR2 ligand (Pam3csk4)-induced JNK activation and IL-12 production in monocytes/macrophages.
Above, we discussed the role of ISGs in the control of HBV replication. However, HBV is able to evade the functions of ISGs by counteracting IFN signaling. HBV pol protein was reported to inhibit TBK1/IKKε activity by disrupting the interaction between IKKε and DDX3[117]. The downstream of JAK-STAT signaling pathway activated by IFNs which resulted in the induction of ISGs could be blocked by HBV. As discussed above, HBV pol inhibited STAT1 translocation in HepG2 cells and thus impaired the IFN-induced MyD88 promoter activity[84]. The response of HBV-infected human hepatocytes in chimeric mice was also reduced compared to that of uninfected human hepatocytes, as shown by the reduced STAT-1 nuclear accumulation[121]. Recently, Chen et al[122] explored the mechanism by which HBV impairs IFN-induced STAT1 translocation. They demonstrated that HBV pol suppresses IFN-α–induced STAT1 serine 727 phosphorylation through the interaction with the catalytic domain of protein kinase C-δ (PKC-δ). HBV pol protein interferes also with nuclear transportation of STAT1/2 by binding to importin-α5[122].
HBV genome mutation might be one factor determining the response to IFN treatment. In vitro studies in hepatoma cell lines showed a lower sensitivity of HBV with precore or BCP mutations to IFN-α[123,124]. In a recent clinical investigation[125], 214 HBeAg-positive CHB (Chronic hepatitis B) patients were recruited and treated with PEG-IFN ± lamivudine for 52 wk, and the relationship between the response to IFN treatment and the presence of wild type or non-WT were calculated. It was concluded that the presence of only WT virus at baseline is a strong predictor of response (HBeAg loss with HBV DNA < 10000 copies/mL) to PEG-IFN for HBeAg-positive CHB. Patients with detectable PC (precore) and/or BCP (basal core promoter) mutants have a lower probability of response and are less optimal candidates for PEG-IFN therapy. The same cohort was followed to 78 wk. The relationship between HBsAg levels and response to IFN depends upon the presence of PC/BCP mutations[126]. Besides, the mutation in HBV ISRE was supposed to influence IFN response in CHB patients[127]. On the other hand, HBV variants can be selected during IFN-α therapy[128].
It is well accepted that activation of TLR and the production of IFNs contribute to the control of HBV replication. Due to the complexity of TLR and IFN signaling and functions, the antiviral actions of these pathways are not completely understood. The stimulation of IFN and TLR signaling pathways may be explored as therapeutic approaches for chronic viral infections. Recently, several preclinical studies were presented to show the potential usefulness of TLR ligands (e.g., TLR7) for the treatment of chronic HBV infection. Furthermore, the identification of the effector molecules downstream of IFN and TLR signaling pathways, especially those involved in HBV cccDNA elimination, will provide more options for the development of anti-HBV drugs.
P- Reviewer: Seya T, Villacres MC, Zeromski J S- Editor: Zhai HH L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64:51-68. [PubMed] |
| 2. | McMahon BJ. Epidemiology and natural history of hepatitis B. Semin Liver Dis. 2005;25 Suppl 1:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 289] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 3. | McMahon BJ. The natural history of chronic hepatitis B virus infection. Semin Liver Dis. 2004;24 Suppl 1:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci USA. 2004;101:6669-6674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 550] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 5. | Wieland SF, Chisari FV. Stealth and cunning: hepatitis B and hepatitis C viruses. J Virol. 2005;79:9369-9380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 346] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 6. | Dunn C, Peppa D, Khanna P, Nebbia G, Jones M, Brendish N, Lascar RM, Brown D, Gilson RJ, Tedder RJ. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology. 2009;137:1289-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 280] [Article Influence: 17.5] [Reference Citation Analysis (1)] |
| 7. | Guidotti LG, Ando K, Hobbs MV, Ishikawa T, Runkel L, Schreiber RD, Chisari FV. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc Natl Acad Sci USA. 1994;91:3764-3768. [PubMed] |
| 8. | Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25-36. [PubMed] |
| 9. | Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825-829. [PubMed] |
| 10. | Grimm D, Thimme R, Blum HE. HBV life cycle and novel drug targets. Hepatol Int. 2011;5:644-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Urban S, Schulze A, Dandri M, Petersen J. The replication cycle of hepatitis B virus. J Hepatol. 2010;52:282-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Abou-Jaoudé G, Sureau C. Entry of hepatitis delta virus requires the conserved cysteine residues of the hepatitis B virus envelope protein antigenic loop and is blocked by inhibitors of thiol-disulfide exchange. J Virol. 2007;81:13057-13066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Le Duff Y, Blanchet M, Sureau C. The pre-S1 and antigenic loop infectivity determinants of the hepatitis B virus envelope proteins are functionally independent. J Virol. 2009;83:12443-12451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Sureau C, Salisse J. A conformational heparan sulfate binding site essential to infectivity overlaps with the conserved hepatitis B virus a-determinant. Hepatology. 2013;57:985-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 15. | Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1280] [Cited by in RCA: 1599] [Article Influence: 123.0] [Reference Citation Analysis (1)] |
| 16. | Macovei A, Petrareanu C, Lazar C, Florian P, Branza-Nichita N. Regulation of hepatitis B virus infection by Rab5, Rab7, and the endolysosomal compartment. J Virol. 2013;87:6415-6427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Rabe B, Glebe D, Kann M. Lipid-mediated introduction of hepatitis B virus capsids into nonsusceptible cells allows highly efficient replication and facilitates the study of early infection events. J Virol. 2006;80:5465-5473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Rabe B, Vlachou A, Panté N, Helenius A, Kann M. Nuclear import of hepatitis B virus capsids and release of the viral genome. Proc Natl Acad Sci USA. 2003;100:9849-9854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 216] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Rabe B, Delaleau M, Bischof A, Foss M, Sominskaya I, Pumpens P, Cazenave C, Castroviejo M, Kann M. Nuclear entry of hepatitis B virus capsids involves disintegration to protein dimers followed by nuclear reassociation to capsids. PLoS Pathog. 2009;5:e1000563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Bock CT, Schranz P, Schröder CH, Zentgraf H. Hepatitis B virus genome is organized into nucleosomes in the nucleus of the infected cell. Virus Genes. 1994;8:215-229. [PubMed] |
| 21. | Liang TJ. Hepatitis B: the virus and disease. Hepatology. 2009;49:S13-S21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 747] [Cited by in RCA: 635] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 22. | Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1202] [Cited by in RCA: 1212] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 23. | Lentz TB, Loeb DD. Roles of the envelope proteins in the amplification of covalently closed circular DNA and completion of synthesis of the plus-strand DNA in hepatitis B virus. J Virol. 2011;85:11916-11927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778-809, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2029] [Cited by in RCA: 2003] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 25. | Tompkins WA. Immunomodulation and therapeutic effects of the oral use of interferon-alpha: mechanism of action. J Interferon Cytokine Res. 1999;19:817-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 88] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Rizza P, Moretti F, Belardelli F. Recent advances on the immunomodulatory effects of IFN-alpha: implications for cancer immunotherapy and autoimmunity. Autoimmunity. 2010;43:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Belloni L, Allweiss L, Guerrieri F, Pediconi N, Volz T, Pollicino T, Petersen J, Raimondo G, Dandri M, Levrero M. IFN-α inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J Clin Invest. 2012;122:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 484] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 28. | Sung JJ, Wong ML, Bowden S, Liew CT, Hui AY, Wong VW, Leung NW, Locarnini S, Chan HL. Intrahepatic hepatitis B virus covalently closed circular DNA can be a predictor of sustained response to therapy. Gastroenterology. 2005;128:1890-1897. [PubMed] |
| 29. | Liu F, Campagna M, Qi Y, Zhao X, Guo F, Xu C, Li S, Li W, Block TM, Chang J. Alpha-interferon suppresses hepadnavirus transcription by altering epigenetic modification of cccDNA minichromosomes. PLoS Pathog. 2013;9:e1003613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 30. | Guo JT, Pryce M, Wang X, Barrasa MI, Hu J, Seeger C. Conditional replication of duck hepatitis B virus in hepatoma cells. J Virol. 2003;77:1885-1893. [PubMed] |
| 31. | Addison WR, Walters KA, Wong WW, Wilson JS, Madej D, Jewell LD, Tyrrell DL. Half-life of the duck hepatitis B virus covalently closed circular DNA pool in vivo following inhibition of viral replication. J Virol. 2002;76:6356-6363. [PubMed] |
| 32. | Zhu Y, Yamamoto T, Cullen J, Saputelli J, Aldrich CE, Miller DS, Litwin S, Furman PA, Jilbert AR, Mason WS. Kinetics of hepadnavirus loss from the liver during inhibition of viral DNA synthesis. J Virol. 2001;75:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 194] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 33. | Civitico GM, Locarnini SA. The half-life of duck hepatitis B virus supercoiled DNA in congenitally infected primary hepatocyte cultures. Virology. 1994;203:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Su H, Yee JK. Regulation of hepatitis B virus gene expression by its two enhancers. Proc Natl Acad Sci USA. 1992;89:2708-2712. [PubMed] |
| 35. | Tur-Kaspa R, Teicher L, Laub O, Itin A, Dagan D, Bloom BR, Shafritz DA. Alpha interferon suppresses hepatitis B virus enhancer activity and reduces viral gene transcription. J Virol. 1990;64:1821-1824. [PubMed] |
| 36. | Nawa T, Ishida H, Tatsumi T, Li W, Shimizu S, Kodama T, Hikita H, Hosui A, Miyagi T, Kanto T. Interferon-α suppresses hepatitis B virus enhancer II activity via the protein kinase C pathway. Virology. 2012;432:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Nakao K, Nakata K, Yamashita M, Tamada Y, Hamasaki K, Ishikawa H, Kato Y, Eguchi K, Ishii N. p48 (ISGF-3gamma) is involved in interferon-alpha-induced suppression of hepatitis B virus enhancer-1 activity. J Biol Chem. 1999;274:28075-28078. [PubMed] |
| 38. | Rang A, Heise T, Will H. Lack of a role of the interferon-stimulated response element-like region in interferon alpha -induced suppression of Hepatitis B virus in vitro. J Biol Chem. 2001;276:3531-3535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Alcantara FF, Tang H, McLachlan A. Functional characterization of the interferon regulatory element in the enhancer 1 region of the hepatitis B virus genome. Nucleic Acids Res. 2002;30:2068-2075. [PubMed] |
| 40. | Tsui LV, Guidotti LG, Ishikawa T, Chisari FV. Posttranscriptional clearance of hepatitis B virus RNA by cytotoxic T lymphocyte-activated hepatocytes. Proc Natl Acad Sci USA. 1995;92:12398-12402. [PubMed] |
| 41. | Guidotti LG, Borrow P, Hobbs MV, Matzke B, Gresser I, Oldstone MB, Chisari FV. Viral cross talk: intracellular inactivation of the hepatitis B virus during an unrelated viral infection of the liver. Proc Natl Acad Sci USA. 1996;93:4589-4594. [PubMed] |
| 42. | Heise T, Guidotti LG, Cavanaugh VJ, Chisari FV. Hepatitis B virus RNA-binding proteins associated with cytokine-induced clearance of viral RNA from the liver of transgenic mice. J Virol. 1999;73:474-481. [PubMed] |
| 43. | Heise T, Guidotti LG, Chisari FV. La autoantigen specifically recognizes a predicted stem-loop in hepatitis B virus RNA. J Virol. 1999;73:5767-5776. [PubMed] |
| 44. | Ehlers I, Horke S, Reumann K, Rang A, Grosse F, Will H, Heise T. Functional characterization of the interaction between human La and hepatitis B virus RNA. J Biol Chem. 2004;279:43437-43447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | McClary H, Koch R, Chisari FV, Guidotti LG. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J Virol. 2000;74:2255-2264. [PubMed] |
| 46. | Wieland SF, Guidotti LG, Chisari FV. Intrahepatic induction of alpha/beta interferon eliminates viral RNA-containing capsids in hepatitis B virus transgenic mice. J Virol. 2000;74:4165-4173. [PubMed] |
| 47. | Pasquetto V, Wieland SF, Uprichard SL, Tripodi M, Chisari FV. Cytokine-sensitive replication of hepatitis B virus in immortalized mouse hepatocyte cultures. J Virol. 2002;76:5646-5653. [PubMed] |
| 48. | Wieland SF, Eustaquio A, Whitten-Bauer C, Boyd B, Chisari FV. Interferon prevents formation of replication-competent hepatitis B virus RNA-containing nucleocapsids. Proc Natl Acad Sci USA. 2005;102:9913-9917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 49. | Xu C, Guo H, Pan XB, Mao R, Yu W, Xu X, Wei L, Chang J, Block TM, Guo JT. Interferons accelerate decay of replication-competent nucleocapsids of hepatitis B virus. J Virol. 2010;84:9332-9340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 50. | Zhang Q, Wang Y, Wei L, Jiang D, Wang JH, Rao HY, Zhu L, Chen H, Fei R, Cong X. Role of ISGF3 in modulating the anti-hepatitis B virus activity of interferon-alpha in vitro. J Gastroenterol Hepatol. 2008;23:1747-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Robek MD, Boyd BS, Wieland SF, Chisari FV. Signal transduction pathways that inhibit hepatitis B virus replication. Proc Natl Acad Sci USA. 2004;101:1743-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Guidotti LG, Morris A, Mendez H, Koch R, Silverman RH, Williams BR, Chisari FV. Interferon-regulated pathways that control hepatitis B virus replication in transgenic mice. J Virol. 2002;76:2617-2621. [PubMed] |
| 53. | Robek MD, Wieland SF, Chisari FV. Inhibition of hepatitis B virus replication by interferon requires proteasome activity. J Virol. 2002;76:3570-3574. [PubMed] |
| 54. | Guidotti LG, McClary H, Loudis JM, Chisari FV. Nitric oxide inhibits hepatitis B virus replication in the livers of transgenic mice. J Exp Med. 2000;191:1247-1252. [PubMed] |
| 55. | Wieland SF, Vega RG, Müller R, Evans CF, Hilbush B, Guidotti LG, Sutcliffe JG, Schultz PG, Chisari FV. Searching for interferon-induced genes that inhibit hepatitis B virus replication in transgenic mouse hepatocytes. J Virol. 2003;77:1227-1236. [PubMed] |
| 56. | Vieira VC, Soares MA. The role of cytidine deaminases on innate immune responses against human viral infections. Biomed Res Int. 2013;2013:683095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 57. | Zhang W, Zhang X, Tian C, Wang T, Sarkis PT, Fang Y, Zheng S, Yu XF, Xu R. Cytidine deaminase APOBEC3B interacts with heterogeneous nuclear ribonucleoprotein K and suppresses hepatitis B virus expression. Cell Microbiol. 2008;10:112-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Gonzalez MC, Suspène R, Henry M, Guétard D, Wain-Hobson S, Vartanian JP. Human APOBEC1 cytidine deaminase edits HBV DNA. Retrovirology. 2009;6:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | Rösler C, Köck J, Kann M, Malim MH, Blum HE, Baumert TF, von Weizsäcker F. APOBEC-mediated interference with hepadnavirus production. Hepatology. 2005;42:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 60. | Köck J, Blum HE. Hypermutation of hepatitis B virus genomes by APOBEC3G, APOBEC3C and APOBEC3H. J Gen Virol. 2008;89:1184-1191. [PubMed] |
| 61. | Jost S, Turelli P, Mangeat B, Protzer U, Trono D. Induction of antiviral cytidine deaminases does not explain the inhibition of hepatitis B virus replication by interferons. J Virol. 2007;81:10588-10596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Bonvin M, Achermann F, Greeve I, Stroka D, Keogh A, Inderbitzin D, Candinas D, Sommer P, Wain-Hobson S, Vartanian JP. Interferon-inducible expression of APOBEC3 editing enzymes in human hepatocytes and inhibition of hepatitis B virus replication. Hepatology. 2006;43:1364-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 212] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 63. | Noguchi C, Hiraga N, Mori N, Tsuge M, Imamura M, Takahashi S, Fujimoto Y, Ochi H, Abe H, Maekawa T. Dual effect of APOBEC3G on Hepatitis B virus. J Gen Virol. 2007;88:432-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Turelli P, Mangeat B, Jost S, Vianin S, Trono D. Inhibition of hepatitis B virus replication by APOBEC3G. Science. 2004;303:1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 372] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 65. | Nguyen DH, Gummuluru S, Hu J. Deamination-independent inhibition of hepatitis B virus reverse transcription by APOBEC3G. J Virol. 2007;81:4465-4472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 66. | Nguyen DH, Hu J. Reverse transcriptase- and RNA packaging signal-dependent incorporation of APOBEC3G into hepatitis B virus nucleocapsids. J Virol. 2008;82:6852-6861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 67. | Baumert TF, Rösler C, Malim MH, von Weizsäcker F. Hepatitis B virus DNA is subject to extensive editing by the human deaminase APOBEC3C. Hepatology. 2007;46:682-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 68. | Rösler C, Köck J, Malim MH, Blum HE, von Weizsäcker F. Comment on “Inhibition of hepatitis B virus replication by APOBEC3G”. Science. 2004;305:1403; author reply 1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 69. | Suspène R, Guétard D, Henry M, Sommer P, Wain-Hobson S, Vartanian JP. Extensive editing of both hepatitis B virus DNA strands by APOBEC3 cytidine deaminases in vitro and in vivo. Proc Natl Acad Sci USA. 2005;102:8321-8326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 259] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 70. | Vartanian JP, Henry M, Marchio A, Suspène R, Aynaud MM, Guétard D, Cervantes-Gonzalez M, Battiston C, Mazzaferro V, Pineau P. Massive APOBEC3 editing of hepatitis B viral DNA in cirrhosis. PLoS Pathog. 2010;6:e1000928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 71. | Henry M, Guétard D, Suspène R, Rusniok C, Wain-Hobson S, Vartanian JP. Genetic editing of HBV DNA by monodomain human APOBEC3 cytidine deaminases and the recombinant nature of APOBEC3G. PLoS One. 2009;4:e4277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 72. | Kitamura K, Wang Z, Chowdhury S, Simadu M, Koura M, Muramatsu M. Uracil DNA glycosylase counteracts APOBEC3G-induced hypermutation of hepatitis B viral genomes: excision repair of covalently closed circular DNA. PLoS Pathog. 2013;9:e1003361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 73. | Proto S, Taylor JA, Chokshi S, Navaratnam N, Naoumov NV. APOBEC and iNOS are not the main intracellular effectors of IFN-gamma-mediated inactivation of Hepatitis B virus replication. Antiviral Res. 2008;78:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 74. | Turelli P, Liagre-Quazzola A, Mangeat B, Verp S, Jost S, Trono D. APOBEC3-independent interferon-induced viral clearance in hepatitis B virus transgenic mice. J Virol. 2008;82:6585-6590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Haller O, Staeheli P, Kochs G. Interferon-induced Mx proteins in antiviral host defense. Biochimie. 2007;89:812-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 254] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 76. | Peltekian C, Gordien E, Garreau F, Meas-Yedid V, Soussan P, Willams V, Chaix ML, Olivo-Marin JC, Bréchot C, Kremsdorf D. Human MxA protein participates to the interferon-related inhibition of hepatitis B virus replication in female transgenic mice. J Hepatol. 2005;43:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 77. | Gordien E, Rosmorduc O, Peltekian C, Garreau F, Bréchot C, Kremsdorf D. Inhibition of hepatitis B virus replication by the interferon-inducible MxA protein. J Virol. 2001;75:2684-2691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 150] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 78. | Li N, Zhang L, Chen L, Feng W, Xu Y, Chen F, Liu X, Chen Z, Liu W. MxA inhibits hepatitis B virus replication by interaction with hepatitis B core antigen. Hepatology. 2012;56:803-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 79. | Fernández M, Quiroga JA, Martín J, Cotonat T, Pardo M, Horisberger MA, Carreño V. Impaired interferon induction of human MxA protein in chronic hepatitis B virus infection. J Med Virol. 1997;51:332-337. [PubMed] |
| 80. | Rosmorduc O, Sirma H, Soussan P, Gordien E, Lebon P, Horisberger M, Bréchot C, Kremsdorf D. Inhibition of interferon-inducible MxA protein expression by hepatitis B virus capsid protein. J Gen Virol. 1999;80:1253-1262. [PubMed] |
| 81. | Fernández M, Quiroga JA, Carreño V. Hepatitis B virus downregulates the human interferon-inducible MxA promoter through direct interaction of precore/core proteins. J Gen Virol. 2003;84:2073-2082. [PubMed] |
| 82. | Xiong W, Wang X, Liu X, Xiang L, Zheng L, Yuan Z. Interferon-inducible MyD88 protein inhibits hepatitis B virus replication. Virology. 2004;319:306-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Li J, Lin S, Chen Q, Peng L, Zhai J, Liu Y, Yuan Z. Inhibition of hepatitis B virus replication by MyD88 involves accelerated degradation of pregenomic RNA and nuclear retention of pre-S/S RNAs. J Virol. 2010;84:6387-6399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 84. | Wu M, Xu Y, Lin S, Zhang X, Xiang L, Yuan Z. Hepatitis B virus polymerase inhibits the interferon-inducible MyD88 promoter by blocking nuclear translocation of Stat1. J Gen Virol. 2007;88:3260-3269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 85. | Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1666] [Cited by in RCA: 1766] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 86. | Iwamoto N, Ito H, Ando K, Ishikawa T, Hara A, Taguchi A, Saito K, Takemura M, Imawari M, Moriwaki H. Upregulation of indoleamine 2,3-dioxygenase in hepatocyte during acute hepatitis caused by hepatitis B virus-specific cytotoxic T lymphocytes in vivo. Liver Int. 2009;29:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 87. | Chen YB, Li SD, He YP, Shi XJ, Chen Y, Gong JP. Immunosuppressive effect of IDO on T cells in patients with chronic hepatitis B*. Hepatol Res. 2009;39:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 88. | Mao R, Zhang J, Jiang D, Cai D, Levy JM, Cuconati A, Block TM, Guo JT, Guo H. Indoleamine 2,3-dioxygenase mediates the antiviral effect of gamma interferon against hepatitis B virus in human hepatocyte-derived cells. J Virol. 2011;85:1048-1057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 89. | Barr SD, Smiley JR, Bushman FD. The interferon response inhibits HIV particle production by induction of TRIM22. PLoS Pathog. 2008;4:e1000007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 236] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 90. | Eldin P, Papon L, Oteiza A, Brocchi E, Lawson TG, Mechti N. TRIM22 E3 ubiquitin ligase activity is required to mediate antiviral activity against encephalomyocarditis virus. J Gen Virol. 2009;90:536-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 91. | Di Pietro A, Kajaste-Rudnitski A, Oteiza A, Nicora L, Towers GJ, Mechti N, Vicenzi E. TRIM22 inhibits influenza A virus infection by targeting the viral nucleoprotein for degradation. J Virol. 2013;87:4523-4533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 199] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 92. | Gao B, Duan Z, Xu W, Xiong S. Tripartite motif-containing 22 inhibits the activity of hepatitis B virus core promoter, which is dependent on nuclear-located RING domain. Hepatology. 2009;50:424-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 172] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 93. | Terenzi F, Hui DJ, Merrick WC, Sen GC. Distinct induction patterns and functions of two closely related interferon-inducible human genes, ISG54 and ISG56. J Biol Chem. 2006;281:34064-34071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 94. | Guo J, Hui DJ, Merrick WC, Sen GC. A new pathway of translational regulation mediated by eukaryotic initiation factor 3. EMBO J. 2000;19:6891-6899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 170] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 95. | Pichlmair A, Lassnig C, Eberle CA, Górna MW, Baumann CL, Burkard TR, Bürckstümmer T, Stefanovic A, Krieger S, Bennett KL. IFIT1 is an antiviral protein that recognizes 5’-triphosphate RNA. Nat Immunol. 2011;12:624-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 385] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 96. | Pei R, Qin B, Zhang X, Zhu W, Kemper T, Ma Z, Trippler M, Schlaak J, Chen X, Lu M. Interferon-induced proteins with tetratricopeptide repeats 1 and 2 are cellular factors that limit hepatitis B virus replication. J Innate Immun. 2014;6:182-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 97. | Zhang X, Kraft A, Broering R, Schlaak JF, Dittmer U, Lu M. Preclinical development of TLR ligands as drugs for the treatment of chronic viral infections. Expert Opin Drug Discov. 2012;7:597-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 98. | Isogawa M, Robek MD, Furuichi Y, Chisari FV. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol. 2005;79:7269-7272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 360] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 99. | Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, Fosdick A, Frey CR, Zheng J, Wolfgang G. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology. 2013;144:1508-1517, 1517.e1-10. [PubMed] |
| 100. | Xia C, Lu M, Zhang Z, Meng Z, Zhang Z, Shi C. TLRs antiviral effect on hepatitis B virus in HepG2 cells. J Appl Microbiol. 2008;105:1720-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 101. | Guo H, Jiang D, Ma D, Chang J, Dougherty AM, Cuconati A, Block TM, Guo JT. Activation of pattern recognition receptor-mediated innate immunity inhibits the replication of hepatitis B virus in human hepatocyte-derived cells. J Virol. 2009;83:847-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 102. | Thompson AJ, Colledge D, Rodgers S, Wilson R, Revill P, Desmond P, Mansell A, Visvanathan K, Locarnini S. Stimulation of the interleukin-1 receptor and Toll-like receptor 2 inhibits hepatitis B virus replication in hepatoma cell lines in vitro. Antivir Ther. 2009;14:797-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 103. | Zhang X, Ma Z, Liu H, Liu J, Meng Z, Broering R, Yang D, Schlaak JF, Roggendorf M, Lu M. Role of Toll-like receptor 2 in the immune response against hepadnaviral infection. J Hepatol. 2012;57:522-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 104. | Wu J, Lu M, Meng Z, Trippler M, Broering R, Szczeponek A, Krux F, Dittmer U, Roggendorf M, Gerken G. Toll-like receptor-mediated control of HBV replication by nonparenchymal liver cells in mice. Hepatology. 2007;46:1769-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 231] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 105. | Chang J, Block TM, Guo JT. The innate immune response to hepatitis B virus infection: implications for pathogenesis and therapy. Antiviral Res. 2012;96:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 106. | Liu J, Jiang M, Ma Z, Dietze KK, Zelinskyy G, Yang D, Dittmer U, Schlaak JF, Roggendorf M, Lu M. TLR1/2 ligand-stimulated mouse liver endothelial cells secrete IL-12 and trigger CD8+ T cell immunity in vitro. J Immunol. 2013;191:6178-6190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 107. | Li Y, Shi X. MicroRNAs in the regulation of TLR and RIG-I pathways. Cell Mol Immunol. 2013;10:65-71. [PubMed] |
| 108. | Quinn SR, O’Neill LA. A trio of microRNAs that control Toll-like receptor signalling. Int Immunol. 2011;23:421-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 241] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 109. | Zhang X, Zhang E, Ma Z, Pei R, Jiang M, Schlaak JF, Roggendorf M, Lu M. Modulation of hepatitis B virus replication and hepatocyte differentiation by MicroRNA-1. Hepatology. 2011;53:1476-1485. [PubMed] |
| 110. | Zhang GL, Li YX, Zheng SQ, Liu M, Li X, Tang H. Suppression of hepatitis B virus replication by microRNA-199a-3p and microRNA-210. Antiviral Res. 2010;88:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 111. | Zhang X, Hou J, Lu M. Regulation of hepatitis B virus replication by epigenetic mechanisms and microRNAs. Front Genet. 2013;4:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 112. | Xie Q, Shen HC, Jia NN, Wang H, Lin LY, An BY, Gui HL, Guo SM, Cai W, Yu H. Patients with chronic hepatitis B infection display deficiency of plasmacytoid dendritic cells with reduced expression of TLR9. Microbes Infect. 2009;11:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 113. | Vincent IE, Zannetti C, Lucifora J, Norder H, Protzer U, Hainaut P, Zoulim F, Tommasino M, Trépo C, Hasan U. Hepatitis B virus impairs TLR9 expression and function in plasmacytoid dendritic cells. PLoS One. 2011;6:e26315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 114. | Xu N, Yao HP, Lv GC, Chen Z. Downregulation of TLR7/9 leads to deficient production of IFN-α from plasmacytoid dendritic cells in chronic hepatitis B. Inflamm Res. 2012;61:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 115. | Huang YW, Lin SC, Wei SC, Hu JT, Chang HY, Huang SH, Chen DS, Chen PJ, Hsu PN, Yang SS. Reduced Toll-like receptor 3 expression in chronic hepatitis B patients and its restoration by interferon therapy. Antivir Ther. 2013;18:877-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 116. | Chen Z, Cheng Y, Xu Y, Liao J, Zhang X, Hu Y, Zhang Q, Wang J, Zhang Z, Shen F. Expression profiles and function of Toll-like receptors 2 and 4 in peripheral blood mononuclear cells of chronic hepatitis B patients. Clin Immunol. 2008;128:400-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 117. | Wang H, Ryu WS. Hepatitis B virus polymerase blocks pattern recognition receptor signaling via interaction with DDX3: implications for immune evasion. PLoS Pathog. 2010;6:e1000986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (2)] |
| 118. | Wu J, Meng Z, Jiang M, Pei R, Trippler M, Broering R, Bucchi A, Sowa JP, Dittmer U, Yang D. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology. 2009;49:1132-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 276] [Article Influence: 17.3] [Reference Citation Analysis (4)] |
| 119. | Xu Y, Hu Y, Shi B, Zhang X, Wang J, Zhang Z, Shen F, Zhang Q, Sun S, Yuan Z. HBsAg inhibits TLR9-mediated activation and IFN-alpha production in plasmacytoid dendritic cells. Mol Immunol. 2009;46:2640-2646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 120. | Wang S, Chen Z, Hu C, Qian F, Cheng Y, Wu M, Shi B, Chen J, Hu Y, Yuan Z. Hepatitis B virus surface antigen selectively inhibits TLR2 ligand-induced IL-12 production in monocytes/macrophages by interfering with JNK activation. J Immunol. 2013;190:5142-5151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (2)] |
| 121. | Lütgehetmann M, Bornscheuer T, Volz T, Allweiss L, Bockmann JH, Pollok JM, Lohse AW, Petersen J, Dandri M. Hepatitis B virus limits response of human hepatocytes to interferon-α in chimeric mice. Gastroenterology. 2011;140:2074-2083, 2083.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 122. | Chen J, Wu M, Zhang X, Zhang W, Zhang Z, Chen L, He J, Zheng Y, Chen C, Wang F. Hepatitis B virus polymerase impairs interferon-α-induced STA T activation through inhibition of importin-α5 and protein kinase C-δ. Hepatology. 2013;57:470-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (2)] |
| 123. | Wang Y, Wei L, Jiang D, Cong X, Fei R, Xiao J, Wang Y. In vitro resistance to interferon of hepatitis B virus with precore mutation. World J Gastroenterol. 2005;11:649-655. [PubMed] |
| 124. | Wang Y, Wei L, Jiang D, Cong X, Fei R, Chen H, Xiao J, Wang Y. In vitro resistance to interferon-alpha of hepatitis B virus with basic core promoter double mutation. Antiviral Res. 2007;75:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 125. | Sonneveld MJ, Rijckborst V, Zeuzem S, Heathcote EJ, Simon K, Senturk H, Pas SD, Hansen BE, Janssen HL. Presence of precore and core promoter mutants limits the probability of response to peginterferon in hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2012;56:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 126. | Sonneveld MJ, Rijckborst V, Zwang L, Zeuzem S, Jenny Heathcote E, Simon K, Zoutendijk R, Akarca US, Pas SD, Hansen BE, Janssen HL. Hepatitis B e antigen levels and response to peginterferon: influence of precore and basal core promoter mutants. Antiviral Res. 2013;97:312-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 127. | Lu JJ, Chen EQ, Yang JH, Zhou TY, Liu L, Tang H. A mutation in the interferon regulatory element of HBV may influence the response of interferon treatment in chronic hepatitis B patients. Virol J. 2012;9:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 128. | Radecke K, Protzer U, Trippler M, Meyer Zum Büschenfelde KH, Gerken G. Selection of hepatitis B virus variants with aminoacid substitutions inside the core antigen during interferon-alpha therapy. J Med Virol. 2000;62:479-486. [PubMed] |