INTRODUCTION
Total mesorectal excision (TME) is the standard treatment for rectal cancer. It achieves locoregional control of the disease, and the rate of local recurrence is below 5%[1]. TME involves low anterior rectal or coloanal resection, very often combined with a protective ostomy, or abdominoperineal resection (Miles operation) and definitive colostomy. However, complications are frequent; associated morbidity is around 33%, mortality 2%[2], and 20%-30% of patients present genitourinary alterations and sexual dysfunction[3,4].
The capacity of local surgery to cure rectal cancer depends on the degree of lymph node invasion. The risk of possible metastatic lymph nodes has been reported to range between 0% and 12% in T1, between 12% and 28% in T2 and between 36% and 79% in T3[5]. In local surgery, endoanal excision is limited by the height of the lesion with respect to the anal verge; it is difficult to control the resection limits and to perform complete removal of the rectal wall. Local surgery via trans-sphincteric exposure as described by Mason[6] has been used to treat lesions in the middle third of the rectum located in the anterior face, but the sectioning of the sphincters raises the morbidity rate. Kraske’s trans-sacral rectal excision[7] made it possible to reach the upper third of the rectum, but it has also been abandoned due to its high morbidity and mortality.
Transanal endoscopic microsurgery (TEM) provides a solution to these problems. First described by Buess et al[8], this endoscopic procedure allows preservation of the anal sphincters and, through its excellent viewing system, allows access to rectal tumors as far as 20 cm from the anal verge. TEM facilitates the maneuvers of dissection, cutting, coagulation and suturing. Postoperative morbidity rates are below 10%, and no mortality, genitourinary alterations or sexual dysfunction have been reported[9,10].
So what is the place of local surgery using TEM in rectal cancer? In this review, we examine the following aspects of its use: patient selection; surgical technique and types of equipment; risk of adenocarcinoma in rectal adenomas; its indication in T1 rectal adenocarcinomas, and its application in T2 tumors.
SELECTION OF PATIENTS FOR TEM: TREATMENT GROUPS
All possible candidates for TEM must undergo full preoperative staging of the tumor: total colonoscopy with multifocal biopsy, and rigid rectoscopy prior to endorectal ultrasound (EUS), to confirm tumor size, the distance of its lower and upper edge from the anal verge, and location by quadrant (anterior, posterior, right or left lateral). EUS allows staging of the lesion according to Hildebrandt’s criteria[11] and pelvic magnetic resonance imaging (MRI) is an important complement, although MRI is not more accurate than EUS, in rectal adenocarcinomas it is needed to confirm the tumor stage and the absence of metastasis to lymph nodes.
If adenocarcinoma is either suspected or has been diagnosed, abdominal and chest computed tomography is performed to rule out distance metastasis and to determine tumor markers carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9. All patients are administered the Wexner sphincter function questionnaire[12], if there are signs of fecal incontinence, anorectal manometry is performed to obtain baseline parameters. We have found that TEM causes manometric alterations but does not affect clinical continence scores[13].
After these complementary examinations, patients are classified into preoperative indication groups from I to IV[10,14]. Group I, with curative intent, includes rectal lesions with biopsy revealing adenoma and staged uT0, uN0 by EUS and pelvic MRI. Group II, also with curative intent, includes adenocarcinomas [either well differentiated (G1) or moderately differentiated (G2)], and staged uT0-1, uN0. Group III, indication by consensus, includes adenocarcinomas [either well differentiated (G1) or moderately differentiated (G2)], with staging uT2, uN0[15]. Group IV includes palliative indications regardless of the tumor stage. Therefore, it is patients in groups I and II who are candidates for TEM.
Certain rectal and pelvic pathologies are habitually treated by laparotomy or laparoscopy via an abdominal approach. The use of TEM by expert groups allows some of these surgeries to be performed using a less aggressive approach which achieves lower morbidity rates. These indications are termed “atypical”, as they do not involve removal of rectal tumors[16,17].
SURGICAL TECHNIQUE
On the day prior to surgery all patients undergo mechanical preparation of the colon and thromboembolism prophylaxis. With the induction of anesthesia, they are administered the standard antibiotic prophylaxis in colorectal surgery.
In the classical technique of TEM[8], correct positioning of the patient on the operating table is vitally important. In TEM the surgeon works with the tumor visible in the lower part of the rectoscope at all times, so the positioning of the patient depends on the location of the rectal tumor. The TEM equipment comprises a 4 cm diameter rectoscope with two different lengths (12 and 20 cm) selected according to the location of the tumor. The pneumorectum is maintained at a constant pressure (10-12 mmHg). The rectal distension created in this way exposes the tumor and the rectal wall. Our group[10] begins the dissection by making a dotted line with the monopolar electric scalpel 10-15 mm from the tumor. We then open the mucosa over the dotted line and begin the full thickness excision of the rectal wall using an ultrasound scalpel (Ultracision, Ethicon, Endo-Surgery, Cincinnati, OH, United States). The defect of the lesion in the rectal wall is sutured to avoid stenosis of the rectal lumen and postoperative bleeding due to feces. The suture is made transversally so as not to compromise the rectal lumen.
Oral diet is initiated on the day after surgery and increased progressively depending on tolerance. Standard analgesia is administered with non-steroid anti-inflammatory drugs and morphine as rescue medication. The bladder catheter is withdrawn after surgery and the patient is mobilized after eight hours. The patient is discharged from hospital between days 2 and 4 post-surgery.
Different types of transanal endoscopic surgery
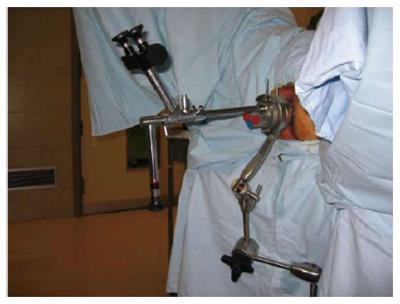
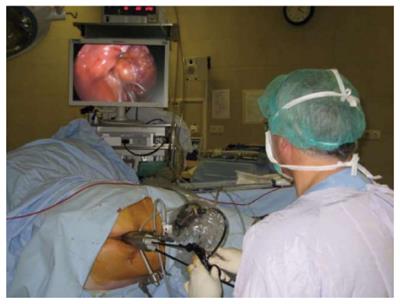
As noted above, TEM[8] is an endoscopic procedure with three-dimensional vision (3-D) (Figure 1). Transanal endoscopic operation (TEO) provides two-dimensional vision also through a 4 cm diameter rectoscope of various lengths (7.5, 15 and 22 cm) and on-screen vision. The introduction of a high-definition camera and its application in a panoramic thin-film transistor screen provides an image that is very similar to 3-D. The surgeon is seated in front of the monitor, as in laparoscopy; this makes the learning process easier (Figure 2). Like Nieuwenhuis et al[18], we performed a comparative study of our experience with the 2-D system (TEO) and the classical 3-D system (TEM). Our results with regard to surgical difficulty, postoperative morbidity, quality of surgical resections were similar for the two approaches, but the economic cost was lower in the case of TEO[19].
Figure 1 Transanal endoscopic microsurgery equipment
Figure 2 Transanal endoscopic operation equipment
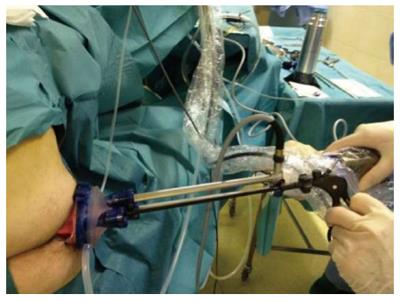
Recently a new transanal endoscopic surgery technique has been introduced termed transanal minimally invasive surgery (TAMIS)[20] or transanal single port microsurgery (TSPM)[21], which uses a single laparoscopic port via the anus (Figure 3). We have considerably less experience with TAMIS/TSPM approaches than with TEM or TEO. TAMIS/TSPM requires an assistant to hold and move the camera. From the technical point of view, the introduction of the single port into the anal canal is more complex than in TEM/TEO; a further disadvantage is that the rectoscope cannot be mobilized at the site of the lesion, a maneuver that can be performed with TEM/TEO.
Figure 3 Transanal minimally invasive surgery or transanal single port microsurgery equipment
We carried out an economic study comparing TEM/TEO/TAMIS based on the following assumptions: The economic aspects are analysed annually [estimating 50 surgical interventions (SI) per year and a useful life of the non-expendable material of 5 years], divided into variable and fixed costs; The variable annual costs are determined by applying the equation (1): n annual surgeries × (surgical time + hospital stay + consumable material). Consumable material: Ultracision scalpel, single port, sutures…; The annual fixed costs are determined by applying the equation (2): non-expendable equipment/time of useful life; The annual cost for each technique is given by the equation (3): annual fixed costs + annual variable costs; the estimated cost of the use of the operating theatre is 10 €/min and the cost of hospitalization in a conventional ward is 220 €/d.
The variable annual costs calculated are [equation (1)]: TEO: 93000 €/year [50 SI × (700 €/SI + 660 €/SI + 500 €/SI)]; TEM: 104500 €/year [50 SI × (830 €/SI + 660 €/SI + 600 €/SI)]; TAMIS: 111000 €/year [50 SI × (760 €/SI + 660 €/SI + 800 €/SI)].
The fixed annual costs calculated are [equation (2)]: TEO: 3000 €/year (15000 €/5 years); TEM: 11000 €/year (55000 €/5 years); TAMIS: 0 €/year.
The total annual costs calculated for each technique are [equation (3)]: TEO: 96000 €/year (93000 €/year + 3000 €/year); TEM: 115500 €/year (104500 €/year + 11000 €/year); TAMIS: 111000 €/year.
Finally, under these assumptions, we obtain the following mean costs: TEO: 1920 €, TEM: 2310 €, TAMIS: 2220 €.
Technical limitations of TEM: Height and morphology
Limitations due to height: The distance of the upper edge of the lesion from the anal verge is of vital importance. Conventional endoanal excision is limited to lesions at distances of up to 7-8 cm. With TEM, classically the limits were set by the risk of perforation of the peritoneal cavity: it was possible to perform the excision with a low risk of perforation at a distance of up to 18-20 cm when the tumor was located in the posterior quadrant, and up to 15 cm when its location was anterior or lateral. Today, perforation of the peritoneal cavity is not considered a contraindication for TEM[22]. There are no limits in terms of the location of the lesion (i.e., anterior, posterior, or lateral). The limit due to height is determined by the length of the rectoscope, and on occasion by anatomical features: narrow rectosigmoidal junctions with a small rectal ampulla (below 10 cm), or a history of abdominal surgical interventions that immobilize the rectosigmoidal junction and impede the progression of the rectoscope further than 10 cm. The limit for low lesions is the anal verge itself.
Limitations due to morphology: It is possible to excise adenomatous lesions that cover up to three quadrants of the circumference (10-12 cm Ø). In fact all four quadrants can sometimes be reached if the lesions are not particularly wide and if the size does not exceed the height permitted. The problem presented by large lesions is the need to suture the defect, due to the risk of stenosis. If the defect cannot be completely closed, it should be reduced to the maximum - especially the upper part, due to the risk of perforation.
Follow-up protocol for rectal adenocarcinomas after TEM
In accordance with international guidelines, in our treatment group II we recommend strict follow-up of these lesions. The follow-up schedule comprises rectosigmoidoscopy-biopsy, EUS and CEA determination every four months during the first and second years; rectosigmoidoscopy-biopsy, EUS and CEA every six months from the third to fifth year; complete colonoscopy, abdominal CT and pelvic MRI annually until the fifth year; and from the fifth year onward, the standard follow-up protocol for colon polyps. The usefulness of EUS after TEM is limited due to the difficulty of interpreting the scar fibrosis, and so it is substituted by pelvic MRI.
HIGH FREQUENCY OF ADENOCARCINOMA IN LARGE RECTAL ADENOMAS
Colorectal adenomatous polyps are considered premalignant lesions with a risk of developing into adenocarcinoma[23]. Early detection and removal are the best means to avoid the appearance of adenocarcinoma[23,24]. In our series and in the study by Absar and Haboubi[25], the rate of invasive adenocarcinomas in adenomatous polyps of the colon was above 18%. Therefore, almost one of every five rectal tumors with a biopsy of adenoma is likely to be an invasive adenocarcinoma. For this reason, piecemeal endoscopic resection or mucosectomy is insufficient treatment[26]. In the case of large rectal adenomas we advocate full-thickness rectal wall resection using TEM, leaving adequate safety margins for correct staging by the pathologist[27]. In our series, half of the infiltrating adenocarcinomas resulting from adenomas were pT1[26]. This means that, with adequate resection and pathological diagnosis, in the absence of factors of poor prognosis, these patients will not require radical rescue surgery[14,28,29].
The factors associated with malignancy in rectal adenomatous tumors have not been clearly established. Among epidemiological variables, it has been postulated that male patients aged under 65 may present a higher risk of adenocarcinoma. Nonetheless, multivariate analyses have not identified age or sex as predictive factors[30,31].
Among morphological factors, an association between lesion size and malignancy risk has often been proposed. Years ago, Muto et al[32] reported that with lesion size above 2 cm the risk rises to 53%. Other authors have suggested an association between the size of colorectal adenomas and the risk of adenocarcinoma, but have not been able to demonstrate it[33]. Several studies have also established that villous adenomas present a high risk of malignancy. In our series we have not found differences with respect to tubular and tubulo-villous adenomas; however, the sessile type presents a higher risk of adenocarcinoma than other morphologies[30,31,34]. As for dysplasia, it is natural to assume that lesions with high-grade dysplasia present a higher risk of malignancy[30,33].
If the preoperative study of these lesions includes only rigid rectoscopy and/or colonoscopy with biopsies there is a high risk of understaging, because these techniques do not provide information on the extent of the invasion of the wall and the possible lymph node involvement in the case of infiltrating adenocarcinoma[35]. For this reason, the preoperative study of these lesions should include EUS and pelvic MRI[34]. Preoperative EUS identifies lesions with invasion beyond the submucosa (T1)[11,36], and we regard pelvic MRI as an important complement to endorectal ultrasound, even though it is less effective in discriminating between lesions affecting the submucosa and the muscle layer (T1 and T2), it identifies lesions of stages above T2, and can also detect the presence of lymph nodes in which metastasis is suspected[37].
T1 RECTAL ADENOCARCINOMA: TUMORS WITH GOOD OR POOR PROGNOSIS
The treatment of rectal tumors depends on several factors of prognostic significance: the penetration of the tumor in the thickness of the rectal wall, the involvement of the mesorectal fascia, and the presence of lymph node and distance metastasis[38]. According to the TNM classification, T1 rectal adenocarcinoma presents invasion of the submucosa, but not beyond[39-41].
Local surgery is an alternative to TME for treatment of T1. In long-term series using classical endoanal resection, local recurrence rates are as high as 29%[9,42,43]. TEM has demonstrated its effectiveness in treating these tumors[44] and achieves initial results for local recurrence below 10%. Recently, however, some alarming figures for local recurrence of T1 with TEM have been published[45], and Doornebosch et al[46] also reported a rate of 20.5%. Tytherleigh et al[39] offered a possible explanation for these high rates by classifying T1 rectal adenocarcinoma according to good or poor prognosis, which may be related to surgical and pathological factors.
The depth of the submucosal invasion is considered the most important predictor of locoregional lymph node involvement[39,41]. Several methods have been described to assess submucosal invasion. All of them present advantages and disadvantages, and there is no single system based on scientific evidence that can be recommended for all situations. At present the Haggitt classification is proposed for polypoid lesions and the Kikuchi classification for non-polypoid lesions[39,40,47,48].
Haggitt et al[48] staged polypoid lesions according to the invasion of the carcinoma, as follows: invading the mucosa (level 0), invading the submucosa but limited to the head of the polyp (level 1), invading the neck (level 2), invading any part of the stalk (level 3), or invading beyond the stalk or base (level 4). Level 4 is associated with a high risk of locoregional lymph node involvement.
Kikuchi et al[47] divided the invasion of the submucosa into three levels: Sm1, submucosal invasion that does not extend beyond 200-300 μm from the muscularis mucosae; Sm2, intermediate submucosal invasion; Sm3, submucosal invasion near the surface of the muscularis propria. In this classification, the frequency of locoregional lymph node involvement varies according to the depth of the submucosal invasion: 2% in Sm1 lesions, 8% in Sm2 lesions, and 23% in Sm3 lesions. So, in the absence of other risk factors, Sm3 is sufficient to indicate radical surgery.
The Kikuchi classification can be related to the Haggitt levels: levels 1, 2 and 3 correspond to Kikuchi Sm1, while Haggitt level 4 may be Sm1, Sm2 or Sm3[39].
In addition to the depth of submucosal invasion, other predictors of locoregional lymph node involvement have also been reported in the literature. These include the degree of tumor differentiation, vascular invasion, lymph node invasion, perineural invasion, involvement of the resection margin (≤ 1 mm), lymphocyte infiltration, tumor budding (presence of neoplastic cells below the invasive front), demarcation of the submucosal invasive front, and tumor differentiation at the leading edge of the lesion[38-41].
Adequate selection of patients for local surgery on the basis of pathological criteria is essential. In addition, the surgical procedure must comply with a series of standards to ensure its effectiveness: complete excision of the lesion in a single piece (i.e., without fragmentation), complete rectal wall excision, and tumor-free resection margins of at least 1 mm from the lesion[38,39,46,49].
In conclusion, if the histological and surgical characteristics are favorable the risk of local recurrence is below 5%, and the lesion is considered to be a T1 rectal adenocarcinoma with good prognosis[39]. T1 with poor prognosis are lesions with predictors of lymph node involvement and deficient surgery (for example, fragmentation, surgical margins affected, or less than 1 mm from the lesion). In these circumstances the risk of local recurrence can rise to 29%.
LOCAL SURGERY IN T2 RECTAL ADENOCARCINOMA
According to the NCCN-2013[50], the standard treatment of rectal adenocarcinoma T2N0M0 (ADC-T2) is TME without adjuvant therapy. These guidelines no longer propose local surgery associated with adjuvant therapy as an alternative, as they did in 2008[51]. The local surgical approaches for ADC-T2 described in the literature are simple local excision (either via endoanal excision or TEM), local surgery with postoperative chemoradiotherapy (CT-RT), and preoperative CT-RT and local excision[14,49,52-55]. As we noted above, radical surgery (TME) reduces quality of life and may lead to death due to causes not directly related to cancer. So it is important to be able to assess the results obtained with these alternatives in order to choose the most suitable approach in each case.
Describing simple local excision with TEM for ADC-T2, Borschitz et al[49] reported a local recurrence rate of 35%. In our study of a series of 11 patients and a mean follow-up of 59 mo, local recurrence was recorded in 22.2%. These results suggest that local excision alone at this stage of the disease should only be used with palliative intent.
The possibilities of postoperative adjuvant therapy have received considerable attention. This is not surprising, since with adequate pathological diagnosis of the lesion (avoiding understaging or overstaging) adjuvant care can control the disease at a lower cost than radical surgery. However, the review of the literature on CT-RT after local surgery presents disappointing results, with local recurrence ranging from 0% to 45%[56]. Our experience has been unfavorable, with two out of six patients (33%) presenting local recurrence despite adequate surgical resection and with tumors defined as low risk on the strength of histological findings[15]. We agree with Baxter et al[56] that although postoperative adjuvant therapy appears to reduce local recurrence compared to simple local excision, the rate still remains higher than with TME.
In a review of the literature on preoperative CT-RT and local surgery in ADC-T2, Borschitz et al[55] observed that when complete pathological response (CPR) is achieved (that is, ypT0), local recurrence (LR) was 0% and systemic recurrence was 4%. With ypT1 tumors, LR was 2% and systemic recurrence was 7%. In ypT2, LR rose to 7% and systemic recurrence also to 7%. However, when there was no response to neoadjuvant therapy (ypT3), the LR rate was 21% and systemic recurrence 12%. Although the experience is limited, promising results have also been reported in T3, although the reports do not specify whether the lesions were superficial or deep[57,58].
The main objective of neoadjuvant treatment is to achieve CPR. The CPR rates reported for T2 and T3 range widely, between 11.7% and 73%[59,60]. The immense majority of CPRs are obtained with a long CT-RT regimen (5-fluorouracil or capecitabine, combined with radiotherapy of 50.4 Gy for five weeks). The adverse effects (AE) due to the toxicity of the CT-RT should be borne in mind. In 11 of the 40 patients (26.5%), Yu et al[57] reported toxicity following neoadjuvant treatment; ten of them had AE grade ≥ 2, although none abandoned treatment. In attempts to improve the CPR, adjuvant treatment regimens have been modified. Garcia-Aguilar et al[59] proposed the association of standard doses of capecitabine and oxaliplatin with radiotherapy, and achieved a CPR of 48%. However, 44% of patients had AE grade ≥ 3, which obliged a reduction in the capecitabine dose; with the new dose the CPR rate was 36%, and 30% presented AE grade ≥ 3.
Complete clinical response (CCR) is not always the same as CPR. Attempts have been made to determine clinical and radiological predictors of CPR, but no definitive conclusions have been reached[59,61,62]. Due to the lack of correlation between CCR and CPR, the combination of CT-RT and local surgery is not suitable for all types of rectal cancer that present CCR. For this reason, we advocate exhaustive selection of patients by a multidisciplinary team[63,64]. The most favorable results reported in the literature were in adenocarcinomas staged by EUS, MRI and abdominal CT as T2N0M0 with degrees of differentiation G1-2[50], size ≤ 4 cm, and CPR after CT-RT.
Adequate excision of the lesion is most important, avoiding fragmentation of the specimen and involvement of the surgical margins (> 1 mm)[49]. As noted above, TEM also achieves better results than conventional local excision with regard to the resection margins and the quality of the specimen[44].
The results obtained so far with TEM are promising. However, the scientific evidence is still limited, and these findings need to be confirmed in prospective randomized control trials.
ACKNOWLEDGMENTS
We thank the other members of the Coloproctology Unit, Dr. Jordi Bombardó and Dr. Isidro Ayguavives. We also thank the members of the multidisciplinary committee for colorectal tumors at Parc Taulí University Hospital: Carlos Pericay, Aleidis Pisa, Emma Dotor, Eugeni Saigi (Oncology Service), Eva Ballesteros, Antonio Malet (Radiodiagnostic Service), Rafael Campos, Enric Brullet, Eva Martinez (Digestive Pathology Service), and the coordinator nurse of the multidisciplinary committee for colorectal tumors, Ms. Maite Martinez. We thank Cristina Gomez Vigo for correcting the manuscript and Michael Maudsley for the translation into English.