Published online Aug 14, 2014. doi: 10.3748/wjg.v20.i30.10606
Revised: March 23, 2014
Accepted: April 30, 2014
Published online: August 14, 2014
Processing time: 176 Days and 6.3 Hours
AIM: To evaluated our management algorithm of the coagulopathy. We evaluated our management algorithm of the coagulopathy.
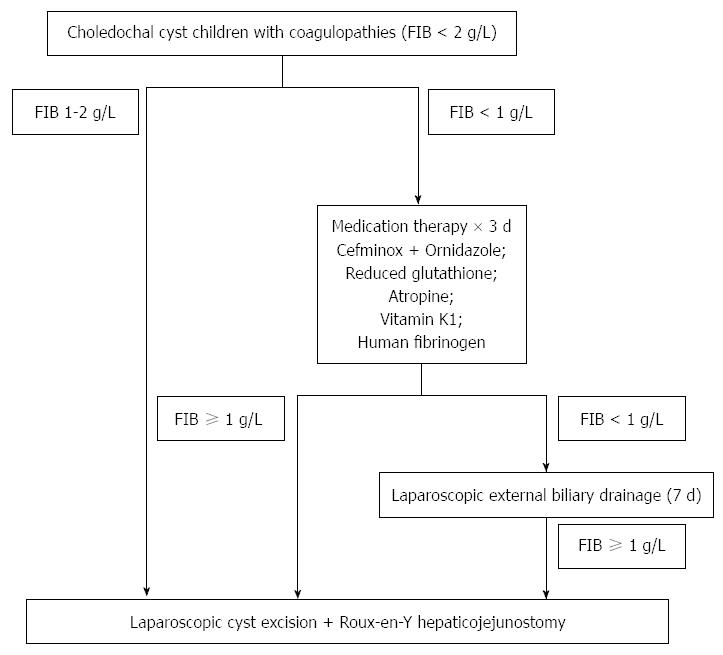
METHODS: Between October 2001 and January 2013, 160 CDC children with coagulopathy (fibrinogen, FIB < 2 g/L) were recruited. FIB ≥ 1 g/L is generally required for safe elective surgery. We used FIB level as an indicator when: (1) patients with FIB levels between 1-2 g/L underwent one-stage definitive operation; and (2) patients with FIB < 1 g/L underwent 3 d of medical treatment. Thereafter, those with FIB ≥ 1 g/L underwent one-stage definitive operation whereas those with FIB < 1 g/L underwent external biliary drainage to allow liver function improvement. Those patients with liver function improvements underwent definitive operation after 7 d of drainage.
RESULTS: After preoperative optimization, 92.5% of CDC children with coagulopathy underwent successful one-stage definitive operation. The remaining 7.5% of CDC children required initial external bile drainage, and underwent definitive operation 11 d after the admission. The mean operative time and postoperative recovery duration were comparable to those with normal coagulations. The median follow-up period was 57 mo. No blood transfusion or other postoperative complications were encountered.
CONCLUSION: Following our management protocol, the majority of CDC children with coagulopathy can be managed with one-stage definitive operation.
Core tip: Children suffering from choledochal cysts with coagulopathy have increased operative risks. We assessed the coagulation profiles in children with different subtypes of choledochal cyst, and established a management strategy whereby patients underwent either medical optimization or laparoscopic external drainage before definitive laparoscopic operation. The results suggest that the treatment protocol allows safe one-stage definitive operation for the majority of choledochal cyst children with coagulopathy, and maximizes the opportunity of definitive operation.
- Citation: Diao M, Li L, Cheng W. Coagulopathy in a subtype of choledochal cyst and management strategy. World J Gastroenterol 2014; 20(30): 10606-10612
- URL: https://www.wjgnet.com/1007-9327/full/v20/i30/10606.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i30.10606
Coagulopathy secondary to biliary obstruction often occurs in children with choledochal cysts (CDC). Our previously published study categorizes CDC into 2 subtypes according to the common bile duct (CBD) outlet size and its associated pathology[1]: (1) CDC with stenotic distal CBD outlet is associated with more severe CBD dilatation (often cystic in morphology) and more deranged liver function; and (2) CDC with non-stenotic distal CBD (often fusiform in morphology) is commonly associated with protein plug formation in the common channels. In the current study, we assessed the coagulation profiles of children with these 2 subtypes of CDC.
Coagulopathy poses a major risk for operation. However, definitive operation is the only way to completely clear biliary obstruction and to reverse coagulopathy. Conventionally, open external biliary drainage and delayed definitive operation is adopted[2,3]. The disadvantages include: (1) two-stage operation; (2) external drainage decompresses the biliary pressure but does not resolve the distal CBD obstruction, which leads to excessive bile drainage and subsequent dehydration and electrolyte imbalance; and (3) missing the opportunity of minimal invasive surgery. Since October 2001, we started definitive laparoscopic operation for CDC children, and formulated a new management strategy for CDC children with coagulopathy secondary to biliary obstructions. Our clinical experiences found the main abnormality in CDC children with coagulopathy was deficient fibrinogen (FIB) rather than prolonged prothrombin time (PT) or partial thromboplastin time (APTT). Acquired FIB deficiency develops when there is severe liver parenchyma damage and impaired synthesis[4]. FIB level below 1 g/L (normal range 2-4 g/L) indicates FIB depletion[5]. FIB level of at least 1 g/L is generally required for safe elective surgery[6]. We herewith used FIB level as an indicator of adequate liver function before operating on CDC children with coagulopathy. Laparoscopic resection of CDC and Roux-en-Y hepatico-jejunal reconstruction were carried out for those CDC children with FIB level ≥ 1 g/L in our series. In this study, we evaluated the safety and efficacy of our management algorithm.
Between October 2001 and January 2013, 456 CDC children were recruited. Ten patients were excluded because they had undergone external drainage for cyst perforation and peritonitis or because of poor general medical condition. Ethics approval from the Ethics Committee of Capital Institute of Pediatrics was obtained. Written informed consents were obtained from the parents of the CDC patients prior to the surgery.
Perioperative ultrasonography, liver function and coagulation tests were performed. Preoperative computed tomography and magnetic resonance cholangiopancreatography were also carried out.
The treatment protocol is shown in Figure 1. CDC children with FIB ≥ 1 g/L underwent laparoscopic cyst excision and Roux-en-Y hepatico-jejunostomy. CDC children with severe coagulopathy (FIB < 1 g/L) underwent 3 d of medical therapy. These patients were referred to our hospital because of poor response to 1-4 wk of routine therapy in local hospitals, including antibiotics and reduced glutathione antioxidant to boost liver function. Since their blood routine tests in our hospital showed significantly elevated white blood cells (16 × 109/L-25 × 109/L, reference range: 4 × 109/L-10 × 109/L) and C-reactive protein values ranged from 38-107 mg/L (reference range: < 8 mg/L), antibiotics were administered. Treatment included antibiotics (cefminoxime, 80 mg/kg per day, intravenous infusion, divided Q12h, and ornidazole, 80 mg/kg per day, intravenous infusion, divided Q12h), reduced glutathione antioxidant (0.3-0.6 g/d, intravenous infusion, QD, to boost liver function), vitamin K1 homologs (5-10 mg/d, intravenous infusion, QD), antispasmodics/anticholinergics (atropine, 0.01 mg/kg, intramuscular injection, Q12h, to reduce bile and pancreatic juice secretion, and to decrease the smooth muscle tone of sphincter of Oddi to facilitate protein plug drainage into duodenum), and lyophilized human fibrinogen (0.5 g/d, intravenous infusion, QD, to improve coagulation). After the optimization, the patients with FIB level ≥ 1 g/L underwent definitive laparoscopic operation. On the other hand, those patients with FIB level less than 1 g/L underwent laparoscopic external biliary drainage to decompress the biliary system and subsequently improve the liver function. They then underwent the definitive laparoscopic operation after 7 d of biliary drainage.
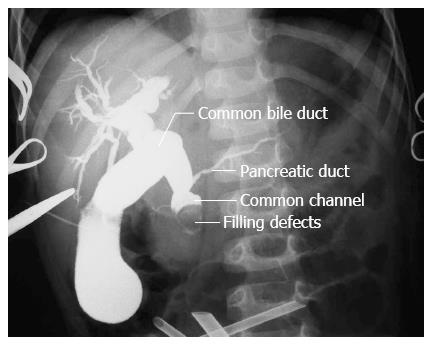
Intraoperative cholangiograms and endoscopies were performed to establish the anatomy of junctions of CBDs, pancreatic ducts, common channels, and the presence of intraluminal protein plugs/calculi. Intraoperative cholangiograms were reviewed by pediatric radiologists. Relative diameter and relative length, defined as the ratio of the bile duct diameter and length to the height of the second lumbar vertebra, were calculated to compare the measurements between children of different ages[7].
Laparoscopic cyst excision and hepatico-jejunostomy, and clearance of protein plugs were performed as previously described[8-10].
Patients were followed up in our clinic at 1, 3, 6, and 12 mo postoperatively and 6 mo thereafter. Physical examination, abdominal ultrasonography and laboratory tests were carried out at each visit. Postoperative complications such as bile/pancreatic leak, anastomotic stenosis, cholangitis, pancreatitis, intestinal obstruction, wound infection, or incisional hernia were evaluated clinically, with abdominal ultrasonographic studies and with blood tests. Upper gastrointestinal contrast studies were performed at one month follow-up to assess the presence and severity of reflux from the Roux loop into the biliary system. The age at operation, blood transfusion, operative time, postoperative hospital stay, time required to resume full diet, duration of drainage, intra- and postoperative complications, and perioperative liver function and coagulation parameters were analyzed.
Data were analyzed with the SPSS 13.0 package. χ2 test was applied to compare the liver function and extent of coagulopathy between patients with and without stenotic distal CBDs. Student’s t tests were used to compare the operative time, postoperative hospital stay, resumption of diet, duration of drainage between CDC patients with normal and abnormal coagulations. Paired t-tests were applied to compare perioperative laboratory values. P < 0.01 was considered to be statistically significant.
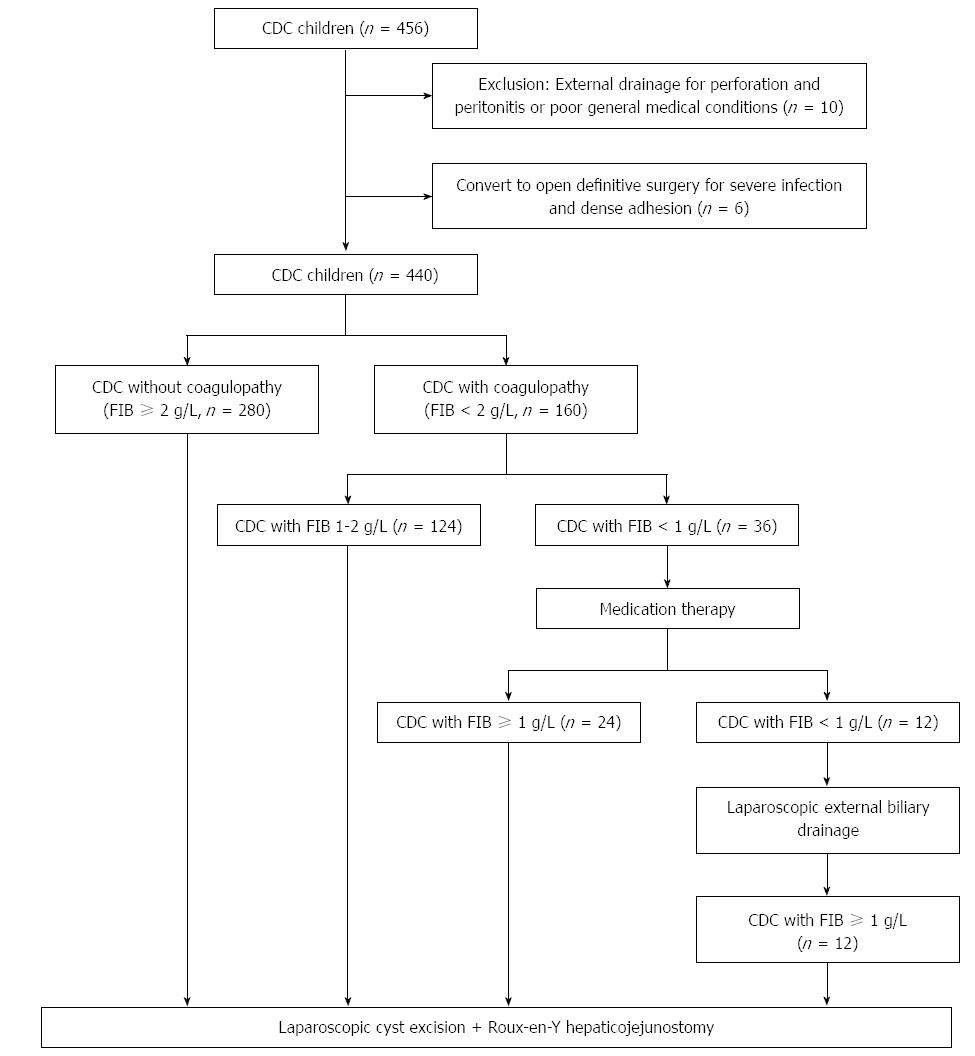
With the exception of 6 patients who were converted to open procedures due to severe infections and dense adhesions, 440 CDC patients successfully underwent laparoscopic definitive operation (median age: 3.39 years, range: 7 d-18 years; F/M: 328/112). Of the 440 CDC patients, 336 (76.4%) patients had stenotic distal CBDs, while the remaining 104 (23.6%) patients had non-stenotic distal CBDs. The mean relative diameters of distal CBDs were 0.19 ± 0.07 in the stenotic group and 0.46 ± 0.16 in the non-stenotic group (P < 0.001). Average relative diameters of maximal dilated CBDs were 4.36 ± 1.74 in the stenotic group and 1.28 ± 0.38 in the non-stenotic group (P < 0.001). The mean relative diameters of common channels were 0.20 ± 0.12 in the stenotic group and 0.86 ± 0.27 in the non-stenotic group (P < 0.001). Dilatation of the CBD in the non-stenotic group was unremarkable compared to that in the stenotic group.
Of these patients, 313 of 336 (93.2%) with stenotic distal CBDs showed abnormal liver function and 292 (86.9%) presented with jaundice; whereas 67 of 104 (64.4%) patients with non-stenotic distal CBDs showed abnormal liver function and 34 (32.6%) presented with jaundice (Table 1). Patients with stenotic distal CBDs were more likely to have abnormal liver function (Table 1, 93.2% vs 64.4%, P < 0.001).
As shown in Figure 2, 160 of 440 (36.4%) patients showed abnormal coagulation with FIB level lower than 2 g/L. Of the 124 (77.5%) patients with FIB value of 1-2 g/L, 88 (70.9%) had stenotic distal CBDs and 36 (29.1%) had non-stenotic distal CBDs. Of the remaining 36 patients with FIB level lower than 1 g/L, 5 (13.9%) had stenotic distal CBDs, and 31 (86.1%) had non-stenotic distal CBDs (Table 2). Coagulopathy secondary to poor hepatic function was more frequently encountered in patients with non-stenotic distal CBDs (Table 2, P < 0.001). Complete biliary obstructions by massive protein plugs in the common channel were detected in 100% of the patients with non-stenotic distal CBDs and severe coagulopathy (n = 31) (Figure 3).
| Stenotic (n = 93) | Non-stenotic (n = 67) | P value | |
| FIB 1-2 g/L (n = 124) | 71 (88) | 36 (29) | < 0.001 |
| FIB < 1 g/L (n = 36) | 5 (13.9) | 31 (86.1) |
Of the 36 patients with FIB level lower than 1 g/L, 34 had normal prothrombin time (PT, 10-12.4 s, reference: 8.8-12.8 s) and partial thromboplastin time (PTT, 30.1-36.2 s, reference range: 24-37 s). One patient showed prolonged PT (19.6 s) but normal PTT (31.7 s). An additional patient had prolonged PT (13.6 s) and PTT (42.8 s).
Following our intervention protocol, 92.5% (148/160) of CDC children with coagulopathy, including 124 CDC children with an FIB value of 1-2 g/L and 24 patients whose FIB increased to a level above 1 g/L after medical therapy, successfully underwent one-stage laparoscopic definitive operation. The coagulopathy of the remaining 7.5% of CDC children (12/160) was successfully corrected after laparoscopic external bile drainage. They subsequently underwent laparoscopic cyst excision and Roux-en-Y hepaticojejunostomy 11 d after hospital admission (Figure 2). Of the 36 patients with FIB level lower than 1 g/L, with the relief of obstructive jaundice, the FIB levels in all patients with stenotic distal CBDs (n = 5) increased to a level above 1 g/L after medical therapy, and they successfully underwent subsequent one-stage definitive laparoscopic operations (serum bilirubin: 233.70 ± 17.48 μmol/L baseline vs 39.64 ± 3.34 μmol/L after medical therapy vs 14.62 ± 2.94 μmol/L after definitive operation, P < 0.001 respectively; FIB: 0.61 ± 0.36 g/L baseline vs 1.63 ± 0.37 g/L after medical therapy vs 2.74 ± 0.18 g/L after definitive operation, P < 0.001 respectively). Of the remaining 31 patients with non-stenotic distal CBDs, with the relief of obstructive jaundice, the FIB level in 19 patients increased to a level above 1 g/L after medical therapy (serum bilirubin: 237.47 ± 29.05 μmol/L baseline vs 41.47 ± 4.10 μmol/L after medical therapy vs 15.54 ± 3.25 μmol/L after definitive operation, P < 0.001 respectively; FIB: 0.50 ± 0.23 g/L baseline vs 1.35 ± 0.25 g/L after medical therapy vs 2.53 ± 0.25 g/L after definitive operations, P < 0.001 respectively). The remaining 12 patients underwent laparoscopic external biliary drainage because FIB levels were still lower than 1 g/L after medical therapy. Their FIB levels were reversed to 1-2 g/L after 7-d external biliary drainage. All of them successfully underwent definitive laparoscopic operation after the drainage and normalization of coagulation (FIB: 0.27 ± 0.21 g/L baseline vs 0.48 ± 0.24 g/L after medical therapy vs 1.76 ± 0.33 g/L after external biliary drainage vs 2.49 ± 0.32 g/L after definitive operation, P < 0.01, P < 0.001 and P < 0.001 respectively).
The mean operative time was 3.11 ± 0.42 h in CDC patients with coagulopathy, which was comparable to that of CDC patients with normal coagulation (3.03 ± 0.49 h, P = 0.11). The average postoperative hospital stay, time to full feed and duration of drainage in CDC patients with coagulopathy were 5.56 ± 1.03 d, 2.18 ± 0.39 d, and 3.21 ± 0.60 d respectively, which did not differ significantly from the postoperative recovery in CDC patients with normal coagulation (5.41 ± 1.39 d, 2.11 ± 0.35 d, and 3.06 ± 0.94 d, P = 0.21, 0.07, 0.12 respectively). The median follow-up period was 57 mo. No blood transfusion was required. There was no mortality. No bile leak, anastomotic stenosis, cholangitis, pancreatic juice leak, pancreatitis, pancreatic or biliary stone formation was encountered. No intrahepatic reflux was observed in postoperative upper gastrointestinal contrast studies. Liver function tests and serum amylase levels normalized by 3 mo postoperatively (Table 3, P < 0.001 respectively).
| CDC without coagulopathy (n = 280) | CDC with coagulopathy (n = 160) | ||||
| FIB 1-2 g/L (n = 124) | FIB ≥ 1 g/L after medication (n = 24) | FIB ≥ 1 g/L after external drainage (n = 12) | |||
| TBIL (µmol/L) | Pre-op | 168.64 ± 90.22 | 201.57 ± 71.64 | 234.77 ± 23.98 | 254.97 ± 17.63 |
| Ref: 3.4-20 | Post-op | 11.03 ± 5.68 | 16.57 ± 4.39 | 17.25 ± 5.88 | 18.75 ± 2.66 |
| ALT (U/L) | Pre-op | 99.15 ± 46.39 | 182.11 ± 74.52 | 219.02 ± 13.72 | 231.04 ± 12.34 |
| Ref: < 40 | Post-op | 16.16 ± 8.74 | 24.80 ± 8.29 | 31.14 ± 10.38 | 36.12 ± 6.01 |
| AST (U/L) | Pre-op | 100.77 ± 45.95 | 184.19 ± 74.58 | 220.26 ± 13.19 | 230.82 ± 10.27 |
| Ref: < 40 | Post-op | 18.11 ± 8.67 | 28.71 ± 9.49 | 32.07 ± 10.55 | 38.92 ± 2.96 |
| ALP (U/L) | Pre-op | 526.42 ± 135.72 | 728.99 ± 118.35 | 755.08 ± 57.35 | 813.75 ± 53.51 |
| Ref: < 400 | Post-op | 140.68 ± 43.72 | 151.75 ± 29.50 | 161.16 ± 47.24 | 188.58 ± 54.88 |
| GGT (U/L) | Pre-op | 295.68 ± 192.57 | 405.93 ± 161.46 | 609.42 ± 87.32 | 688.83 ± 39.03 |
| Ref: 7-50 | Post-op | 26.72 ± 12.30 | 30.38 ± 9.76 | 36.75 ± 3.45 | 38.70 ± 2.02 |
| SAMY (U/L) | Pre-op | 130.96 ± 111.76 | 312.31 ± 286.55 | 678.91 ± 298.26 | 801.13 ± 92.68 |
| Ref: 25-125 | Post-op | 27.28 ± 9.52 | 32.22 ± 13.89 | 38.62 ± 16.38 | 51.25 ± 10.68 |
| P value (pre- vs post-operation) | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
CDC children may have abnormal coagulation if biliary obstruction continues for a substantial period of time[11]. Although patients with stenotic distal CBDs are more likely to have liver dysfunction, the hepatic dysfunction and consequent coagulopathy were more severe in patients with non-stenotic distal CBDs and complete obstruction by massive protein plugs in the common channel. The explanation might be that in the stenotic group, the severe dilatation of CBD may reduce the pressure of intrahepatic ducts, whereas in the non-stenotic group of patients, limited dilatation of CBD may not adequately relieve the pressure of intrahepatic ducts. Furthermore, with the accumulation of massive protein plugs in a moderately dilated common channel, the CBD is more prone to spontaneous perforation, which is often underestimated. Therefore, early definitive operation is advocated, particularly for patients with non-stenotic distal CBDs.
Hemorrhage may increase the morbidity of the operation and the conversion rate in laparoscopic surgery. Adequate preoperative preparation and right timing of the operation are essential. We herewith established a protocol for safe surgical correction of CDC for those children with coagulopathy, the first attempt to standardize the surgical management of CDC with coagulopathy. The advantages of the treatment protocol are: (1) being able to apply safe one-stage definitive operation for the majority of CDC children; (2) to reduce the external biliary drainage which is conventionally used in CDC with severe coagulopathy; and (3) to maximize the opportunity of definitive operation and minimal invasive surgery.
Acquired FIB deficiency develops in a patient with profound liver parenchyma damage[4]. Besides declining FIB levels, hyperfibrinolysis may also occur. FIB value < 1 g/L reflects FIB depletion[5]. This low level presents a risk of spontaneous bleeding[6]. FIB level ≥ 1 g/L is generally sufficient for elective surgery[6]. Two screening tests, PT and PTT, are also indicative of FIB level[6]. However, the tests were found to be abnormal only when FIB is below the critical threshold value of 1 g/L[6]. Our results verified that the majority of CDC children with FIB level lower than 1 g/L still present normal PT and PTT. We proved that FIB value was a more sensitive indicator of coagulopathy in CDC children. Hence, in the case of suspected bleeding, it is recommended to determine FIB concentrations directly[6]. Our outcomes suggest that FIB level can be used to monitor the liver function improvement. FIB value ≥ 1 g/L may be an indicator that CDC children with coagulopathy can undergo definitive operation.
Early coagulopathy in patients with biliary obstruction is attributed to deficiency of vitamin K-dependent clotting factors due to malabsorption of fat-soluble vitamin K[12]. When complete biliary obstruction occurs, a sharply increased biliary pressure aggravates canalicular cholestasis and the subsequent bile plug formations, leading to bile ductule rupture. The bile leak leads to severe hepatocellular injury. Hepatic synthetic function subsequently shuts down. Deficiencies of non-vitamin K dependent-clotting factors, including FIB, are present[12]. Administration of vitamin K early in the course of biliary obstruction or fresh frozen plasma late in the course of biliary obstruction cannot sufficiently reverse coagulation dysfunction unless the biliary obstruction is relieved. Conventionally, percutaneous transhepatic biliary drainage or open external biliary drainage is considered as first choice for biliary decompression in this situation[2,3]. However, coagulation defects increase the risk of blood loss in any invasive procedure[12]. To resolve this dilemma, atropine, a routine premedication used in general anesthesia in children including small infants, was adopted in our treatment protocol to reduce bile and pancreatic juice secretion, to relax the sphincter of Oddi and facilitate protein plug drainage into the duodenum, hence relieving the pressure in the biliary tract. Our results verified that our treatment protocol was safe and effective, especially for patients who have a poor response to 1-4 wk of routine combination therapy in the local hospital, including antibiotics and reduced glutathione antioxidant to boost liver function. Atropine can be used as an initial attempt to decompress the biliary tract. External biliary drainage is utilized when patients have a poor response to atropine. The hepatic functions were remarkably reversed in a short period of time. The rate of external biliary drainage was decreased. Following our intervention protocol, the majority of CDC children with coagulopathy successfully underwent one-stage laparoscopic definitive operation. None of the CDC patients with abnormal FIB required blood transfusions. Postoperative recovery and morbidities in CDC patients with aberrant FIB are comparable to those with normal FIB. Liver function tests in all patients normalized within 3 mo postoperatively.
For those patients whose coagulation functions failed to respond to medical therapy, laparoscopic external biliary drainage is advocated as early as possible to decompress the pressure in the biliary tract and subsequently improve liver function and coagulation. Moreover, it allows an opportunity for future laparoscopic cyst excision and Roux-en-Y hepaticojejunostomy.
In the era of open surgery, delayed primary excision Roux-en-Y hepaticojejunostomy is recommended 1-3 mo after external biliary drainage[2,13]. While external biliary drainage is a “bridge procedure” to decompress the biliary tract, the hepatic dysfunction and coagulopathy cannot be completely reversed until distal biliary obstruction is cleared by definitive operation. Furthermore, constant distal CBD obstruction and extended duration of external drainage often result in excessive bile loss and subsequent fluid-electrolyte imbalance. Patients often have to be re-admitted to hospital for intravenous re-hydration. In addition, drain tube dislodgement frequently occurs when children resume normal activities, and this requires urgent surgical intervention. In the era of laparoscopic surgery, less surgical trauma accelerates post-drainage recovery. We aimed to keep drainage duration as short as was safe. Our results showed that 3-day medical therapy and 7-d external bile drainage effectively improve the coagulation function for CDC patients with coagulopathy. None of the patients suffered from water-electrolyte imbalance or drain tube dislocation.
The drawback of the current study is the lack of a control group, i.e., CDC patients with FIB < 1 g/L who would undergo one-stage definitive operation directly. However, doing so may place these patients at high risk-for intra- and postoperative hemorrhages.
In conclusion, early surgical intervention is advocated, particularly for patients with non-stenotic distal CBDs and moderate dilation of the proximal CBD. Patients with massive protein plugs in a moderately dilated common channel and CBD may have more severe liver damage and coagulopathy. They may benefit from medical treatment or preoperative drainage. Our protocol offers a safe and effective treatment algorithm to manage CDC children with coagulopathy. Following this strategy, the majority of CDC children with liver dysfunction and abnormal coagulation function can be successfully treated with one-stage definitive laparoscopic operation. The remaining children with persistent severe coagulopathy can be successfully managed with a short period of laparoscopic external bile drainage followed by definitive laparoscopic operation.
Choledochal cyst (CDC) children with coagulopathies secondary to liver dysfunction are at high risk when they undergo definitive laparoscopic surgeries. We herewith established a management logarithm whereby patients underwent either medical optimization or laparoscopic external drainage before definitive laparoscopic surgery, and evaluated its safety and efficacy.
Conventionally, open external biliary drainage and delayed definitive operation is adopted for CDC children with coagulopathies. The disadvantages include: (1) two-stage operation; (2) external drainage decompresses the biliary pressure but does not resolve the distal common bile duct obstruction, which leads to excessive bile drainage and subsequent dehydration and electrolyte imbalance; and (3) missing the opportunity of minimal invasive surgery. The current study is the first attempt to assess the coagulation profiles of children with different subtypes of CDC, to establish a protocol for safe surgical correction of CDC for those children with coagulopathy, and to standardize the surgical management of CDC with coagulopathy.
Acquired fibrinogen (FIB) deficiency develops when there is severe liver parenchyma damage and impaired synthesis. FIB level of at least 1 g/L is generally required for safe elective surgery. The authors herewith used FIB level as an indicator of adequate liver function before operating on CDC children with coagulopathies. Patients with FIB levels between 1-2 g/L underwent one-stage definitive laparoscopic surgeries; the remaining patients with FIB < 1 g/L underwent 3 d of medical treatment. Thereafter, those with FIB ≥ 1 g/L underwent one-stage definitive laparoscopic surgeries whereas those with FIB < 1 g/L underwent laparoscopic external biliary drainages to allow liver function improvement. Those patients with liver function improvement then underwent definitive laparoscopic surgeries after 7 d of drainage. After preoperative optimization, the majority (92.5%) of CDC children with coagulopathy underwent successful one-stage definitive surgery. The remaining 7.5% of CDC children underwent definitive surgery after 7 d of external bile drainage. The mean operative time and postoperative recovery duration were comparable to those with normal coagulation. No blood transfusion or other postoperative complications were encountered.
The authors’ protocol offers a safe and effective treatment algorithm to manage CDC children with coagulopathy. The advantages of the treatment protocol are: (1) being able to apply safe one-stage definitive operation for the majority of CDC children; (2) to reduce the external biliary drainage which is conventionally used in CDC with severe coagulopathy; and (3) to maximize the opportunity of definitive operation and minimal invasive surgery.
FIB value < 1 g/L reflects FIB depletion, indicating a risk of spontaneous bleeding. These outcomes suggest that FIB level can be used to monitor the liver function improvement. FIB value ≥ 1 g/L may be an indicator that CDC children with coagulopathy can undergo definitive operation.
Children suffering from choledochal cysts with coagulopathy have increased operative risks. The current study assessed the coagulation profiles in different subtypes of choledochal cyst, introduced a new concept of using fibrinogen as an indicator and established a management strategy whereby patients either underwent medical optimization or laparoscopic external drainage before definitive laparoscopic operation. The results of a larger number of patients suggest that the treatment protocol allows safe one-stage definitive operation for the majority of choledochal cyst children with coagulopathy, and maximizes the opportunity of definitive operation.
P- Reviewer: David JS S- Editor: Qi Y L- Editor: Logan S E- Editor: Ma S
| 1. | Diao M, Li L, Cheng W. Congenital biliary dilatation may consist of 2 disease entities. J Pediatr Surg. 2011;46:1503-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Miyano T, Li L, Yamada K. Choledochal cyst. Pediatric Surgery-Diagnosis and Management 1st ed. New Delhi, India: Jayee Brother Medical Publishers (P) LTD 2009; p. 1013-25. |
| 3. | Wang HZ. Congenital Biliary Dilatation. Practical Pediatric Surgery. The First Edition ed. Beijing: The People’s Health Publishing House 2001; p. 1060-75. |
| 4. | Scherer RU, Giebler RM. [Perioperative coagulation disorders]. Anasthesiol Intensivmed Notfallmed Schmerzther. 2004;39:415-43; quiz 444-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Giangrande PLF, Littlewood TJ. Haematological Problems. Oxford Textbook of Surgery. New York: Oxford University Press 1994; p. 214. |
| 6. | 7 Procoagulators. Transfus Med Hemother. 2009;36:419-436. [PubMed] |
| 7. | Ceia F, Fonseca C, Brito D, Madeira H. Heart failure treatment in Portuguese hospitals: results of a survey. Rev Port Cardiol. 2001;20:1259-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Li L, Feng W, Jing-Bo F, Qi-Zhi Y, Gang L, Liu-Ming H, Yu L, Jun J, Ping W. Laparoscopic-assisted total cyst excision of choledochal cyst and Roux-en-Y hepatoenterostomy. J Pediatr Surg. 2004;39:1663-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Diao M, Li L, Cheng W. Laparoscopic versus Open Roux-en-Y hepatojejunostomy for children with choledochal cysts: intermediate-term follow-up results. Surg Endosc. 2011;25:1567-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Diao M, Li L, Zhang JS, Cheng W. Laparoscopic-assisted clearance of protein plugs in the common channel in children with choledochal cysts. J Pediatr Surg. 2010;45:2099-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | O’Neill JA, Goran AG, Fonkalsrud E. Choledochal Cyst. 6th ed. United States: Mosby; 2006; p. 1620-34. |
| 12. | Rege RV. Adverse effects of biliary obstruction: implications for treatment of patients with obstructive jaundice. AJR Am J Roentgenol. 1995;164:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Miyano T, Yamataka A. Choledochal cysts. Curr Opin Pediatr. 1997;9:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |