Published online Aug 14, 2014. doi: 10.3748/wjg.v20.i30.10531
Revised: March 23, 2014
Accepted: April 28, 2014
Published online: August 14, 2014
Processing time: 290 Days and 12.3 Hours
AIM: To illustrate the critical techniques and feasibility of laparoscopic extended right hemicolectomy (LERH), according to our previous experience.
METHODS: Anatomical relationship and operative techniques were demonstrated. One hundred and five consecutive patients who underwent extended right hemicolectomy with D3 lymphadenectomy between January 2008 and May 2011 were included in the present study [laparoscopic group (n = 48) vs open group (n = 57)].
RESULTS: The right retrocolic space was the main surgical plan of the LERH. The superior mesenteric vein was the most important anatomical landmark for vascular dissection. The medial-to-lateral dissection approach made the LERH performed efficiently. Compared with the open group, the LERH group had less blood loss (111.7 ± 127.8 mL vs 170.2 ± 49.7 mL, P = 0.023), faster return of flatus (3.0 ± 1.6 d vs 3.7 ± 1.3 d, P = 0.019), and earlier diet (4.2 ± 1.4 d vs 5.0 ± 1.2 d, P = 0.005). Five patients (10.4%) underwent conversion during laparoscopic surgery. The cancer recurrence rates between the two groups were comparable (laparoscopic vs open, 8.6% vs 9.1%, P = 0.335).
CONCLUSION: For an advanced tumor located at the hepatic flexure or proximal transverse colon, LERH with D3 lymphadenectomy using a medial-to-lateral approach seems to be safe and feasible when the superior mesenteric vein serves as the main anatomical landmark and the right retrocolic space severed as the surgical plan.
Core tip: Laparoscopic extended right hemicolectomy with D3 lymphadenectomy is technically demanding for complex vascular anatomy. D3 lymphadenectomy can be implemented concisely and safely when the superior mesenteric vein serves as the anatomical landmark and the right retrocolic space severed as the surgical plan.
- Citation: Zhao LY, Liu H, Wang YN, Deng HJ, Xue Q, Li GX. Techniques and feasibility of laparoscopic extended right hemicolectomy with D3 lymphadenectomy. World J Gastroenterol 2014; 20(30): 10531-10536
- URL: https://www.wjgnet.com/1007-9327/full/v20/i30/10531.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i30.10531
The laparoscopic approach has been currently accepted as an alternative to open surgery for colon cancer[1-4]. A tumor located at or within 10 cm distal to the hepatic flexure has an increased risk of infrapyloric lymph node metastasis[5]. However, most previous studies excluded tumors located at the hepatic flexure or within 10 cm distal to the hepatic flexure due to the technical difficulty in a complete lymphadenectomy around the origins of the middle colic vessels and the right gastroepiploic vessels. Consequently, open extended right hemicolectomy (OERH) with D3 lymphadenectomy has been recommended as the optional procedure[6]. Laparoscopic D3 lymphadenectomy around the above mentioned vessels is a highly technique. Thus, we introduced the pivotal techniques and feasibility of the laparoscopic extended right hemicolectomy (LERH) with D3 lymphadenectomy according to our previous experience.
Patients with a tumor located at the hepatic flexure or within 10 cm distal to it who underwent extended right hemicolectomy with D3 lymphadenectomy between January 2008 and May 2011 were retrieved from the established database[7]. The surgical approach was chosen based on an understanding of the risks and benefits inherent to laparoscopic and open resection, without any pressure from the surgeon. After excluding distant metastases, multiple primary tumors, acute surgery, previous colon surgery, and previous malignant disease, 105 patients were included for the final analysis (laparoscopic vs open: 48 vs 57). All patients provided written informed consent.
Clinical data like age, sex, operation time, total blood loss during surgery, liquid diet start time, time of first flatus, postoperative hospital stay, and complications within 30 d after surgery were collected and compared between the two groups. To evaluate the oncologic quality of the resection, tumor size, distal and proximal resection margin, and number of lymph nodes harvested were collected and compared consequently.
The D3 lymphadenectomy in this approach was defined as lymphadenectomy by ligating the ileocolic, right colic, middle colic, and right gastroepiploic vessels successively at their origins.
The patient was placed in the Trendelenburg position, with the surgeon standing between the patient’s legs, the camera operator standing on the patient’s left side, the assistant standing on the right of the camera operator, and the scrub nurse standing on the patient’s right side. A 30º-angled scope placed through an umbilical port was used to get an adequate view. A 10-mm trocar was introduced 10 cm below the umbilicus for the surgeon’s right hand and a 5-mm McBurney’s point port was placed for the left-hand instrument. An additional two 5-mm trocars were placed at the opposite McBurney’s point and the right subcostal position for the assistant to retract and display the colon and mesocolon.
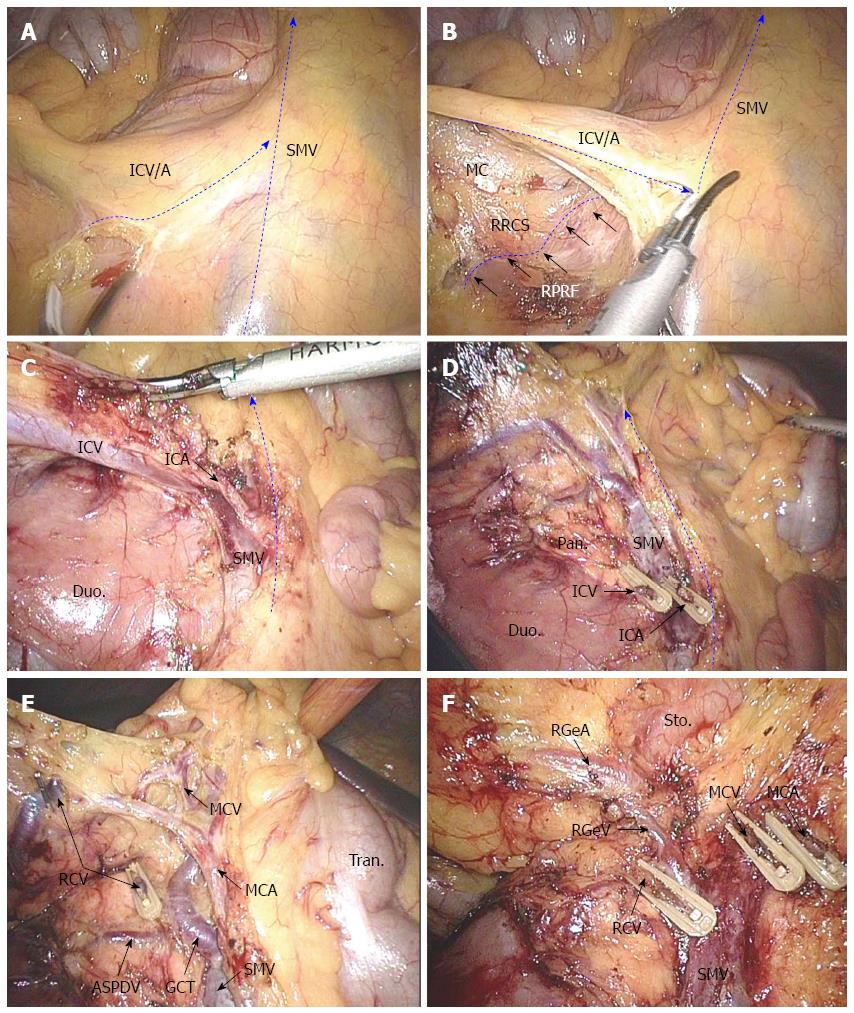
Locating the ileocolic and superior mesenteric vessel pedicles: The small bowel was displaced to the left and the omentum was turned up to the upper quadrant. The transverse colon and the ileocecal junction were towed cranially and laterally respectively. These retractions tented up the root of the mesentery and the right mesocolon, displaying the ileocolic and superior mesenteric vessels clearly, even in very obese patients (Figure 1A).
Opening the “mesenteric window” and exploring the right retrocolic space: A medial-to-lateral approach was useful to facilitate the exposure of the mesentery with the assistance of the peritoneal fixation of the right colon laterally. The “mesenteric window” was opened just at the inferior edge of the ileocolic vascular pedicle, which stood out clearly (Figure 1A). The right retrocolic space (RRCS) between the mesocolon and the right pre-renal fascia was the natural surgical plan of the extended right hemicolectomy. The RRCS was extended laterally and cranially via the “mesenteric window” (Figure 1B).
Dissecting the ileocolic vessels and superior mesenteric vein: The origins of the ileocolic vessels was identified and then ligated at their origin point from the superior mesenteric vessels. The ventral aspect of the caudal portion of the superior mesenteric vein (SMV) was first exposed in this process. The spatial relationship of the ileocolic vein and ileocolic artery was used to locate the superior mesenteric artery (Figure 1B and C).
Dissecting the gastrocolic vein trunk and middle colic artery: The inferior part of the duodenum was the first exposed structure in the course of extending the RRCS cranially, with the uncinate process of pancreas exposed subsequently. The right colic artery (if it existed) and the gastrocolic venous trunk were then located and skeletonized when dissecting the ventral of the SMV caudally to cranially. The gastrocolic venous trunk was described as the confluence of the right colic vein and the right gastroepiploic vein (RGeV) draining into the SMV at an average distance of 2.2 cm from the inferior pancreatic border[8]. The middle colic vessels were the first branches of the superior mesenteric vessels when they came outside of the pancreatic neck. In our center, in order to locate the middle colic vessels, we usually skeletonized the SMV cranially and regarded the inferior of the pancreatic neck as the anatomical landmark. The right colic vessels, RGeV, and middle colic vessels were divided at their origins one by one carefully (Figure 1D and E). Dissection continued between the mesocolon and the right pre-renal fascia throughout in favor of security and oncologic quality.
Dissecting the gastrocolic artery: The gastrocolic venous trunk was the landmark for locating the RGeV. The gastroepiploic artery (RGeA) was the anterior of the superior edge of the pancreatic neck, and located at the right superior of the RGeV. Hypopyloric lymphadenectomy was performed along with the original ligation of the gastroepiploic vessels (Figure 1F).
Mobilization of the transverse colon and hepatic flexure: The transverse colon 10 cm distal to the tumor was drawn caudally by the left hand of the surgeon and the greater gastric curvature was drawn crucially by the assistant. The gastrocolic ligament was divided rightwards near to the greater gastric curvature border until the hepatocolic ligament was divided completely, and then the transverse colon and hepatic flexure were mobilized. The transverse mesocolon was divided downwards caudally until it joined the plan of RRCS dissected previously.
Mobilization of the ascending colon: The ileocecus was drawn upwards and an incision was made at the peritoneal reflection laterally to medially to join the RRCS dissected initially. At least 10 cm of the terminal ileum was mobilized. The ascending colon was mobilized from the ileocecus to the hepatic flexure along the lateral peritoneal attachment and rightwards extended to join the dissected plan of RRCS. Finally, the terminal ileum, right colon, and proximal transverse colon, as well as the hepatic flexure, were fully mobilized.
Anastomosis: A functional end-to-end ileocolic extracorporeal anastomosis between the ileum and the transverse colon was performed through the right subcostal incision using a transverse liner stapler. The length of the incision was about 5-6 cm.
The OERH was defined as lymphadenectomy simultaneously with the ligation of the ileocolic, right colic, middle colic, and gastroepiploic vessels at their origins, mobilizing 10 cm length of the terminal ileum, ascending colon, hepatic flexure, and transverse colon at least 10 cm distal to the tumor. The open surgeries were performed by the same surgery team. The difference between OERH and LERH is that open procedure followed a lateral-to-middle approach while LERH followed a middle-to-lateral one.
SPSS 16.0 was used for all analyses. Fisher’s exact test or χ2 test for parametric value and Student’s t test for continuous value were performed as appropriate. A P value < 0.050 was considered significant.
There were no statistical differences in age, gender distribution, or tumor stage between the two groups (Table 1). Surgical time was significantly longer in the LERH group than in the OERH group. However, blood loss was statistically less in the LERH group. In addition, the mean time to first flatus and the mean time to the start of a liquid diet were shorter in the LERH group. Although there was no statistical difference between the two groups, the mean length of postoperative hospital stay was shorter in the LERH group (10.1 ± 8.3 vs 11.3 ± 7.4, P = 0.328)
| LERH (n = 48) | OERH (n = 57) | P value | |
| Female | 18 (37.5) | 26 (45.6) | 0.401 |
| Age (yr, mean ± SD) | 60.5 ± 11.2 | 64.1 ± 14.2 | 0.153 |
| Tumor stage | 0.644 | ||
| I | 3 (6.3) | 2 (3.5) | |
| II | 24 (50.0) | 33 (57.9) | |
| III | 21 (43.8) | 22 (38.6) |
Five cases (10.4%) in the LERH group were converted to open surgery because of extensive adhesions (n = 3), size of tumor (n = 1), and uncontrolled bleeding (n = 1). No deaths occurred during the surgery in either group. Pathologically, no differences were observed between the two groups in terms of tumor size, length of proximal or distal resection margins, or number of lymph nodes harvested (Table 2).
| LERH(n = 48) | OERH (n = 57) | P value | |
| Operating time (min) | 244.4 (84.8) | 170.7 (49.7) | < 0.001 |
| Estimated blood loss (mL) | 111.7 (127.8) | 170.2 (49.7) | 0.023 |
| Time to flatus (d) | 3.0 (1.6) | 3.7 (1.3) | 0.019 |
| Time to diet (d) | 4.2 (1.4) | 5.0 (1.2) | 0.005 |
| Postoperative hospital stay (d) | 10.1 (8.3) | 11.3 (7.4) | 0.328 |
| Tumor size(cm) | 4.5 (1.4) | 5.1 (2.2) | 0.124 |
| Proximal resection margin (cm) | 17.3 (3.6) | 17.2 (3.3) | 0.904 |
| Distal resection margin (cm) | 13.5 (2.9) | 14.8 (4.2) | 0.080 |
| No. of lymph nodes harvested | 17.1 (10.0) | 17.3 (11.1) | 0.910 |
Postoperative complications occurred in 6 (12.5%) patients in the LERH group vs 11 (19.3%) in the OERH group (P = 0.346). One case with a major complication (defined as any complication requiring reoperation) was observed in the LERH group (anastomotic leak) and one case in the OERH group (bleeding). Minor complications in 5 cases of the LERH group and in 10 cases of the OERH group were treated successfully and conservatively (Table 3). No deaths associated with complications were observed in either group.
| LERH (n = 48) | OERH (n = 57) | P value | |
| Complications | 6 (12.5) | 11 (19.3) | 0.346 |
| Wound infection | 1 (2.1) | 3 (5.3) | |
| Ileus | 1 (2.1) | 3 (5.3) | |
| Anastomotic leak | 2 (4.2) | 2 (3.5) | |
| Abdominal infection | 1 (2.1) | 1 (1.8) | |
| Anastomotic stenosis | 1 (2.1) | 1 (1.8) | |
| Hemorrhage | 0 (0.0) | 1 (1.8) |
No patients were lost to follow-up in either group. There were no differences in the median follow-up time (14.6 mo vs 16.6 mo, P = 0.227) or cancer recurrence rate (8.6% vs 9.1%, P = 0.335) between the two groups. Three cancer related deaths were observed in the LERH group and four in the OERH group.
The laparoscopic approach for hepatic flexure or proximal transverse colon cancer is challenging, requiring advanced laparoscopic skills as well as comprehensive anatomical knowledge of minimally-invasive surgery. In the present study, there were no significant differences in terms of tumor stage distribution, tumor size, length of proximal and distal resection margin, number of lymph nodes harvested, postoperative hospital stay, or postoperative complications between the two groups. Reduced blood loss during operation and faster recovery of gastrointestinal function after surgery were observed in the LERH group.
The main challenges of performing LERH include the lack of tactile sensation, as well as the complicated anatomical structures at the relevant areas. Thus, effective landmarks guiding the dissection are crucial in order to keep the dissection secure. SMV and the RRCS served as the landmark and surgical plan, respectively, in this study. Meanwhile, the medial-to-lateral, caudal-to-cranial, and posterior-to-anterior procedures were followed to make the exposure and location adequate and precise, the dissection distinct, and the operation easier[9,10]. The key points of the LERH procedure include: (1) identifying the proper anatomical landmarks; (2) locating relevant blood vessels accurately and ligating the vessels at their origins; (3) following the proper surgical plan; and (4) maintaining the integrity of the fascia.
In our center, the SMV was considered the most important landmark of LERH using a medial-to-lateral method, which could guide the vascular dissection and lymphadenectomy, as well as be considered the middle boundary of the surgical plan (RRCS). The unique blue bulge of the SMV and the invariable ileocolic vessels makes the intersection of the SMV and the ileocolic vein easily distinguishable when the transverse colon and the ileocecal junction were towed cranially and laterally, respectively. Consequently, the LERH approach was started from the intersection. An incision was made on the lower boundary of the ileocolic vein in order to enter the RRCS. Next, with skeletonizing of the ventral aspect of the SMV caudally to cranially, vessels originating from the SMV or SMA (ileocolic, right colic, and middle colic vessels) were exposed and ligated one-by-one just at their origins. The spatial relationship between the ileocolic artery and vein was used to identify the spatial relationship between the SMV and the SMA, which can be used to understand the arteriovenous spatial relationship of the right and middle colic vessels.
The RRCS is the fusional interfascial space between the mesocolon and pre-renal fascia that is nearly exempt of nerves and blood vessels and was the natural surgical plan of LERH. Keeping the operation in this space was an effective way to preserve the intact mesocolon and pre-renal fascia which could protect the ureter, gonadal vessels, duodenum, and pancreas, as well as reduce blood loss during surgery. The most important thing is that separation of the mesocolon from the pre-renal fascia and D3 lymphadenectomy of the supplying arteries and draining veins will mostly likely ensure maximal harvest of the regional lymph nodes, which is associated with improved survival[11-14].
Compared with OERH, LERH also showed short-term advantages in reduced blood loss and faster recuperation of the gastrointestinal function, although its operation time was significantly longer. Furthermore, due to Chinese culture, most Chinese patients tended to leave hospital after stitches have been taken out, which may explain why there was no statistical difference in postoperative hospital stay between the two groups, which is uncommon in Western studies. This may also contribute to the slightly longer postoperative hospital stay compared with other laparoscopic colectomies[15]. Compared with previous findings, the mean time to liquid diet was longer in our series, partially due to the fast-track approach not being routinely used in our department[16]. The pathologic results between the two groups were similar and complied with the criteria of oncologic resection.
Five cases in the LERH group were converted to open surgery, mainly because of the presence of large and invasive cancer. For conversion cases, previous studies have shown there was an increase in operating time, hospital stay, morbidity, and and poor short-term survival[2,17,18]. The report also showed that locally advanced tumors were an independent risk factor for conversion[19].
In our study, about 43.8% of patients were in stage III. All conversional cases were in stage III, with the mean diameter of the tumors being 6.2 cm. All surgeries in the present study were performed by an experienced laparoscopic team in which every surgeon had been reached the stable level of the learning curve[20]. This could be helpful in relatively decreasing our conversion rate.
The main limitations of this study included retrospective design, single-center site, relatively short median follow-up time, and small sample size. However, this study showed that for an advanced tumor located at the hepatic flexure or proximal transverse colon, LERH with D3 lymphadenectomy using a medial-to-lateral approach seems to be both safe and feasible when the superior mesenteric vein serves as the main anatomical landmark and the right retrocolic space severed as the surgical plan.
The main factor that restricts the application of laparoscopic extended right hemicolectomy (LERH) with D3 lymphadenectomy for colon cancer was that it was technologically demanding in achieving a very high tie of the middle colic vessels and right gastroepiploic vessels at their origins laparoscopically.
Due to being highly technically demanding and having a complex vascular anatomic relation, LERH with D3 lymphadenectomy was performed cautiously. With the improvements in surgical techniques, surgeon experience, and renewal instruments, extended right hemicolectomy with D3 lymphadenectomy was subsequently attempted in laparoscopic surgery.
LERH with D3 lymphadenectomy for tumors located at the hepatic flexure or within 10 cm distal to it is challenging, mainly because of the technical difficulty in dissecting the lymph nodes around the origin of the middle colic vessels and right gastroepiploic vessel, and the handling of the intricacies of venous anatomy at the gastrocolic trunk of Henle. LERH with D3 lymphadenectomy using a medial-to-lateral approach seems to be both safe and feasible when the superior mesenteric vein serves as the main anatomical landmark for lymphadenectomy and the right retrocolic space severed as the surgical plan.
Based on the experience, LERH with D3 lymphadenectomy should be both easy and safe when the superior mesenteric vein is served as the main anatomical landmark for lymphadenectomy and the right retrocolic space severed as the surgical plan.
Tumors located at or within 10 cm distal to the hepatic flexure have an increased risk of infra-pyloric lymph node metastasis. Extended right hemicolectomy with D3 lymphadenectomy, which was defined as the ileocolic, right colic, middle colic, and right gastroepiploic vessels, were ligated at their origins in sequence and bowel mobilization was performed at least 10 cm from the margins of the tumor.
This manuscript is about the feasibility of laparoscopic extended right hemicolectomy with D3 lymphadenectomy. The authors concluded that this technique is feasible, although the final long-term outcome is not available due to a short follow-up period.
P- Reviewer: Pyo H S- Editor: Qi Y L- Editor: Rutherford A E- Editor: Ma S
| 1. | Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1681] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 2. | Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW, Hellinger M, Flanagan R, Peters W, Nelson H. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007;246:655-62; discussion 662-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 802] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 3. | Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 965] [Cited by in RCA: 1057] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 4. | Bai HL, Chen B, Zhou Y, Wu XT. Five-year long-term outcomes of laparoscopic surgery for colon cancer. World J Gastroenterol. 2010;16:4992-4997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Toyota S, Ohta H, Anazawa S. Rationale for extent of lymph node dissection for right colon cancer. Dis Colon Rectum. 1995;38:705-711. [PubMed] |
| 6. | Block GE, Moossa AR. Operative colorectal surgery. Philadelphia: WB Saunders Pub 1994; 143-145. |
| 7. | Liang YZ, Yu J, Zhang C, Wang YN, Cheng X, Huang F, Li GX. [Construction and application of evaluation system of laparoscopic colorectal surgery based on clinical data mining]. Zhonghua Wei Chang Wai Ke Zazhi. 2010;13:741-744. [PubMed] |
| 8. | Ignjatovic D, Spasojevic M, Stimec B. Can the gastrocolic trunk of Henle serve as an anatomical landmark in laparoscopic right colectomy? A postmortem anatomical study. Am J Surg. 2010;199:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Poon JT, Law WL, Fan JK, Lo OS. Impact of the standardized medial-to-lateral approach on outcome of laparoscopic colorectal resection. World J Surg. 2009;33:2177-2182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Lee SD, Lim SB. D3 lymphadenectomy using a medial to lateral approach for curable right-sided colon cancer. Int J Colorectal Dis. 2009;24:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 780] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 12. | Vather R, Sammour T, Zargar-Shoshtari K, Metcalf P, Connolly A, Hill A. Lymph node examination as a predictor of long-term outcome in Dukes B colon cancer. Int J Colorectal Dis. 2009;24:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009;11:354-64; discussion 364-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1106] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 14. | West NP, Morris EJ, Rotimi O, Cairns A, Finan PJ, Quirke P. Pathology grading of colon cancer surgical resection and its association with survival: a retrospective observational study. Lancet Oncol. 2008;9:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 321] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 15. | Akiyoshi T, Kuroyanagi H, Fujimoto Y, Konishi T, Ueno M, Oya M, Yamaguchi T. Short-term outcomes of laparoscopic colectomy for transverse colon cancer. J Gastrointest Surg. 2010;14:818-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Tsikitis VL, Holubar SD, Dozois EJ, Cima RR, Pemberton JH, Larson DW. Advantages of fast-track recovery after laparoscopic right hemicolectomy for colon cancer. Surg Endosc. 2010;24:1911-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Hewett PJ, Allardyce RA, Bagshaw PF, Frampton CM, Frizelle FA, Rieger NA, Smith JS, Solomon MJ, Stephens JH, Stevenson AR. Short-term outcomes of the Australasian randomized clinical study comparing laparoscopic and conventional open surgical treatments for colon cancer: the ALCCaS trial. Ann Surg. 2008;248:728-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 272] [Article Influence: 16.0] [Reference Citation Analysis (1)] |
| 18. | Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2298] [Article Influence: 114.9] [Reference Citation Analysis (0)] |
| 19. | Thorpe H, Jayne DG, Guillou PJ, Quirke P, Copeland J, Brown JM. Patient factors influencing conversion from laparoscopically assisted to open surgery for colorectal cancer. Br J Surg. 2008;95:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Akiyoshi T, Kuroyanagi H, Oya M, Konishi T, Fukuda M, Fujimoto Y, Ueno M, Yamaguchi T, Muto T. Safety of laparoscopic total mesorectal excision for low rectal cancer with preoperative chemoradiation therapy. J Gastrointest Surg. 2009;13:521-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |