Published online Aug 14, 2014. doi: 10.3748/wjg.v20.i30.10495
Revised: March 29, 2014
Accepted: May 12, 2014
Published online: August 14, 2014
Processing time: 189 Days and 17.8 Hours
AIM: To compare endoscopic retrograde cholangio-pancreatography (ERCP), intraductal ultrasound (IDUS), endosonography (EUS), endoscopic transpapillary forceps biopsies (ETP) and computed tomography (CT) with respect to diagnosing malignant bile duct strictures.
METHODS: A patient cohort with bile duct strictures of unknown etiology was examined by ERCP and IDUS, ETP, EUS, and CT. The sensitivity, specificity, and accuracy rates of the diagnostic procedures were calculated based on the definite diagnoses proved by histopathology or long-term follow-up in those patients who did not undergo surgery. For each of the diagnostic measures, the sensitivity, specificity, and accuracy rates were calculated. In all cases, the gold standard was the histopathologic staging of specimens or long-term follow-up of at least 12 mo. A comparison of the accuracy rates between the localization of strictures was performed by using the Mann-Whitney U-test and the χ2 test as appropriate. A comparison of the accuracy rates between the diagnostic procedures was performed by using the McNemar’s test. Differences were considered statistically significant if P < 0.05.
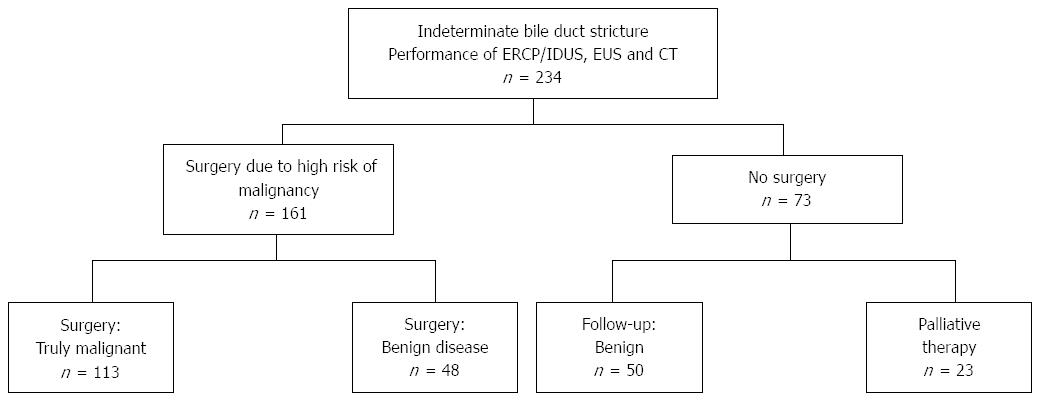
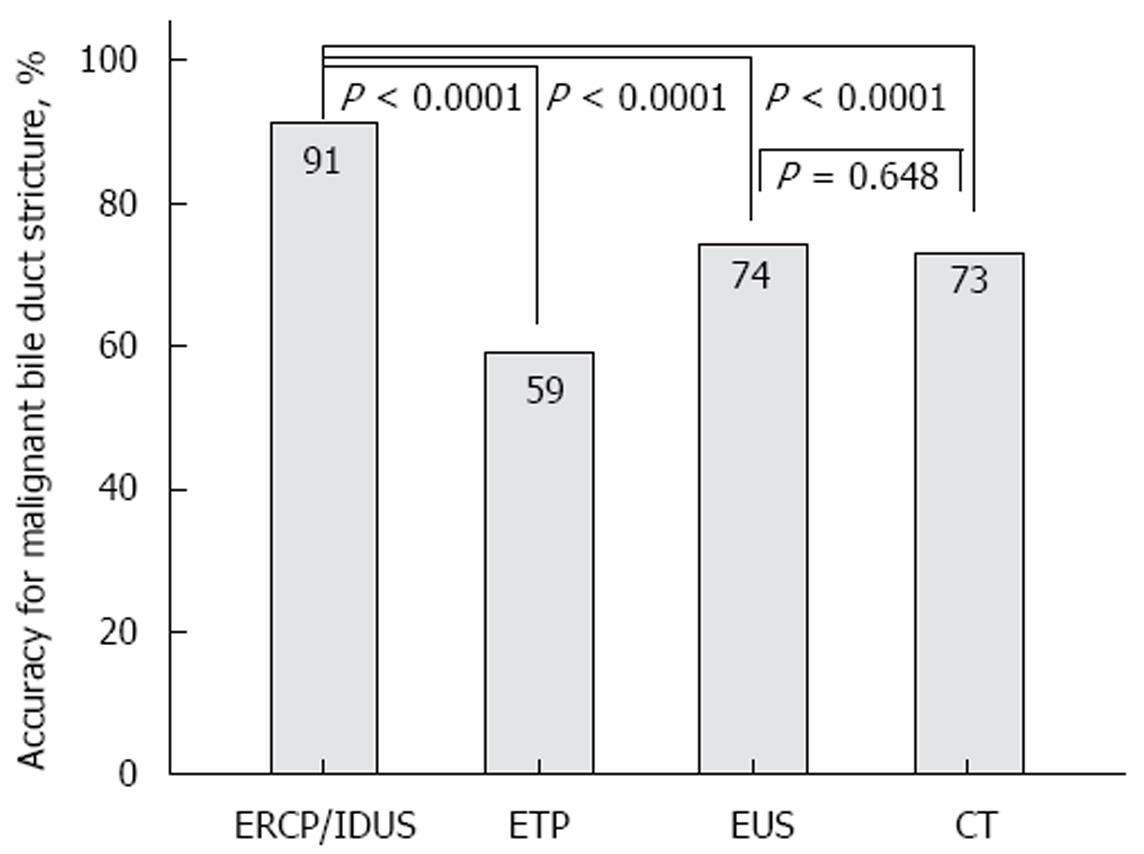
RESULTS: A total of 234 patients (127 males, 107 females, median age 64, range 20-90 years) with indeterminate bile duct strictures were included. A total of 161 patients underwent operative exploration; thus, a surgical histopathological correlation was available for those patients. A total of 113 patients had malignant disease proven by surgery; in 48 patients, benign disease was surgically found. In these patients, the decision for surgical exploration was made due to the suspicion of malignant disease in multimodal diagnostics (ERCP, CT, or EUS). Fifty patients had a benign diagnosis and were followed by a surveillance protocol with a follow-up of at least 12 mo; the median follow-up was 34 mo. Twenty-three patients had extended malignant disease, and thus were considered palliative. A comparison of the different diagnostic tools for detecting bile duct malignancy resulted in accuracy rates of 91% (ERCP/IDUS), 59% (ETP), 92% (IDUS + ETP), 74% (EUS), and 73% (CT), respectively. In the subgroup analysis, the accuracy rates (%, ERCP + IDUS/ETP/IDUS + ETP; EUS; CT) for each tumor entity were as follows: cholangiocellular carcinoma: 92%/74%/92%/70%/79%; pancreatic carcinoma: 90%/68%/90%/81%/76%; and ampullary carcinoma: 88%/90%/90%/76%/76%. The detection rate of malignancy by ERCP/IDUS was superior to ETP (91% vs 59%, P < 0.0001), EUS (91% vs 74%, P < 0.0001) and CT (91% vs 73%, P < 0.0001); EUS was comparable to CT (74% vs 73%, P = 0.649). When analyzing accuracy rates with regard to localization of the bile duct stenosis, the accuracy rate of EUS for proximal vs distal stenosis was significantly higher for distal stenosis (79% vs 57%, P < 0.0001).
CONCLUSION: ERCP/IDUS is superior to EUS and CT in providing accurate diagnoses of bile duct strictures of uncertain etiology. Multimodal diagnostics is recommended.
Core tip: A cohort of 234 patients with bile duct strictures of unknown etiology was examined to determine the diagnostic efficiency of endoscopic retrograde cholangio-pancreatography (ERCP), intraductal ultrasound (IDUS), endosonography (EUS), endoscopic transpapillary forceps biopsies (ETP) and computed tomography (CT). The detection rate of malignancy by ERCP/IDUS was superior to ETP (91% vs 59%, P < 0.0001), EUS (91% vs 74%, P < 0.0001), and CT (91% vs 73%, P < 0.0001); EUS was comparable to CT (74% vs 73%, P = 0.649). Taking the findings of our study into account, we suggest that ERCP/IDUS is superior to EUS and CT in providing accurate diagnoses of bile duct strictures of uncertain etiology, thus allowing for adequate further clinical management.
- Citation: Heinzow HS, Kammerer S, Rammes C, Wessling J, Domagk D, Meister T. Comparative analysis of ERCP, IDUS, EUS and CT in predicting malignant bile duct strictures. World J Gastroenterol 2014; 20(30): 10495-10503
- URL: https://www.wjgnet.com/1007-9327/full/v20/i30/10495.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i30.10495
There are no definite guidelines for the management and diagnosis of biliary strictures of indeterminate etiology to date. Various endoscopic and radiographic imaging modalities, such as endoscopic retrograde cholangio-pancreatography (ERCP), intraductal ultrasound (IDUS), endosonography (EUS), and computed tomography (CT), are available and compete with each other[1-3]. Due to its widespread availability, CT is commonly performed on patients with indeterminate strictures of the biliary tract[4]. In a small non-comparative study with 58 patients, the overall accuracy of CT cholangiography for the diagnosis of biliary obstruction was 89.8%[5]. However, a small comparative study of 40 patients only revealed a sensitivity and specificity rate for CT of 77% and 63%, respectively[6]. With respect to vascular infiltration and lymph node invasiveness, CT was found to be favorable compared with the other techniques[7].
ERCP has significant diagnostic and therapeutic value in bile duct strictures in which there is an additional need for intervention and specimen acquisition occurs[8]. Yet, trials on biliary brush cytology or forceps biopsies have described poor results, thus indicating the need for further studies analyzing appropriate tissue sampling[9,10]. The use of IDUS performed during ERCP enables the investigator to obtain additional information concerning the bile duct wall and the periductal tissue[11] and has demonstrated its tremendous clinical importance by estimating the extent of potentially cancerous infiltration[12]. EUS is capable of visualizing the local extent of tumors, and the status of regional lymph nodes has shown promising results for the detection of malignant stenoses of the distal bile duct with a sensitivity of up to 96%[13]. Its role for the evaluation of proximal bile duct lesions still remains uncertain[14,15]. In contrast, cholangioscopy is a fairly new and promising diagnostic technique for use on patients with indeterminate pancreaticobiliary pathology. However, so far, only limited data evaluating the diagnostic impact of cholangioscopy are available, and they show a sensitivity of 71% up to 89%[16,17].
Comparative studies of the various imaging studies with only limited patient numbers are available so far. Therefore, we aimed to evaluate the diagnostic yield of IDUS, EUS, and CT in a large patient cohort scheduled for ERCP with IDUS, CT, and EUS due to indeterminate strictures or filling defects of the common bile duct. The objectives of this study were to observe the diagnostic quality of the imaging procedures in differentiating malignant from benign strictures. A postoperative diagnosis based on a histological assessment of surgical resection specimens or alternatively a follow-up period of at least 12 mo was considered the gold standard.
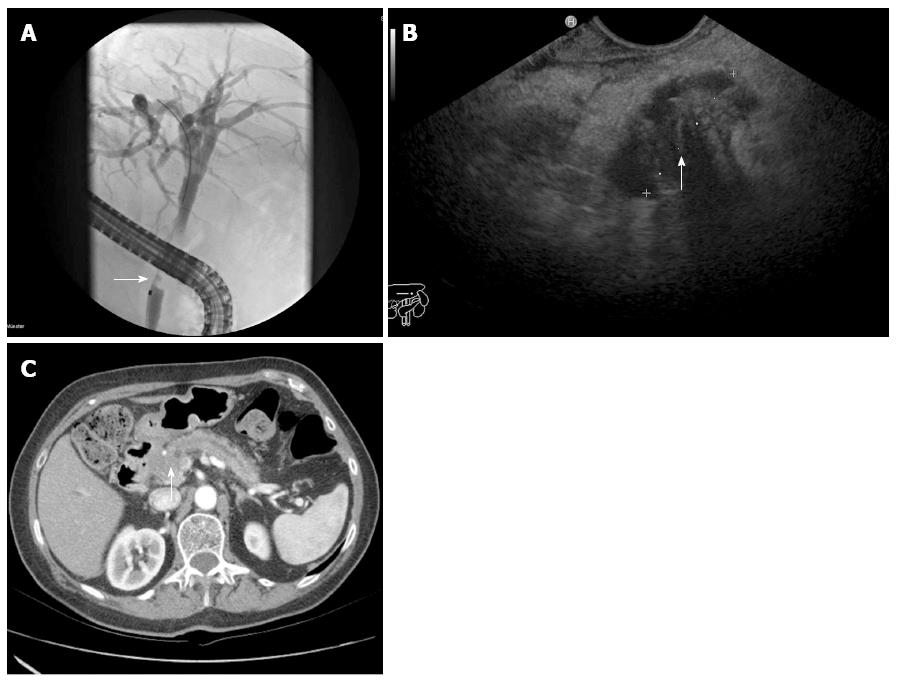
This retrospective analysis was performed on 234 patients. We included all patients with jaundice of unknown etiology and suspected biliary stricture. Patients did not have a history of previous biliary surgery. According to the study protocol, patients underwent diagnostic laparotomy; alternatively, a long follow-up of at least 12 mo was accepted. We examined patients having undergone ERCP, IDUS, EUS, and CT at Münster University Hospital from 2002 to 2010. Figure 1 demonstrates the identification of a malignant bile duct stricture in the three mentioned diagnostic imaging modalities. The presence of a uniform (homogeneous) echo of the tumor with smooth margins and no signs of infiltrative processes were considered as benign characteristics on IDUS or EUS. Signs of malignancy involved an inhomogeneous echo of the tumor with infiltration of the adjacent tissue. A double-duct-sign was also considered to be a malignant characteristic. The characterization of a malignant biliary obstruction by ERCP followed the known criteria: irregularly shaped intraluminal filling defect of the bile duct, promptly and irregularly altered shape of a distal narrow segment, and a proportionally dilated biliary tree proximally. According to the established criteria of bile duct malignancy, CT signs range from the eccentric thickness of the bile duct wall, leading to stenosis of the bile duct lumen and proximal dilatation of the biliary tract, to tumor formations primarily with hypodensic density aspects. Signs of invasion into neighboring tissue, blurred borders, tumoral compression, or blunt termination of biliary tracts and vessels and lymphogenic or hematogenic tumor spread are usually characteristic signs of malignancy. In contrast, the absence of neighboring lymph node involvement and metastases and signs of progressive narrowing and sharply defined tissue borders or other causes of extrahepatic cholestasis like bile duct stones suggests a benign tumor.
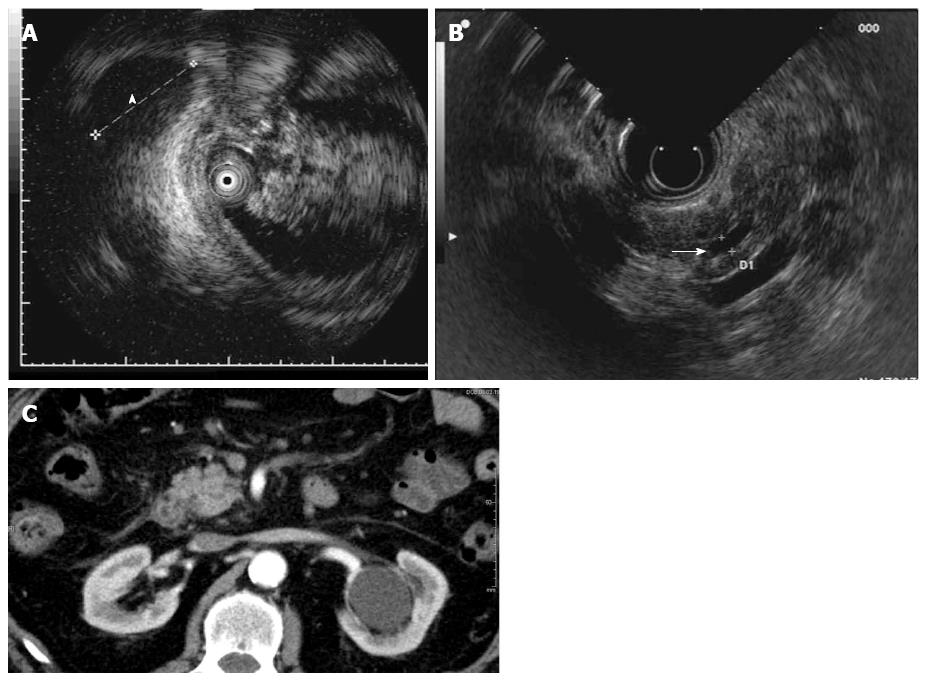
In Figure 2, malignancy could only be observed through IDUS (Figure 2B). This pancreatic tumor could not be visualized by EUS or CT (Figure 2B and C).
The clinical records of patients were collected and carefully analyzed. Baseline characteristics, diagnostic techniques employed, and histopathology were retrieved, as shown in Table 1.
| Variable | Value |
| Patients, n | 234 |
| Median age, yr (IQR) | 64 (54-73) |
| Age range, yr | 20-90 |
| Sex (M/F) | 127/107 |
| ERCP + IDUS performed, n | 234 |
| ETP performed | 194 (83) |
| EUS performed, n | 234 |
| CT performed, n | 234 |
| Median follow-up, mo (IQR) | 34 (19-52) |
| Follow-up range, mo | 12-100 |
| Procedures, n | |
| Clinical follow-up | 50 |
| Surgery | 161 |
| Palliative therapy | 23 |
| Localization of stricture (CBD) | |
| Proximal third | 28 (12) |
| Middle third | 21 (9) |
| Distal third | 185 (79) |
| Final diagnosis (n) | |
| Benign disease | 98 |
| Normal bile duct | 10 |
| Papillitis | 15 |
| Ampullary adenoma | 8 |
| Cholangitis | 10 |
| PSC | 3 |
| Mirizzi syndrome | 3 |
| Choledocholithiasis | 2 |
| Pancreatitis | 39 |
| Pseudocyst | 2 |
| Choledochal adenofibroma | 1 |
| IPMN | 2 |
| Choledochal cyst | 1 |
| Pancreatic cystadenoma | 1 |
| Caroli’s syndrome | 1 |
| Malignant disease | 136 |
| Cholangiocarcinoma | 56 |
| Pancreatic carcinoma | 55 |
| Ampullary carcinoma | 19 |
| Gallbladder carcinoma | 3 |
| Hepatocellular carcinoma | 3 |
The following patients were excluded from this study: patients with prior biliary surgery; patients not having been histopathologically controlled by surgery, forceps biopsy, or with an endoscopic follow-up of less than 1 year in suspected benign biliary stenosis.
All patients enrolled in the present study underwent ERCP with the additional application of IDUS, EUS, and CT. The individual procedure was performed after written informed consent had been obtained from the patients or related persons for the endoscopic procedures. All endoscopic maneuvers were executed by highly experienced investigators according to the generally accepted guidelines with an ERCP case volume above 200/year[18]. The ERCP and IDUS procedures were performed under fluoroscopic guidance using a side-viewing duodenoscope (Olympus TJF 160, Olympus, Ltd., Tokyo, Japan).
All endoscopic procedures were performed under conscious sedation (propofol combined with pethidine) according to the German guidelines[19]. For IDUS, a 6F or 8F ultrasound miniprobe was employed with a radial scanner of 15-20 MHz at the tip of the probe (Aloka Co., Tokyo, Japan). Thus, a radial real-time image of 360° view was possible for optimum investigation of the area surrounding the probe. The visible depth was approximately 20 mm with a resolution of up to 0.1 mm. A total of 194 procedures included additional transpapillary forceps biopsies (n = 4-8 specimens) from the biliary strictures obtained using straight or angled endoscopic forceps (MTW Endoscopy, Wesel, Germany). If insertion of the forceps into the stricture was not feasible, biopsies were retrieved from the distal margins of the bile duct stenosis[20]. Patients who were eligible for surgery were transferred to the Department of General and Visceral Surgery, Münster University Hospital.
The EUS procedures were performed using a radial or linear echoendoscope with up to a 10 MHz frequency (EG-3670 URK and EG-3870 UTK, Pentax Medical/Hitachi Medical Systems, Tokyo, Japan) with the patient lying in the left lateral position.
CT examinations were performed using multi-slice CT scanners (16- and 64-slice scanner, Siemens AG, Forchheim, Germany) using a standardized tube voltage at 120 kV with a detector configuration of 16 mm × 0.75 mm and 32 mm × 0.6 mm, respectively, and a pitch of 0.75. In the majority of cases, the images were reconstructed with a slice thickness of 1.0 mm (reconstruction increment of 0.8) with a soft tissue reconstruction kernel (B30). A volume of 120-150 mL intravenous contrast agent (Ultravist 370®, Bayer Schering Pharma AG, Leverkusen, Germany) was applied at a constant flow rate of 3-4 mL/s depending on the acquired phases of contrast enhancement. Dual-phasic images were acquired with a scan delay of 45 s (late arterial, as obtained by test- or care bolus technique) and 75 s (portal-venous). The imaging protocol did not differ from the standardized protocol used in clinical routines.
For image assessment, the reconstructed images were assessed on a standard workstation (Syngo, Siemens AG, Forchheim, Germany) with cine mode and multiplanar reformation capabilities. In 46 cases, image analysis was only possible on hard copies. Image data were analyzed independently by two radiologists, blinded to the final diagnosis, who evaluated the bile duct stricture characteristics (benign or malignant, stricture location) and the involvement of regional lymph nodes or distant metastases in case of malignancy. Furthermore, tumor size and localization was assessed.
All patients with suspected benign strictures had routine follow-up the day following intervention and every three months for the first year, every six months the second year, and annually up to the third year after intervention at our department. The follow-up procedure was performed according to a surveillance protocol including laboratory testing and abdominal ultrasound. In cases of biliary plastic stent insertion, the follow-up procedures included ERCP and possible biliary plastic stent changing.
Data were analyzed using SPSS 17.0 (Chicago, IL, United States). The results were expressed as medians (interquartile range) and ranges. For each of the diagnostic measures, the sensitivity, specificity, and accuracy rates were calculated. In all cases, the gold standard was the histopathologic staging of specimens. A comparison of the accuracy rates between the localization of the stricture was performed by using the Mann-Whitney U-test and the χ2 test as appropriate. A comparison of the accuracy rates between the diagnostic procedures was performed by using McNemar’s test. Differences were considered statistically significant if P < 0.05.
A total of 234 patients (127 males, 107 females, median age 64, range 20-90 years) with indeterminate bile duct strictures were included. Fifty patients received a benign diagnosis based on imaging modalities without having undergone surgery and were instead followed by a surveillance protocol with a follow-up of at least 12 mo; the median follow-up was 34 mo (Table 1). In twenty-three patients, extended tumor disease was found using the imaging modalities, with consecutive palliative treatment. The remaining 161 patients with a suspected malignancy based on at least one of the analyzed imaging modalities were referred to the department of surgery for operative exploration; thus, a surgical histopathological correlation was available for those patients. A total of 113 patients had malignant disease proven by surgery, whereas in 48 patients, benign disease was found during surgery. A flow chart showing the enrollment of the study patients is presented in Figure 3.
A comparison of the different diagnostic tools for detecting bile duct malignancy resulted in accuracy rates of 91% (ERCP/IDUS), 59% (ETP), 92% (IDUS + ETP), 74% (EUS), and 73% (CT), respectively. In the subgroup analysis, the accuracy rates (%, ERCP + IDUS/ETP/IDUS + ETP; EUS; CT) for each tumor entity were as follows: cholangiocellular carcinoma: 92%/74%/92%/70%/79%; pancreatic carcinoma: 90%/68%/90%/81%/76%; and ampullary carcinoma: 88%/90%/90%/76%/76% (Table 2). The detection of malignancy by ERCP/IDUS was superior to ETP (91% vs 59%, P < 0.0001), EUS (91% vs 74%, P < 0.0001), and CT (91% vs 73%, P < 0.0001). The accuracy rate of EUS was comparable to CT (74% vs 73%, P = 0.649) (Figure 4). When analyzing accuracy rates with regard to localization of the bile duct stenosis, the accuracy rate for proximal vs distal stenosis did not differ statistically for ETCP/IDUS, EPT and CT, whereas by EUS, the detection rate of malignancy was significantly higher for distal stenosis (79% vs 57%, P < 0.0001) (Table 3).
| Index | Diagnostic parameter | ||||||||||||||
| ERCP/IDUS | ETP | IDUS + ETP | EUS | CT | |||||||||||
| 95%CI | 95%CI | 95%CI | 95%CI | 95%CI | |||||||||||
| All patients | |||||||||||||||
| Sensitivity | 93% | 88-97 | 126/136 | 37% | 29-45 | 47/127 | 94% | 90-98 | 128/136 | 71% | 63-78 | 96/136 | 67% | 59-75 | 91/136 |
| Specificity | 89% | 83-95 | 87/98 | 100% | NA | 67/67 | 89% | 83-95 | 87/98 | 78% | 69-86 | 76/98 | 82% | 74-89 | 80/98 |
| Accuracy | 91% | 87-95 | 213/234 | 59% | 52-66 | 114/194 | 92% | 88-95 | 215/234 | 74% | 68-79 | 172/234 | 73% | 67-79 | 171/234 |
| Subgroup only considering CCC as a malignancy | |||||||||||||||
| Sensitivity | 96% | 92-100 | 54/56 | 42% | 29-55 | 23/55 | 96% | 92-100 | 54/56 | 57% | 44-70 | 32/56 | 73% | 62-85 | 41/56 |
| Specificity | 89% | 83-95 | 87/98 | 100% | NA | 67/67 | 89% | 83-95 | 87/98 | 78% | 69-86 | 76/98 | 82% | 74-89 | 80/98 |
| Accuracy | 92% | 87-96 | 141/154 | 74% | 66-81 | 90/122 | 92% | 87-96 | 141/154 | 70% | 63-77 | 108/154 | 79% | 72-85 | 121/154 |
| Subgroup only considering pancreatic carcinoma as a malignancy | |||||||||||||||
| Sensitivity | 93% | 86-100 | 51/55 | 26% | 14-38 | 13/50 | 93% | 86-100 | 51/55 | 87% | 78-96 | 48/55 | 65% | 53-78 | 36/55 |
| Specificity | 89% | 83-95 | 87/98 | 100% | NA | 67/67 | 89% | 83-95 | 87/98 | 78% | 69-86 | 76/98 | 82% | 74-89 | 80/98 |
| Accuracy | 90% | 85-95 | 138/153 | 68% | 60-77 | 80/117 | 90% | 85-95 | 138/153 | 81% | 75-87 | 124/153 | 76% | 69-78 | 116/153 |
| Subgroup only considering ampullary carcinoma as a malignancy | |||||||||||||||
| Sensitivity | 84% | 68-100 | 16/19 | 53% | 29-77 | 9/8 | 95% | 85-100 | 18/19 | 68% | 48-89 | 13/19 | 47% | 25-70 | 9/19 |
| Specificity | 89% | 83-95 | 87/98 | 100% | NA | 67/67 | 89% | 83-95 | 87/98 | 78% | 69-86 | 76/98 | 82% | 74-89 | 80/98 |
| Accuracy | 88% | 82-94 | 103/117 | 90% | 84-97 | 76/84 | 90% | 84-95 | 105/117 | 76% | 68-84 | 89/117 | 76% | 68-84 | 89/117 |
| Method | Stenosis (localization) | Accuracy | 95%CI | P value |
| (prox. vs distal) | ||||
| IDUS | Proximal | 28 (100) | 100-100 | 0.419 |
| Distal | 165 (89) | 85-94 | ||
| ETP | Proximal | 13 (46) | 28-65 | 0.275 |
| Distal | 93 (62) | 55-70 | ||
| IDUS + ETP | Proximal | 28 (100) | 100-100 | 0.505 |
| Distal | 167 (90) | 86-95 | ||
| EUS | Proximal | 16 (57) | 39-75 | < 0.0001 |
| Distal | 147 (79) | 74-85 | ||
| CT | Proximal | 25 (89) | 79-100 | 0.09 |
| Distal | 132 (71) | 65-78 |
Newly diagnosed bile duct strictures must usually be considered malignant until proven otherwise in patients with no history of previous biliary surgery. Various endoscopic and radiological imaging modalities of the bile tract are available, thus often leading to extensive use of the modalities. However, because of increasing health care expenses, choosing the appropriate imaging technique from the wide range of endoscopic and radiological modalities available seems crucial. For this purpose, doctors should have information about each technique and when it is best to use each of them.
A couple of previously published studies have been devoted to a comparison of the various imaging modalities, leading to an ongoing debate about which diagnostic tool is best for detecting malignancy in bile duct strictures of uncertain etiology. In a prospective study conducted by Rösch and colleagues in 2002[6], ERCP, MRCP, CT, and EUS were compared with respect to the diagnostic accuracy of biliary strictures. They found sensitivity and specificity rates for ERCP/MRCP/CT/EUS of 85%/75%, 85%/71%, 77%/63%, and 79%/62%, respectively. In another prospective study performed by Domagk et al[7], ERCP/IDUS, EUS, multidetector-row computed tomography, and magnetic resonance imaging were prospectively compared, with ERCP/IDUS found to be superior to all other imaging techniques in assessing malignant bile duct strictures. However, these studies were conducted with only a limited number of patients. In a multimodal approach, we retrospectively analyzed the sensitivity and specificity for differentiating between malignant and benign strictures in a group of 234 patients. Differentiation between malignant and benign strictures was best performed by the application of ERCP/IDUS, an approach that was significant superiority to CT and EUS. A comparison of the different diagnostic tools for detecting bile duct malignancy resulted in accuracy rates of 91% (ERCP/IDUS), 59% (ETP), 92% (IDUS + ETP), 74% (EUS), and 73% (CT), respectively (Table 2).
In terms of tumor entity, IDUS demonstrated the best results for cholangiocellular carcinoma, with accuracy and sensitivity rates of 92% and 96%, respectively (Table 2). The findings of this present study are in agreement with the hypothesis that the accuracy of ERCP/IDUS is superior to other imaging investigations[21]. However, due to the minor ultrasonic penetration depth, IDUS tends to understage tumors of the pancreaticobiliary tract and does not seem useful in extensive lymph node staging[12,22]. This calls for a multimodal approach, which in our experience is common clinical practice. However, no official guidelines exist that describe which diagnostic tool should preferably be used for each tumor entity[23]. According to our and previous results[7,12], the combination of ERCP and IDUS seems to be the most accurate imaging modality for diagnosing biliary strictures. An imaging modality, such as EUS or CT, should be added in the case of preoperative evaluation to detect lymph node invasion or distant metastasis[6,24]. In our study, 48 patients referred to the department of surgery for operative exploration due to a suspected malignancy were found to have a benign stricture (Figure 3). In 39 of these patients, malignancy was suspected by ERCP, EUS, and/or CT. In the remaining 9 patients, malignancy was either clinically suspected or a premalignant condition was given: 2 patients with ampullary adenoma underwent papillectomy due to premalignant potential, one patient with PSC received a liver transplant due to extended disease, one patient with chronic pancreatitis and weight loss underwent surgery, 5 patients with indeterminate pancreatic cystic lesions underwent surgery due to malignant potential, and one patient with Caroli disease underwent biliary surgery due to recurrent cholangitis. To avoid unnecessary surgery, a targeted use of diagnostic procedures is mandatory in which the localization of the stenosis and the suspected tumor entity have to be taken into account. When analyzing accuracy rates with regard to localization of the bile duct stenosis, the accuracy rate for proximal vs distal stenosis did not differ statistically for ERCP/IDUS and CT, whereas with EUS, the detection rate of malignancy was significantly higher for distal stenosis (79% vs 57%, P < 0.0001) (Table 3). It needs to be mentioned that preoperative EUS is often insufficient and highly dependent on the investigator′s expertise. Thus, the use of radiological imaging modalities such as CT scans adequately mirrors clinical reality[23].
ERCP/IDUS is superior to EUS and CT in providing accurate diagnoses of bile duct strictures of uncertain etiology, thus allowing for adequate further clinical management. However, one should bear in mind that ERCP complemented with IDUS and EUS are invasive diagnostic procedures compared with CT. Acute pancreatitis is considered a relevant major complication in approximately 3% of cases following endoscopic retrograde cholangiopancreatography[25]. Moreover, IDUS is considered to be a further independent risk factor of post-ERCP pancreatitis. EUS, however, has a complication rate of only 0.03% up to 0.15%[26]. Thus, EUS can be considered a very safe diagnostic imaging tool that is comparable to CT. Taking the diagnostic yield of the three imaging modalities into account and the patient cohort with a probability of therapeutic ERCP in 50% of the cases, we consider the use of ERCP with adjunct IDUS as justified. However, the use of multimodal diagnostics is recommended due to the even better accuracy rates. We are aware that according to the study protocol, there is a methodological bias in our study. First, some of the patients were referred to our hospital because of acute cholangitis; thus, a plastic stent was placed immediately during ERCP, possibly altering the results of further applied diagnostic imaging modalities. Second, three cross-sectional techniques and one ductal imaging technique supplemented with a limited cross-sectional modality were compared. Furthermore, we investigated patients over a time period of seven years. The development and improvement of Multi-Slice-CT in this time period has led to variations of the technical preconditions, especially in the beginning of the study, because of inferior technical requirements due to restrictions in the spatial resolution and not having access to multiplanar 3-dimensional reconstructions. This condition might have led to results favoring other imaging modalities compared with CT.
Definite guidelines for the management and diagnostics of biliary strictures of indeterminate etiology do not exist so far. Moreover, comparative studies of the various imaging studies with only small patient cohorts are available. Therefore, authors aimed to evaluate the diagnostic yield of intraductal ultrasound (IDUS), endosonography (EUS) and computed tomography (CT) in a large patient cohort scheduled for endoscopic retrograde cholangio-pancreatography (ERCP) with IDUS, CT and EUS due to indeterminate strictures or filling defects of the common bile duct. The objectives of this study were to observe the diagnostic quality of the imaging procedures in differentiating malignant from benign strictures.
IDUS, EUS and CT are well established imaging techniques in medicine. However, its combined use in the diagnostics of bile duct strictures is a matter of dabate. The research hotspot is how to combine different imaging tools in order to optimally differentiate malignant from benign bile duct strictures.
Previously published studies have been devoted to comparison of various imaging modalities in the diagnostics of indeterminate bile duct strictures. However, so far only studies of limited patient numbers exist. The combination of ERCP and IDUS seems to be the most accurate imaging modality in diagnosing biliary strictures. However, due to the minor ultrasonic penetration depth IDUS tends to understage tumors of the pancreaticobiliary tract, thus further imaging modality such as EUS or CT should be added in case of preoperative evaluation to detect lymph node invasion or distant metastasis. The analysis of the large patient cohort adds valuable information to the ongoing debate which diagnostic tool is best for detecting malignancy in bile duct strictures of uncertain etiology.
The study results suggest a multimodal approach in the work-up of bile duct strictures of uncertain etiology.
Intraductal ultrasound miniprobes can be inserted into the working channel of the gastroscope or sideviewing duodenoscope. It can be performed within an ERCP session. Its advantage is the high resolution due to frequencies of up to 30 MHz. giving a high resolution picture of the papilla, the bile duct wall and the adjacent tissue.
The authors present an interesting research. The paper is well written. The authors analyzed influence of different diagnostic techniques in predicting malignant bile duct strictures. The results of this study are useful for management of patients with bile duct stricture.
P- Reviewer: Bartalena T, Casciaro S, Markic D S- Editor: Gou SX L- Editor: A E- Editor: Ma S
| 1. | Victor DW, Sherman S, Karakan T, Khashab MA. Current endoscopic approach to indeterminate biliary strictures. World J Gastroenterol. 2012;18:6197-6205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Gabbert C, Warndorf M, Easler J, Chennat J. Advanced techniques for endoscopic biliary imaging: cholangioscopy, endoscopic ultrasonography, confocal, and beyond. Gastrointest Endosc Clin N Am. 2013;23:625-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Yeo D, Perini MV, Muralidharan V, Christophi C. Focal intrahepatic strictures: a review of diagnosis and management. HPB (Oxford). 2012;14:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Choi SH, Han JK, Lee JM, Lee KH, Kim SH, Lee JY, Choi BI. Differentiating malignant from benign common bile duct stricture with multiphasic helical CT. Radiology. 2005;236:178-183. [PubMed] |
| 5. | Kim HJ, Park DI, Park JH, Cho YK, Sohn CI, Jeon WK, Kim BI, Kim SK. Multidetector computed tomography cholangiography with multiplanar reformation for the assessment of patients with biliary obstruction. J Gastroenterol Hepatol. 2007;22:400-405. [PubMed] |
| 6. | Rösch T, Meining A, Frühmorgen S, Zillinger C, Schusdziarra V, Hellerhoff K, Classen M, Helmberger H. A prospective comparison of the diagnostic accuracy of ERCP, MRCP, CT, and EUS in biliary strictures. Gastrointest Endosc. 2002;55:870-876. [PubMed] |
| 7. | Domagk D, Wessling J, Conrad B, Fischbach R, Schleicher C, Böcker W, Senninger N, Heinecke A, Heindel W, Domschke W. Which imaging modalities should be used for biliary strictures of unknown aetiology? Gut. 2007;56:1032. [PubMed] |
| 8. | Choudari CP, Fogel E, Gottlieb K, Sherman S, Lehman GA. Therapeutic biliary endoscopy. Endoscopy. 1998;30:163-173. [PubMed] |
| 9. | Weber A, von Weyhern C, Fend F, Schneider J, Neu B, Meining A, Weidenbach H, Schmid RM, Prinz C. Endoscopic transpapillary brush cytology and forceps biopsy in patients with hilar cholangiocarcinoma. World J Gastroenterol. 2008;14:1097-1101. [PubMed] |
| 10. | Ferrari Júnior AP, Lichtenstein DR, Slivka A, Chang C, Carr-Locke DL. Brush cytology during ERCP for the diagnosis of biliary and pancreatic malignancies. Gastrointest Endosc. 1994;40:140-145. [PubMed] |
| 11. | Menzel J, Domschke W. Intraductal ultrasonography (IDUS) of the pancreato-biliary duct system. Personal experience and review of literature. Eur J Ultrasound. 1999;10:105-115. [PubMed] |
| 12. | Meister T, Heinzow HS, Woestmeyer C, Lenz P, Menzel J, Kucharzik T, Domschke W, Domagk D. Intraductal ultrasound substantiates diagnostics of bile duct strictures of uncertain etiology. World J Gastroenterol. 2013;19:874-881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Sugiyama M, Hagi H, Atomi Y, Saito M. Diagnosis of portal venous invasion by pancreatobiliary carcinoma: value of endoscopic ultrasonography. Abdom Imaging. 1997;22:434-438. [PubMed] |
| 14. | Fritscher-Ravens A, Broering DC, Knoefel WT, Rogiers X, Swain P, Thonke F, Bobrowski C, Topalidis T, Soehendra N. EUS-guided fine-needle aspiration of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am J Gastroenterol. 2004;99:45-51. [PubMed] |
| 15. | Mohamadnejad M, DeWitt JM, Sherman S, LeBlanc JK, Pitt HA, House MG, Jones KJ, Fogel EL, McHenry L, Watkins JL. Role of EUS for preoperative evaluation of cholangiocarcinoma: a large single-center experience. Gastrointest Endosc. 2011;73:71-78. [PubMed] |
| 16. | Shah RJ, Langer DA, Antillon MR, Chen YK. Cholangioscopy and cholangioscopic forceps biopsy in patients with indeterminate pancreaticobiliary pathology. Clin Gastroenterol Hepatol. 2006;4:219-225. [PubMed] |
| 17. | Chen YK, Parsi MA, Binmoeller KF, Hawes RH, Pleskow DK, Slivka A, Haluszka O, Petersen BT, Sherman S, Devière J. Single-operator cholangioscopy in patients requiring evaluation of bile duct disease or therapy of biliary stones (with videos). Gastrointest Endosc. 2011;74:805-814. [PubMed] |
| 18. | Faigel DO, Baron TH, Lewis B, Petersen B, Petrini J. Ensuring competence in endoscopy. Prepared by the ASGE taskforce on ensuring competence in endoscopy and American College of Gastroenterology executive and practice management committees. ASGE policy and procedures manual for gastrointestinal endoscopy: guidelines for training and practice on CD-ROM. ASGE. 2005;1-36. |
| 19. | Riphaus A, Wehrmann T, Weber B, Arnold J, Beilenhoff U, Bitter H, von Delius S, Domagk D, Ehlers A, Faiss S. S3 Guideline: Sedation for gastrointestinal endoscopy. Endoscopy. 2009;41:787-815. |
| 20. | Heinzow HS, Woestmeyer C, Domschke W, Domagk D, Meister T. Endoscopic transpapillary biopsies are of limited value in the diagnostics of bile duct strictures of unknown etiology-results of a histopathologically controlled study in 312 patients. Hepatogastroenterology. 2013;60:1569-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 21. | Domagk D, Poremba C, Dietl KH, Senninger N, Heinecke A, Domschke W, Menzel J. Endoscopic transpapillary biopsies and intraductal ultrasonography in the diagnostics of bile duct strictures: a prospective study. Gut. 2002;51:240-244. [PubMed] |
| 22. | Menzel J, Domschke W. Gastrointestinal miniprobe sonography: the current status. Am J Gastroenterol. 2000;95:605-616. [PubMed] |
| 23. | Adler G, Seufferlein T, Bischoff SC, Brambs HJ, Feuerbach S, Grabenbauer G, Hahn S, Heinemann V, Hohenberger W, Langrehr JM. [S3-Guidelines “Exocrine pancreatic cancer” 2007]. Z Gastroenterol. 2007;45:487-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 24. | Rösch T, Hofrichter K, Frimberger E, Meining A, Born P, Weigert N, Allescher HD, Classen M, Barbur M, Schenck U. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest Endosc. 2004;60:390-396. [PubMed] |
| 25. | Meister T, Heinzow H, Heinecke A, Hoehr R, Domschke W, Domagk D. Post-ERCP pancreatitis in 2364 ERCP procedures: is intraductal ultrasonography another risk factor? Endoscopy. 2011;43:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Jenssen C, Alvarez-Sánchez MV, Napoléon B, Faiss S. Diagnostic endoscopic ultrasonography: assessment of safety and prevention of complications. World J Gastroenterol. 2012;18:4659-4676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |