Published online Aug 14, 2014. doi: 10.3748/wjg.v20.i30.10223
Revised: May 8, 2014
Accepted: May 25, 2014
Published online: August 14, 2014
Processing time: 220 Days and 18.1 Hours
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and leading cause of death among patients with cirrhosis. Treatment guidelines are based according to the Barcelona Clinic Liver Cancer staging system. The choice among therapeutic options that include liver resection, liver transplantation, locoregional, and systemic treatments must be individualized for each patient. The aim of this paper is to review the outcomes that can be achieved in the treatment of HCC with the heterogeneous therapeutic options currently available in clinical practice.
Core tip: Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and its incidence is rising in Western countries. The choice among therapeutic options that include liver resection, liver transplantation, locoregional, and systemic treatments must be individualized for each patient. The aim of this paper is to review the outcomes that can be achieved in the treatment of HCC with the heterogeneous therapeutic options currently available in clinical practice.
- Citation: Tabrizian P, Roayaie S, Schwartz ME. Current management of hepatocellular carcinoma. World J Gastroenterol 2014; 20(30): 10223-10237
- URL: https://www.wjgnet.com/1007-9327/full/v20/i30/10223.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i30.10223
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and the third most frequent cause of cancer-related death[1]. With increasing incidence in Western countries, HCC remains the leading cause of death among patients with cirrhosis[2,3]. The implementation of surveillance programs has allowed early tumor diagnosis, resulting in increased curative treatments achieving 5 year survival rates up to 75%[4,5]. The choice among therapeutic options that include liver resection, liver transplantation, locoregional, and systemic treatments must be individualized for each patient. The majority of trials now choose the Barcelona Clinic Liver Cancer (BCLC) staging system to guide treatment decisions[6-8]. Despite improved outcomes, recurrence after curative treatments remains a significant problem and is reported as high as 70% at 5 years after resection; the optimal treatment of recurrent HCC remains unclear[4]. The aim of this paper is to review the outcomes that can be achieved in the treatment of HCC with the heterogeneous therapeutic options currently available in clinical practice.
In the past, the diagnosis of HCC was usually made at an advanced stage with patients presenting with symptomatic disease and liver impairment[6,9]. Treatment was futile with median survival rates of less than 3 mo[6,9]. In addition, morbidity associated with therapy was significantly high[10,11]. Recent advances in technology and surveillance programs have led to more frequent early HCC diagnosis offering curative treatments with 5 year survival rates ranging from 50% to 75%[4,5].
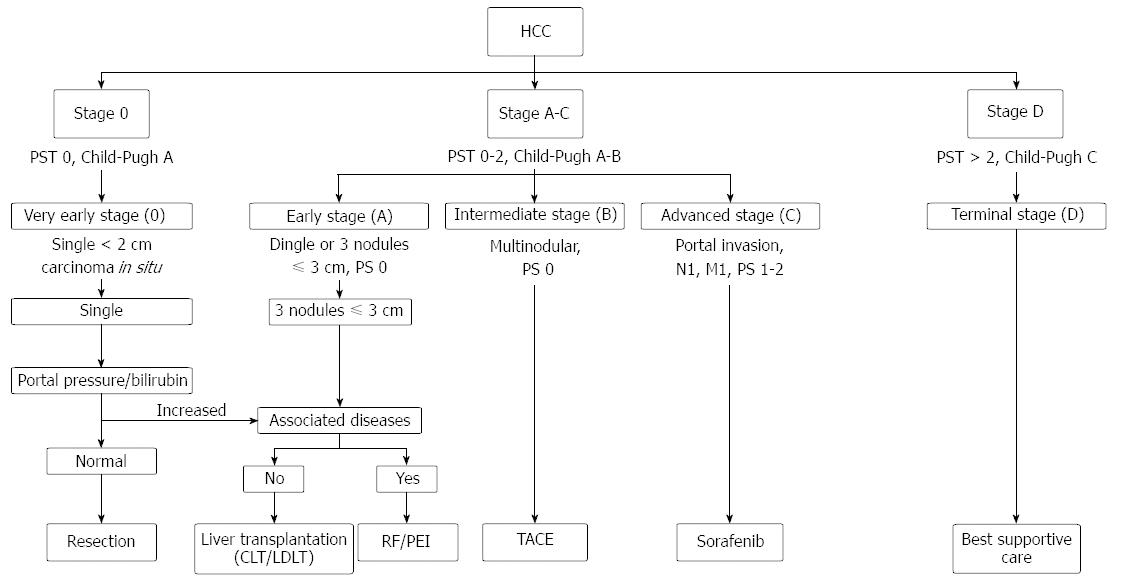
Among the many HCC staging systems that have been developed around the world, the BCLC staging system has emerged as the most useful to guide treatment decisions (Figure 1). The BCLC staging was first developed based on analysis of independent studies of the various treatment modalities applied in various clinical settings. It includes variables related to tumor stage, liver functional status, performance status, and cancer-related systems, its key feature being evidence-based linkage of clinical stage with treatment modalities, allowing an estimation of life expectancy based on published results. BCLC identifies patients with early HCC who are potentially curable, those at intermediate or advanced disease stage for whom noncurative treatment offers the likelihood of extended survival, and those at end stage for whom treatment would provide more harm than benefit.
Ongoing molecular studies are rapidly shedding light on the heterogeneous underlying mechanisms that drive the development and progression of HCC and offer promise of therapies that target the specific abnormalities that lead to and sustain the growth of HCC in individual patients[12-14].
Given the complexity of the disease and the large number of useful therapies, patients with HCC should be cared for by multidisciplinary teams. It is important to note that the level of evidence for most of the therapeutic options is limited to cohort investigations with few RCTs, most of which are limited to the treatment of advanced disease; as with most cancers, surgical treatment for early stage disease has not been compared to no treatment in prospective trials[15,16].
Table 1 summarizes existing series and the level of evidence for efficacy according to trial design and end-points for all available treatments in HCC[4,5,17-31]. Availability of resources also has to be considered in developing treatment strategies. This is particularly relevant when considering liver transplantation, which is unavailable in some areas of the world.
| Treatment | Ref. | Year | Number | 5 yr survival |
| Liver resection (evidence 2A) | Llovet et al[4] | 1999 | 77 | 51% |
| 1Fong et al[5] | 1999 | 154 | 57% | |
| 2Roayaie et al[17] | 2012 | 132 | 70% | |
| Liver transplantation (evidence 2A) | Mazzaferro et al[18] | 1996 | 444 | 73.3% |
| Llovet et al[4] | 1999 | 87 | 69% | |
| Yao et al[19] | 2001 | 70 | 72.4% | |
| 3Roayaie et al[20] | 2002 | 43 | 44% | |
| 4Mazzaferro et al[21] | 2009 | 48 | 75% | |
| Radiofrequency ablation (evidence 2A) | 2Sala et al[22] | 2004 | 34 | 63% |
| Vivarelli et al[23] | 2004 | 79 | 33% (3 yr) | |
| 1Chen et al[24] | 2006 | 71 | 67.9% (4 yr) | |
| Transarterial chemoembolization (evidence 1A) | DETCH[25] | 1995 | 50 | 38% (2 yr) |
| Llovet et al[26] | 2002 | 40 | 29% (3 yr) | |
| Lo et al[27] | 2002 | 40 | 31% (2 yr) | |
| Radiotherapy (evidence 1A) | 5Carr[28] | 2004 | 65 | Median 21.3 mo |
| 6Salem et al[29] | 2011 | 123 | Median 20.7 mo | |
| Systemic therapy (evidence 1A) | Llovet et al[30] | 2008 | 299 | 10.7 mo |
| Cheng et al[31] | 2009 | 150 | 6.5 mo |
Selection criteria: Hepatic resection is widely accepted as first-line treatment for patients with early-stage HCC and preserved liver function[4,32]. The choice of treatment modality in cirrhotic patients (who comprise the majority HCC cases) is challenging and requires assessment of both liver function reserve and tumor extension. Child-Pugh class is commonly used as a basis for estimating hepatic reserve, with resection confined to patients who are Child-Pugh class A; in Asia indocyanine green retention rate at 15 min (ICG 15) is often used as a direct measure of liver function[33,34]. Portal hypertension as assessed by platelet count or direct measurement of hepatic venous pressure gradient has been recognized as a major prognostic factor in the treatment of HCC[35,36]. The BCLC group showed 70% 5-year survival for patients without portal hypertension and with bilirubin < 1 compared with 50% in patients with both risk factors present[4].
Preoperative portal vein embolization (PVE) is often employed to induce growth of the remnant liver prior to major resection, typically right hepatectomy[11,37,38]. The regenerative response to PVE in cirrhosis is less-reliable and takes longer than with normal parenchyma; indeed, response to PVE may be considered a “stress test” for the liver. While anatomic resection is of proven benefit in HCC, it is preferable to perform limited anatomic resection, i.e., segmentectomy or sectorectomy, when possible and to avoid right hepatectomy in cirrhotic patients[39].
The main prognostic predictors after liver function status are tumor size, number of tumors, presence of satellite lesions, and vascular invasion[4,40-42]. These factors should be carefully assessed by contrast-enhanced computed tomography and/or magnetic resonance imaging. Intraoperative ultrasonography is routine in resection of HCC, enabling detection of additional small nodules and defining the relationship of the tumor to intrahepatic structures.
Perioperative outcome: The perioperative mortality of major resection has decreased in the past few decades from 15% in the 1980s to 3%-5% in the majority of large referral centers, with some centers reporting < 1% mortality[10,11,32]. Predictors of perioperative mortality are primarily related to liver function and include Child-Pugh class, degree of hepatic fibrosis, total bilirubin level, presence of clinically relevant portal hypertension, and platelet count.
High operative blood loss and transfusions have repeatedly been shown to correlate with poor outcome; refinement of patient selection as well as of surgical technique has led to improvement in this regard. Inflow occlusion (Pringle maneuver) is widely applied, and many studies have compared different occlusion methods[43,44]. Intermittent inflow occlusion has been shown to decrease ischemia/reperfusion injury with subsequently reduced morbidity and mortality rates, and is the procedure of choice at most centers. Total hepatic vascular exclusion has a role in resection of tumors that are adjacent to the vena cava and/or large hepatic veins, but is rarely applied in cirrhotic patients. In all cases, maintenance of low central venous pressure (< 5 mmHg) by the anesthesiologist is the best way to limit bleeding from hepatic veins during the division of the liver[11].
There is an array of techniques and technologies available for dividing liver parenchyma[45]. Since no one method is suitable for all situations, it is important to master a few techniques and to have a flexible approach.
These combined strategies, in addition to improved perioperative management, have led to a decrease in blood transfusion from 80% to 90% to less than 10% over the past two decades[11].
Recurrence and survival: Recurrence after resection is common; even with early-stage HCC recurrence develops in approximately 20%, 50% and 75% of patients at 1 year, 3 years and 5 years, respectively[4,16,32]. Recurrence of HCC may be the result either of metastasis from the primary tumor that was resected (true recurrence), or de novo HCC due to the underlying predisposition; while molecular testing is required to definitively distinguish between the two, most true recurrence manifests within 2 years of resection, and thus 2 years is often adopted as a practical cut-off to distinguish between true recurrence and de novo HCC[7,42]. Predictors of true recurrence include tumor grade, microscopic and macroscopic vascular invasion, tumor size, number of tumors, presence of satellites, alpha-fetoprotein level, transfusion, and positive surgical margin[4,40-42]. Postresection survival rates are in the range of 80%-92% at 1 year, 61%-86% at 3 years, and 41%-74% at 5 years (Table 2)[4,5,17,46-49]. The nature of the underlying liver disease is important: patient survival is higher and HCC recurrence is less-common in patients with hepatitis B compared to those with hepatitis C[42]. The independent predictors of recurrence and survival are summarized in Table 3[4,41,42,47,50-52].
| Treatment | No. of patients | 1 yr survival | 5 yr survival |
| Surgical resection | |||
| Fong et al[5] 1999 | 154 | 81% | 37% |
| Llovet et al[4] 1999 | 771 | 85% | 51% |
| Poon et al[46] 2002 | 1352 | 90% | 70% |
| Wayne et al[47] 2002 | 249 ( ≤ 5 cm) | 83% | 41% |
| Shrager et al[48] 2012 | 2061 | 60% | 46% |
| Roayaie et al[17] 2013 | 132 ( ≤ 2 cm) | - | 70% |
| Liver transplantation | |||
| Mazzaferro et al[18] 1996 | 48 | 84% | 74% |
| Bismuth et al[82] 1999 | 45 | 82% | 74% |
| Llovet et al[4] 1999 | 79 | 86% | 75% |
| Jonas et al[83] 2001 | 120 | 90% | 71% |
| Yao et al[19] 2001 | 64 | 87% | 73% |
| Living donor transplantation | |||
| Gondolesi et al[84] 2004 | 15 | - | 86% (3 yr) |
| Todo et al[85] 2004 | 137 | - | 79% (3 yr) |
| Ref. | No. of patients | Variables |
| Belghiti et al[50] 1991 | 47 | Tumor size ≥ 5 cm |
| AFP levels ≥ 100 ng/mL | ||
| Llovet et al[4] 1999 | 77 | Differentiation degree |
| Multinodularity | ||
| Satellites | ||
| Imamura et al[42] 2003 | 184 | Early recurrence (< 2 yr) |
| Microvascular invasion | ||
| AFP levels ≥ 32 ng/mL | ||
| Non-anatomical resection | ||
| Late recurrence (> 2 yr) | ||
| Higher grade of hepatitis activity | ||
| Multinodularity | ||
| Gross tumor classification | ||
| Roayaie et al[41] 2009 | 131 | Invasion of a vessel with a muscular wall |
| Tumor size > 10 cm | ||
| Survival | ||
| Llovet et al[4] 1999 | 77 | Portal hypertenstion |
| Bilirubin level (> 1 mg/dL) | ||
| Wayne et al[47] 2002 | 249 | Child-Pugh (B) |
| Differentiation degree | ||
| (Edmonson-Steiner 1-2 vs 3-4) | ||
| Fibrosis | ||
| (Ishak score 0-4 vs 5-6) | ||
| Vauthey et al[51] 2002 | 557 | Vascular invasion |
| Multinodularity | ||
| Tumor size (> 5 cm) | ||
| Fibrosis | ||
| Poon et al[52] 2003 | 518 | Vascular invasion |
| Tumor size (> 5 cm) | ||
| Multinodularity | ||
| Cirrhosis | ||
| AST > 50 U/L | ||
| Invasion of adjacent organs | ||
| Roayaie et al[41] 2009 | 131 | Invasion of a vessel with a muscular wall |
| Invasion > 1 cm from tumor |
Adjuvant therapies to prevent recurrence: The only adjuvant therapy of proven value is treatment of underlying viral hepatitis. Remarkable advances have been made in the past decade in preventing recurrent HCC with the use of antiviral treatment, either after local resection or locoregional tumor ablation. Antiviral therapy of hepatitis B, especially with nucleoside analogues, has been shown to reduce de novo HCC recurrence as well as to retard progression of cirrhosis[16,53,54]. Similarly, interferon-based therapy has been shown to reduce de novo HCC in patients who comply with the treatment regimen[55,56]. Several RCT have been conducted to explore strategies to prevent true recurrence (Table 4)[57-64]. Chemoembolization, systemic chemotherapy, and interferon did not provide benefit[59,65]. Intraarterial radiotherapy with iodine-131 (131I) iodized oil showed improved outcome in two small RCT’s but is currently unavailable[66,67]. Decreased HCC recurrence was reported with acyclic retinoids in 1996, but a follow-up study has proven negative[68]. Samuel et al[69] published a review of 12 RCTs and demonstrated no clear evidence for the efficacy of any of the adjuvant or neoadjuvant protocol. The result of a large randomized trial evaluating sorafenib in the adjuvant setting is currently underway[70].
| Ref. | Treatment | Recurrence rate-3 yr |
| Izumi et al[57] 1994 | Adjuvant arterial lipiodolization (23) vs control (27) | No differences |
| Lai et al[58] 1998 | Adjuvant chemoembolization (30) vs control (36) | 82% vs 52%, P = NS |
| Yamasaki et al[59] 1996 | Neoadjuvant chemoembolization (50) vs control (47) | 54% vs 66%, P = NS |
| Lau et al[60] 1999 | Adjuvant intraarterial lipiodol 131I (21) vs control (22) | 75% vs 38%, P = 0.03 |
| Lygidakis et al[61] 1996 | Adjuvant chemoembolization + immunotherapy (49) vs control (42) | 36 mo vs 18 mo (OS) |
| Takayama et al[62] 2000 | Adjuvant immunotherapy (76) vs control (74) | 33% vs 48%, P = 0.008 |
| Yamamoto et al[63] 1996 | Adjuvant 5-FU (35) vs control (32) | 52% vs 75%, P = NS |
| Kubo et al[64] 2001 | Adjuvant Interferon alpha (15) vs control (15) | 30% vs 60%, P= 0.03 |
Treatment of recurrent HCC after resection: In 65%-80% of cases of recurrent HCC the liver is the sole site of disease[42]. Repeat resection has been widely accepted as the treatment of choice for recurrent intrahepatic HCC in patients with well preserved liver function and solitary tumors[71]. Applicability of repeat hepatectomy in published series ranges from 10% to 35%, with 5-year survival reported from 37%-87%[71,72]. Roayaie et al[71] identified time from primary resection to recurrence (< 1 year) and presence of gross vascular invasion at second hepatectomy as predictors of poor outcome in patients undergoing repeat hepatectomy, providing some guidance in case selection.
OLT may be applied secondarily to treat recurrent HCC after hepatectomy and several authors have explored the possibilities of “salvage” transplantation[73-75]. Cherqui et al[76] found that 61% of patients with recurrent HCC after resection had recurrence within transplant eligibility criteria, and that 5-year survival after retransplant was 70%.
Transcatheter chemoembolization (TACE) is the most widely used treatment modality for recurrent intrahepatic HCC in patients with unresectable disease. Several retrospective studies have reported 1-year survival of 64%-88% with 5-year survival ranging from 0%-27%[77,78]. These results are not dissimilar to those reported in primary BCLC B HCC.
There are few data available on the management of extrahepatic recurrence after hepatectomy, all from small case series. Independent predictors of survival after recurrence include time from primary resection to recurrence > vs < 1 year, size and number of recurrent tumor nodules, site of recurrence, alpha-fetoprotein (AFP) level at recurrence, and type of treatment[77-80]. These studies suggest that aggressive surgical treatment may be of benefit in patients with isolated metastasis, with the most common site being the lung: Lam et al[81] reported 67% 5-year survival after resection of solitary lung metastasis.
Selection criteria: Orthotopic liver transplantation (OLT) is an appealing option for cirrhotic patients with early stage HCC, since it allows removal of both detectable and undetectable HCC in the liver and also treats the underlying cirrhosis. OLT is, however, limited by graft shortage, and appropriate patient selection is critical to achieving satisfactory results[4,18]. The outcomes have improved in the past decades with 5-year survival rates from 18%-40% in the 1980s rising to 85% 1-year and 70% 5-year survival in recent reports (Table 2)[4,18,19,82-85]. In a landmark study, Mazzaferro et al[18] proposed a strict criteria for OLT that result in survival rates equal to those in cirrhotic patients without HCC, with 5 year overall and recurrence free survival of 75% and 83% respectively. The “Milan Criteria” (solitary tumor ≤ 5 cm, or 2-3 nodules all < 3 cm and without gross vascular invasion or extrahepatic spread) are now widely accepted as the basis for selecting candidates with HCC for transplantation and have been adopted in the United States by United Network for Organ Sharing (UNOS) as the basis for prioritizing HCC patients for OLT[18]. Consensus guidelines suggest that OLT is the treatment choice for patients with Child’s B cirrhosis and/or portal hypertension and HCC within Milan criteria; hepatectomy remains the accepted first-line treatment for patients with preserved liver function and early-stage HCC.
Based on the success of OLT for HCC within Milan criteria, controversy has arisen over expansion of the Milan criteria (Table 5)[18,19,21,86-91]. The most widely-recognized study (UCSF criteria) was proposed by Yao and colleagues as the UCSF criteria (single nodule < 6.5 cm, or ≤ 3 nodules the largest of which is ≤ 4.5 cm with the cumulative tumor diameters ≤ 8 cm)[19]. Small studies evaluating post-OLT survival rates in patients who meet the UCSF but exceed the Milan criteria have shown 5-year survival ranging from 38%-93%[92,93]. Although there is no question that many patients would be cured with the adoption of broader criteria, opponents challenge the expansion due to concern that it will lead to increased risk of vascular invasion and tumor recurrence that they consider unacceptable in light of the widespread donor organ shortage[92].
| Ref. | No. of patients | Selection criteria | Survival rate at 5 yr |
| Mazzaferro et al[18] 1996 | 48 | Milan criteria: single HCC < 5 cm or up to 3 nodules < 3 cm | 75% (4 yr) |
| Yao et al[19] 2001 | 70 | UCSF criteria: a maximum tumor size of 6.5 cm or 2 lesions ≤ 4.5 cm in diameter with a total tumor diameter of ≤ 8 m | 75% |
| Kwon et al[86] 2007 | 114 | HCC ≤ 5 cm | 87% |
| AFP ≤ 400 ng/mL | |||
| Lee et al[87] 2008 | 186 | Up to 6 nodules with a maximum diameter of < 5 cm | 76% |
| Mazzaferro et al[21] 2009 | 283 | Up to 7 criteria: 7 as the sum of the largest size (cm) and the number of tumors | 71% |
| Herrero et al[88] 2001 | 154 | HCC ≤ 6 cm or ≤ 3 HCC ≤ 5 cm | 73% |
| Jonas et al[89] 2007 | 21 | Any number, each ≤ 6 cm with cumulated diameter ≤ 15 cm | 62% at 3 yr |
| Toso et al[90] 2008 | 288 | TTV ≤ 115 cm3 | 72% |
| Sugawara et al[91] 2007 | 78 | ≤ 5 HCC ≤ 5 cm | 70% at 3 yr |
Drop-out rates and downstaging: For patients without HCC, prioritization for OLT is based on the Model for End Stage Liver Disease (MELD) score[94]. The MELD score, which ranges from 6 to 40 and is calculated based on total bilirubin, creatinine, and prothrombin time international normalized ratio (INR), provides an objective and reliable index of 3-mo mortality in cirrhotic patients[94]. For patients with HCC, however, the primary risk is not death due to liver failure but rather progression of HCC to the point where transplant is no longer worthwhile. Current UNOS policy accords patients with T2 HCC (single nodule between 2-5 cm or 2-3 nodules all < 3 cm) an initial score of 22 points (“MELD exception”) that rises every three months as long as the tumor is maintained within Milan criteria[95]. This has resulted in both increased transplant rates and excellent long-term outcomes[94].
Despite this policy, however, in many regions of the US patients must wait a year or more for a donor liver. As a result, drop-out from the waiting list due to tumor progression is an important problem that can significantly decrease the survival of transplant for HCC when viewed on an intention-to-treat basis[4]. Identified risk for drop-out include multinodular tumors, failed neoadjuvant therapy, baseline AFP > 200 ng/mL, or steady increase of > 15 ng/mL per month[96]. Numerous studies have examined the role of locoregional therapies as a “bridge” to OLT in order to prevent tumor progression and drop-out and possibly to improve posttransplant outcomes, but none are of adequate design or sufficient power to provide strong evidence in support of this approach[7,97]. Thermal ablation techniques are typically employed to treat solitary nodules > 3 cm, while TACE is commonly preferred for larger or multinodular tumors[98,99]. Based on the available evidence, guidelines recommend locoregional treatment of HCC in patients awaiting OLT when the estimated waiting time will exceed 6 mo.
In an attempt to better define a subset of patients with HCC beyond Milan criteria who could benefit from OLT, a number of reports have explored the concept of downstaging, i.e., nonsurgical tumor treatment to reduce the size and/or number of viable lesions to within acceptable criteria, typically the Milan criteria[99,100]. The rationale of downstaging per se is dubious; all reported protocols include a minimum waiting period after locoregional therapy, typically 3-6 mo, to allow for assessment of tumor behavior. Several single-center studies have reported excellent results with patient survival and the incidence of HCC recurrence comparable those achieved in patients initially within Milan criteria; to this point the evidence is not strong enough for downstaging to have been accepted into guidelines or organ allocation policy[100-103].
Living donor transplantation (LDLT) is, for patients with a suitable and willing donor, a way to eliminate waiting time and the attendant risk of drop-out. In patients fulfilling the Milan criteria, 5-year survival after LDLT is similar to after deceased donor OLT (Table 2), though there is a suggestion of a higher rate of HCC recurrence[84,85,104,105]. Because there is no competition for living donor organs, many centers also offer this option to patients with HCC that is modestly beyond Milan criteria, with reported 5 year survival rates up to 60%[106,107].
Recurrence and outcome: Post-OLT HCC recurrence is seen in 10%-15% of patients transplanted within the Milan criteria[4,18]. It is the result of extrahepatic dissemination of HCC that has occurred before or during the transplant procedure, although interestingly intrahepatic recurrence is common site. The large majority of recurrences are within 2 years of OLT[4,18]. Reported predictors of post-OLT HCC recurrence are summarized in Table 6, foremost among them being the finding of vascular invasion on examination of the explanted liver[83,108-112].
| Ref. | No. of patients | Variables |
| Recurrence | ||
| Iwatsuki et al[108] 2000 | 344 | Bilobar disease |
| Vascular invasion | ||
| Tumor size (2-5 cm, > 5 cm) | ||
| Bismuth et al[109] 1993 | 60 | Tumor size |
| Number of tumors | ||
| Portal thrombus | ||
| Roayaie et al[110] 2000 | 119 | Tumor size |
| Vascular invasion | ||
| Hemming et al[111] 2002 | 112 | Vascular invasion |
| Survival | ||
| Iwatsuki et al[108] 2000 | 344 | Number of tumors (> 3) |
| Vascular invasion | ||
| Jonas et al[83] 2001 | 120 | Vascular invasion |
| Tumor grade |
Overall post-OLT 5-year survival for patients with HCC within Milan criteria is in the range of 70%-75%, though results in patients with associated hepatitis C are around 10% lower than in patients with other underlying diseases[4,18,113]. When considering OLT as an alternative to resection in patients eligible for both, it becomes important to view OLT on an intention-to-treat basis, incorporating waiting list drop-out. When waiting time is > 6 mo, 5-year intention-to-treat survival has been shown to be reduced by 10%-20% (from 58%-81% to 47%-62%)[114].
Post-OLT survival in patients who develop HCC recurrence is approximately 22% at 5 years[115]. Independent predictors of poor survival from the time of recurrence include tumor grade (poor), time to recurrence < 1 year, and presence of bone metastasis[115]. Patients who undergo locoregional or surgical treatment for recurrent HCC enjoy significantly better outcomes than other patients, but it is difficult to fully account for case selection bias in the available retrospective reports. Isolated hepatic recurrence, observed in 15%-20% of cases, is the pattern most amenable to locoregional therapy[116]. Hepatic resection for isolated recurrent disease post-OLT can achieve 5-year survival up to 66%[115-117]. Response to TACE for treatment of recurrent HCC has been demonstrated to be similar to that observed in primary HCC[116,118]. A few Asian reports describe retransplantation for post-OLT HCC recurrence, but there is a broad consensus against this approach[119,120].
Thermal (RFA, microwave) or chemical (ethanol, acetic acid) is the treatment of choice in patients with single small tumors who are not candidates for surgery, and may be curative in well-selected candidates[16,114].
Percutaneous ethanol injection (PEI) is a long-established technique for the treatment of nodular-type HCC, inducing coagulation necrosis of the lesion as a result of cellular dehydration, protein denaturation, and chemical occlusion of small tumor vessels[121]. For tumors < 2 cm, PEI has been shown to yield equivalent results to thermal ablation; for larger tumors PEI is inferior to thermal ablation and is now rarely performed[122].
Thermal ablation has now largely supplanted PEI, initially with RFA and more recently with microwave ablation[121,123]. While most commonly performed percutaneously, ablation can also be done via an open or laparoscopic surgical approach. The thermal damage caused by heating is dependent on both the tissue temperature achieved and the duration of heating. In order to achieve adequate tumor destruction, the entire lesion must be exposed to cytotoxic temperatures; in order to assure this, a rim of nontumor tissue surrounding the lesion being treated is included in the ablation zone. Thermal ablation is associated with low major morbidity (2.2%-3.1%) and mortality (0.1%-0.5%) rates[124,125]. Major complications include intraperitoneal hemorrhage, hepatic abscess, bile duct injury, and liver decompensation. Tumor seeding along the needle track has been reported as a rare (0.5%) late complication of RFA[126].
The efficacy of RFA is reduced with increasing tumor size and the presence of large (3 mm or more) abutting vessels. Complete tumor necrosis in explanted liver specimens was shown in 83% of lesions > 3 cm and in 88% of tumors in nonperivascular locations[16,125,127]. Five RCTs and a meta-analysis have confirmed the superiority of RFA over PEI except in very small lesions (Table 7)[122,128-131].
| Ref. | No. of patients | Initial response | Failure | 3 yr survival | P value |
| Lencioni et al[128] 2003 | RFA (52) | 91% | 8% | 81% | NS |
| PEI (50) | 82% | 34% | 73% | ||
| Lin et al[129] 2004 | RFA (52) | 96% | 17% | 74% | 0.014 |
| 88% | |||||
| PEI (52) | 88% | 45% | 50% | ||
| Shiina et al[122] 2005 | RFA (118) | 100% | 2% | 80% | 0.020 |
| PEI (114) | 100% | 11% | 63% | ||
| Lin et al[130] 2005 | RFA (62) | 97% | 16% | 74% | 0.031 |
| PEI (62) | 89% | 42% | 51% | ||
| Brunello et al[131] 2008 | RFA (70) | 96% | 34% | 59% | NS |
| PEI (69) | 66% | 64% | 57% |
A number of studies have reported on long-term outcomes of RFA in the treatment of HCC. Lencioni et al[125,132] have demonstrated 61% 5-year survival in patients with Child A cirrhosis and solitary HCC, compared with 51% in patients with Child A cirrhosis and multiple tumors and 31% in patients with Child B cirrhosis.
The role of thermal ablation vis-à-vis resection has been the subject of a number of randomized trials. Chen et al[24]. demonstrated no difference in overall or recurrence-free survival in patients with solitary HCC < 5 cm Huang et al[133], on the other hand, showed a significant survival benefit for surgical resection (75.6% 5-year OS, 28.7% RFS) over RFA (54.8% 5-year OS, 21.3% RFS) in patients with HCC within Milan criteria. Both of these studies have been criticized due to insufficient sample size and lack of non-inferiority design[24,133]. Recently, Hasegawa et al[134] published data from a large Japanese cohort study and concluded that resection was associated with higher overall survival and lower recurrence rate than RFA or PEI in the treatment of HCC ≤ 3 cm. Livraghi et al[135] has reported complete tumor response in 97% of tumors ≤ 2 cm, with 5-year survival in patients with preserved hepatic function of 68%, in the process challenging resection as the first-line approach in such cases. Roayaie et al[17] reported the outcomes of resection at two large Western centers in patients for HCC ≤ 2 cm and showed a substantial incidence of vascular invasion, with anatomic resection resulting in significantly less recurrence than nonanatomic resection. The best results of resection, with 5-year survival of 81%, were achieved when the platelet count was > 150000.
Microwave ablation (MWA) is an emerging alternative method to RFA, inducing thermal injury using microwaves with a frequency of 900 kHz. Compared to RFA, MWA is less-susceptible to the heat sink effect of nearby blood vessels. In the one RCT thus far reported RFA proved superior with respect to local recurrence and complications rates, but with the rapid evolution of the technology the outcome of MWA has improved[136].
The use of other ablative technologies including laser ablation, cryoablation, and irreversible electroporation, remains under clinical investigation[16].
TACE, the image-guided delivery of embolic particles +/- chemotherapeutic agents via selective injection into vessels feeding tumors in order to achieve cytoreduction, is a well-established treatment for intermediate stage (BCLC stage B) HCC[16,26,137]. TACE has been shown both in RCTs and in a meta-analysis of RCT’s to provide survival benefit in patients with preserved liver function and HCC confined to the liver without macrovascular invasion: one RCT demonstrated 2-year survival of 63% vs 27% in untreated controls[26,27]. Based on this evidence, TACE has been incorporated into guidelines on HCC management.
Complications of TACE include nontarget embolization, postembolization syndrome (fever, abdominal pain, ileus), liver failure, cholecystitis, and acute portal vein thrombosis[138]. Treatment-related mortality is seen in less than 5% of cases[138]. Main portal vein thrombosis, poor liver function, and extrahepatic spread have been shown to be predictors of poor outcome and are considered contraindications for chemoembolization[139]. Due to increased risk of hepatic necrosis and abscess formation, a total bilirubin level greater than 3 mg/mL should be considered a relative contraindication to TACE unless selective embolization can be performed.
There is no universal standard technique for the performance of TACE; the choice of embolic agent, whether lipiodol is used, the choice of drugs, and the schedule (on demand vs at fixed intervals) all vary among centers. Most commonly, TACE is performed by injection of chemotherapy with or without lipiodol, followed by the injection of embolic particles to near stasis. More recently, the use of calibrated particles that adsorb chemotherapy when mixed with the drug prior to injection and slowly elute the drug after the procedure have been shown to result in fewer side-effects and, in high-risk cases, better results[140].
Whether the addition of the chemotherapy in TACE provides benefit over bland embolization has been the subject of a number of trials (Table 8)[122-124,141-143]. In a recent study, patients treated TACE using drug-eluting beads with epirubicin had a higher rate of complete necrosis (77% vs 27%) and a significantly decreased tumor progression rate compared to patients treated with bland embolization alone[144].
| Ref. | No. of patients | 1 yr survival | 2 yr survival | P value |
| Lin et al[141] 1988 | ||||
| TAE (gelfoam + ivalon) | 21 | 42% | 25 | NS |
| TAE + IV 5-FU | 21 | 20% | 20 | |
| IV 5-FU | 21 | 13% | 13 | |
| Pelletier et al[142] 1990 | ||||
| TACE (doxorubicin, gelfoam) | 21 | 24% | - | NS |
| Conservative treatment | 21 | 33% | - | |
| GETCH[25] 1995 | ||||
| TACE (cisplatin, gelfoam) | 50 | 62% | 38 | NS |
| Conservative treatment | 46 | 43% | 26 | |
| Bruix et al[143] 1998 | ||||
| TAE (gelfoam, coils) | 40 | 70 | 49 | NS |
| Conservative treatment | 40 | 72 | 50 | |
| Lo et al[27] 2002 | ||||
| TACE (cisplatin, gelfoam) | 40 | 57 | 31 | 0.002 |
| Conservative treatment | 39 | 32 | 11 | |
| Llovet et al[26] 2002 | ||||
| TACE (doxorubicin, gelfoam) | 40 | 82 | 63 | 0.009 |
| TAE (gelfoam) | 37 | 75 | 50 | |
| Conservative treatment | 35 | 63 | 27 |
There is growing interest supported by a number of nonrandomized studies in the use of stereotactic body radiation therapy (SBRT) to treat HCC in a variety of settings (Table 9)[145-149]. The best-defined role for SBRT is to treat isolated lesions of HCC, either as definitive therapy or neoadjavant to transplant, when the conventional modalities including TACE and thermal ablation are either not applicable or have failed to achieve tumor control.
| Treatment | Ref. | Year | Number | Overall survival |
| Radiotherapy | Kwon et al[145] | 2010 | 42 | 58.6% (3 yr) |
| Andolino et al[146] | 2011 | 60 | 67% (2 yr) | |
| Huang et al[147] | 2012 | 36 | 64% (2 yr) | |
| Kang et al[148] | 2012 | 47 | 68.7% (2 yr) | |
| Facciuto et al[149] | 2012 | 117 | 1Median 32 mo | |
| Radioembolization | Carr et al[28] | 2004 | 65 | Median 21.3 mo |
| Sangro et al[150] | 2006 | 24 | Median 7 mo | |
| Kulik et al[151] | 2008 | 108 | 2Median 15.6 mo | |
| 3Median 4.4 mo | ||||
| Hilgard et al[152] | 2010 | 108 | Median 16.4 mo | |
| Salem et al[153] | 2010 | 291 | 4Median 7.7 mo | |
| Salem et al[29] | 2011 | 123 | Median 20.7 mo |
Radioembolization via the hepatic artery using microspheres impregnated with yttrium-90 (Y90) has been investigated in the treatment of unresectable HCC (Table 9)[28,29,150-153]. The safety of this technique has been demonstrated in multiple studies, and as there is minimal embolic effect it may be safely applied to patients with tumoral invasion of the portal vein. The treatment protocol requires preliminary investigations to exclude significant hepatopulmonary shunting and to assure that the arterial anatomy is suitable to allow treatment of the involved liver without deposition of microspheres in the gastrointestinal tract.
Cohort and retrospective studies have evaluated the efficacy of radioembolization in the treatment of HCC[28,29,150-153]. Pathologic examination of livers removed at transplant in which HCC had been treated with Y90 has demonstrated complete response in 61% of cases. Median survival for patients with macroscopic portal invasion has been reported at 12 mo. These data are encouraging, and a number of RCT’s are underway to clarify the role of Y90 treatment.
Another agent, 131I iodized oil, has also been used for radioembolization. A small RCT from Hong Kong as well as a French study have shown increased survival after hepatic resection for HCC with postoperative adjuvant infusion of 131I lipiodol into the hepatic artery of the remnant liver[60,67]. These results have yet to be replicated on a larger scale, and due to complexities in its production the agent is no longer available for clinical use.
Prior to 2007, there was no first-line systemic therapy with proven efficacy in HCC. In that year Sorafenib, an oral tyrosine kinase inhibitor that suppresses tumor proliferation and angiogenesis, was shown in a large Western placebo-controlled RCT to significantly prolong survival (from 7.9 mo to 10.7 mo) in patients with Child’s A cirrhosis and advanced HCC (extrahepatic spread, macroscopic vascular invasion, or failure of locoregional treatment)[30]. These findings were confirmed in a second trial conducted in Asia[31]. Sorafenib was overall well-tolerated in both trials with the most common grade 3 drug-related side effects being diarrhea, weight loss, and hand/foot skin reactions[30,31].
Data on the efficacy and tolerability of sorafenib in patients with Child’s B cirrhosis are limited as the majority of patients enrolled in trials have been Child’s class A; such data as are available suggest markedly lower survival (median 3.2 mo for Child’s B compared to 9.5 mo in Child’s A cirrhosis)[154-157]. Furthermore, data on safety and dosing in Child’s B patients are inadequate, particularly when the bilirubin level is elevated[16,155]. Systemic therapy should be administered with caution in these patients.
Encouraged by the success of sorafenib, a number of other studies have been undertaken using other targeted agents in combination with sorafenib, head-to-head against sorafenib, or as second-line after progression on or inability to tolerate sorafenib; to date, all completed studies have been negative (Table 10)[158-163]. A RCT looking at sorafenib after chemoembolization in an attempt to prolong time to progression also provided no meaningful benefit. A large RCT of sorafenib vs placebo as adjuvant therapy after resection or ablation is currently underway[70]. In view of these multiple failures in unselected populations of patients, attention is increasingly shifting to trial enrichment using molecular studies to identify subgroups of patients with identified drivers of tumor progression that may be rationally targeted with specific drugs.
| Treatment | Acronym | Treatment | Primary outcome |
| First line treatment | 1SEARCH | Erlotinib + Sorafenib vs Sorafenib | OS |
| 1BRISK-FL | Brivanib vs Sorafenib | OS | |
| - | Linifanib vs Sorafenib | OS | |
| Second line treatment | 1EVOLVE-1 | Everolimus vs Placebo | OS |
| 1BRISK | Brivanib vs Placebo | OS | |
| 1BRISK-APS | Brivanib vs Placebo | OS | |
| Toh et al[158] 2010 | Linifanib | OS: 9.7 mo | |
| Phase 2 trial (reported) | Hsu et al[159] 2010 | Sorafenib + Tegafur/Uracil | OS: 7.4 mo |
| Thomas et al[160] 2007 | Erlotinib | OS: 6.25 mo | |
| Thomas et al[161] 2009 | Erlotinib + Bevacizumab | OS: 15.7 mo | |
| Faivre et al[162] 2009 | Sunitinib | OS: 8 mo |
P- Reviewer: Hann HW, Zhang ZM, Zhu X S- Editor: Ding Y L- Editor: A E- Editor: Wang CH
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] |
| 2. | Ioannou GN, Splan MF, Weiss NS, McDonald GB, Beretta L, Lee SP. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2007;5:938-45, 945.e1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 240] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 3. | Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, Del Ninno E, Morabito A, Colombo M. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 444] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 4. | Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1271] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 5. | Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790-79; discussion 799-800. [PubMed] |
| 6. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2876] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 7. | Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1325] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 8. | Cillo U, Vitale A, Grigoletto F, Farinati F, Brolese A, Zanus G, Neri D, Boccagni P, Srsen N, D’Amico F. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol. 2006;44:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 332] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 9. | Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 349] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 10. | Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38-46. [PubMed] |
| 11. | Makuuchi M, Sano K. The surgical approach to HCC: our progress and results in Japan. Liver Transpl. 2004;10:S46-S52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Boyault S, Rickman DS, de Reyniès A, Balabaud C, Rebouissou S, Jeannot E, Hérault A, Saric J, Belghiti J, Franco D. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45:42-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 927] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 13. | Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, Gupta S, Moore J, Wrobel MJ, Lerner J. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995-2004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1044] [Cited by in RCA: 1006] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 14. | Chiang DY, Villanueva A, Hoshida Y, Peix J, Newell P, Minguez B, LeBlanc AC, Donovan DJ, Thung SN, Solé M. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008;68:6779-6788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 570] [Cited by in RCA: 565] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 15. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3282] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 16. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4521] [Article Influence: 347.8] [Reference Citation Analysis (2)] |
| 17. | Roayaie S, Obeidat K, Sposito C, Mariani L, Bhoori S, Pellegrinelli A, Labow D, Llovet JM, Schwartz M, Mazzaferro V. Resection of hepatocellular cancer ≤2 cm: results from two Western centers. Hepatology. 2013;57:1426-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 314] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 18. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5312] [Article Influence: 183.2] [Reference Citation Analysis (0)] |
| 19. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1695] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 20. | Roayaie S, Frischer JS, Emre SH, Fishbein TM, Sheiner PA, Sung M, Miller CM, Schwartz ME. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002;235:533-539. [PubMed] |
| 21. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1573] [Article Influence: 92.5] [Reference Citation Analysis (1)] |
| 22. | Sala M, Llovet JM, Vilana R, Bianchi L, Solé M, Ayuso C, Brú C, Bruix J. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. 2004;40:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 338] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 23. | Vivarelli M, Guglielmi A, Ruzzenente A, Cucchetti A, Bellusci R, Cordiano C, Cavallari A. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg. 2004;240:102-107. [PubMed] |
| 24. | Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1104] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 25. | A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire. N Engl J Med. 1995;332:1256-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 596] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 26. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2611] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 27. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 1987] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 28. | Carr BI. Hepatic arterial 90Yttrium glass microspheres (Therasphere) for unresectable hepatocellular carcinoma: interim safety and survival data on 65 patients. Liver Transpl. 2004;10:S107-S110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 188] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, Sato KT, Gupta R, Nikolaidis P, Miller FH. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497-507.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 504] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 30. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10271] [Article Influence: 604.2] [Reference Citation Analysis (2)] |
| 31. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4652] [Article Influence: 273.6] [Reference Citation Analysis (0)] |
| 32. | Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 665] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 33. | Okamoto E, Kyo A, Yamanaka N, Tanaka N, Kuwata K. Prediction of the safe limits of hepatectomy by combined volumetric and functional measurements in patients with impaired hepatic function. Surgery. 1984;95:586-592. [PubMed] |
| 34. | Miyagawa S, Makuuchi M, Kawasaki S, Kakazu T. Criteria for safe hepatic resection. Am J Surg. 1995;169:589-594. [PubMed] |
| 35. | Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, Visa J, Bru C, Rodés J. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018-1022. [PubMed] |
| 36. | Cucchetti A, Piscaglia F, Caturelli E, Benvegnù L, Vivarelli M, Ercolani G, Cescon M, Ravaioli M, Grazi GL, Bolondi L. Comparison of recurrence of hepatocellular carcinoma after resection in patients with cirrhosis to its occurrence in a surveilled cirrhotic population. Ann Surg Oncol. 2009;16:413-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 37. | Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 343] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 38. | Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, Denys A, Sauvanet A. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 429] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 39. | Arii S, Tanaka S, Mitsunori Y, Nakamura N, Kudo A, Noguchi N, Irie T. Surgical strategies for hepatocellular carcinoma with special reference to anatomical hepatic resection and intraoperative contrast-enhanced ultrasonography. Oncology. 2010;78 Suppl 1:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Katz SC, Shia J, Liau KH, Gonen M, Ruo L, Jarnagin WR, Fong Y, D’Angelica MI, Blumgart LH, Dematteo RP. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 335] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 41. | Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, Labow DM, Llovet JM, Schwartz ME. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850-855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 519] [Cited by in RCA: 539] [Article Influence: 33.7] [Reference Citation Analysis (1)] |
| 42. | Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200-207. [PubMed] |
| 43. | Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli F, Marty J, Farges O. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1999;229:369-375. [PubMed] |
| 44. | Figueras J, Llado L, Ruiz D, Ramos E, Busquets J, Rafecas A, Torras J, Fabregat J. Complete versus selective portal triad clamping for minor liver resections: a prospective randomized trial. Ann Surg. 2005;241:582-590. [PubMed] |
| 45. | Gurusamy KS, Pamecha V, Sharma D, Davidson BR. Techniques for liver parenchymal transection in liver resection. Cochrane Database Syst Rev. 2009;CD006880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373-382. [PubMed] |
| 47. | Wayne JD, Lauwers GY, Ikai I, Doherty DA, Belghiti J, Yamaoka Y, Regimbeau JM, Nagorney DM, Do KA, Ellis LM. Preoperative predictors of survival after resection of small hepatocellular carcinomas. Ann Surg. 2002;235:722-30; discussion 730-1. [PubMed] |
| 48. | Shrager B, Jibara G, Schwartz M, Roayaie S. Resection of hepatocellular carcinoma without cirrhosis. Ann Surg. 2012;255:1135-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 49. | Sogawa H, Shrager B, Jibara G, Tabrizian P, Roayaie S, Schwartz M. Resection or transplant-listing for solitary hepatitis C-associated hepatocellular carcinoma: an intention-to-treat analysis. HPB (Oxford). 2013;15:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Belghiti J, Panis Y, Farges O, Benhamou JP, Fekete F. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg. 1991;214:114-117. [PubMed] |
| 51. | Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, Mirza N, Curley SA, Ellis LM, Regimbeau JM, Rashid A. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527-1536. [PubMed] |
| 52. | Poon RT, Fan ST. Evaluation of the new AJCC/UICC staging system for hepatocellular carcinoma after hepatic resection in Chinese patients. Surg Oncol Clin N Am. 2003;12:35-50, viii. [PubMed] |
| 53. | European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1152] [Cited by in RCA: 1155] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 54. | Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1164] [Cited by in RCA: 1174] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 55. | Miyake Y, Takaki A, Iwasaki Y, Yamamoto K. Meta-analysis: interferon-alpha prevents the recurrence after curative treatment of hepatitis C virus-related hepatocellular carcinoma. J Viral Hepat. 2010;17:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 56. | Shen YC, Hsu C, Chen LT, Cheng CC, Hu FC, Cheng AL. Adjuvant interferon therapy after curative therapy for hepatocellular carcinoma (HCC): a meta-regression approach. J Hepatol. 2010;52:889-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 57. | Izumi R, Shimizu K, Iyobe T, Ii T, Yagi M, Matsui O, Nonomura A, Miyazaki I. Postoperative adjuvant hepatic arterial infusion of Lipiodol containing anticancer drugs in patients with hepatocellular carcinoma. Hepatology. 1994;20:295-301. [PubMed] |
| 58. | Lai EC, Lo CM, Fan ST, Liu CL, Wong J. Postoperative adjuvant chemotherapy after curative resection of hepatocellular carcinoma: a randomized controlled trial. Arch Surg. 1998;133:183-188. [PubMed] |
| 59. | Yamasaki S, Hasegawa H, Kinoshita H, Furukawa M, Imaoka S, Takasaki K, Kakumoto Y, Saitsu H, Yamada R, Oosaki Y. A prospective randomized trial of the preventive effect of pre-operative transcatheter arterial embolization against recurrence of hepatocellular carcinoma. Jpn J Cancer Res. 1996;87:206-211. [PubMed] |
| 60. | Lau WY, Lai EC, Leung TW, Yu SC. Adjuvant intra-arterial iodine-131-labeled lipiodol for resectable hepatocellular carcinoma: a prospective randomized trial-update on 5-year and 10-year survival. Ann Surg. 2008;247:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 61. | Lygidakis NJ, Tsiliakos S. Multidisciplinary management of hepatocellular carcinoma. Hepatogastroenterology. 1996;43:1611-1619. [PubMed] |
| 62. | Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 653] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 63. | Yamamoto M, Arii S, Sugahara K, Tobe T. Adjuvant oral chemotherapy to prevent recurrence after curative resection for hepatocellular carcinoma. Br J Surg. 1996;83:336-340. [PubMed] |
| 64. | Kubo S, Nishiguchi S, Hirohashi K, Tanaka H, Shuto T, Yamazaki O, Shiomi S, Tamori A, Oka H, Igawa S. Effects of long-term postoperative interferon-alpha therapy on intrahepatic recurrence after resection of hepatitis C virus-related hepatocellular carcinoma. A randomized, controlled trial. Ann Intern Med. 2001;134:963-967. [PubMed] |
| 65. | Mazzaferro V, Romito R, Schiavo M, Mariani L, Camerini T, Bhoori S, Capussotti L, Calise F, Pellicci R, Belli G. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology. 2006;44:1543-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 295] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 66. | Lau WY, Leung TW, Ho SK, Chan M, Machin D, Lau J, Chan AT, Yeo W, Mok TS, Yu SC. Adjuvant intra-arterial iodine-131-labelled lipiodol for resectable hepatocellular carcinoma: a prospective randomised trial. Lancet. 1999;353:797-801. [PubMed] |
| 67. | Boucher E, Corbinais S, Rolland Y, Bourguet P, Guyader D, Boudjema K, Meunier B, Raoul JL. Adjuvant intra-arterial injection of iodine-131-labeled lipiodol after resection of hepatocellular carcinoma. Hepatology. 2003;38:1237-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 68. | Muto Y, Moriwaki H, Ninomiya M, Adachi S, Saito A, Takasaki KT, Tanaka T, Tsurumi K, Okuno M, Tomita E. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. N Engl J Med. 1996;334:1561-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 477] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 69. | Samuel M, Chow PK, Chan Shih-Yen E, Machin D, Soo KC. Neoadjuvant and adjuvant therapy for surgical resection of hepatocellular carcinoma. Cochrane Database Syst Rev. 2009;CD001199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 70. | Printz C. Clinical trials of note. Sorafenib as adjuvant treatment in the prevention of disease recurrence in patients with hepatocellular carcinoma (HCC) (STORM). Cancer. 2009;115:4646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | Roayaie S, Bassi D, Tarchi P, Labow D, Schwartz M. Second hepatic resection for recurrent hepatocellular cancer: a Western experience. J Hepatol. 2011;55:346-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 72. | Marín-Hargreaves G, Azoulay D, Bismuth H. Hepatocellular carcinoma: surgical indications and results. Crit Rev Oncol Hematol. 2003;47:13-27. [PubMed] |
| 73. | Majno PE, Sarasin FP, Mentha G, Hadengue A. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: an outcome-oriented decision analysis. Hepatology. 2000;31:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 262] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 74. | Belghiti J, Cortes A, Abdalla EK, Régimbeau JM, Prakash K, Durand F, Sommacale D, Dondero F, Lesurtel M, Sauvanet A. Resection prior to liver transplantation for hepatocellular carcinoma. Ann Surg. 2003;238:885-92; discussion 892-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 319] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 75. | Adam R, Azoulay D, Castaing D, Eshkenazy R, Pascal G, Hashizume K, Samuel D, Bismuth H. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg. 2003;238:508-18; discussion 518-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 239] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 76. | Cherqui D, Laurent A, Mocellin N, Tayar C, Luciani A, Van Nhieu JT, Decaens T, Hurtova M, Memeo R, Mallat A. Liver resection for transplantable hepatocellular carcinoma: long-term survival and role of secondary liver transplantation. Ann Surg. 2009;250:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 77. | Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216-222. [PubMed] |
| 78. | Ho CM, Lee PH, Shau WY, Ho MC, Wu YM, Hu RH. Survival in patients with recurrent hepatocellular carcinoma after primary hepatectomy: comparative effectiveness of treatment modalities. Surgery. 2012;151:700-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 79. | Chen WT, Chau GY, Lui WY, Tsay SH, King KL, Loong CC, Wu CW. Recurrent hepatocellular carcinoma after hepatic resection: prognostic factors and long-term outcome. Eur J Surg Oncol. 2004;30:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 80. | Kishi Y, Saiura A, Yamamoto J, Koga R, Seki M, Morimura R, Yoshioka R, Kokudo N, Yamaguchi T. Repeat treatment for recurrent hepatocellular carcinoma: is it validated? Langenbecks Arch Surg. 2011;396:1093-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 81. | Lam CM, Lo CM, Yuen WK, Liu CL, Fan ST. Prolonged survival in selected patients following surgical resection for pulmonary metastasis from hepatocellular carcinoma. Br J Surg. 1998;85:1198-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 82. | Bismuth H, Majno PE, Adam R. Liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 1999;19:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 303] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 83. | Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 706] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 84. | Gondolesi GE, Roayaie S, Muñoz L, Kim-Schluger L, Schiano T, Fishbein TM, Emre S, Miller CM, Schwartz ME. Adult living donor liver transplantation for patients with hepatocellular carcinoma: extending UNOS priority criteria. Ann Surg. 2004;239:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 162] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 85. | Todo S, Furukawa H; Japanese Study Group on Organ Transplantation. Living donor liver transplantation for adult patients with hepatocellular carcinoma: experience in Japan. Ann Surg. 2004;240:451-49; discussion 459-61. [PubMed] |
| 86. | Kwon CH, Kim DJ, Han YS, Park JB, Choi GS, Kim SJ, Joh JW, Lee SK. HCC in living donor liver transplantation: can we expand the Milan criteria? Dig Dis. 2007;25:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 87. | Lee SG, Hwang S, Moon DB, Ahn CS, Kim KH, Sung KB, Ko GY, Park KM, Ha TY, Song GW. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl. 2008;14:935-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 260] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 88. | Herrero JI, Sangro B, Quiroga J, Pardo F, Herraiz M, Cienfuegos JA, Prieto J. Influence of tumor characteristics on the outcome of liver transplantation among patients with liver cirrhosis and hepatocellular carcinoma. Liver Transpl. 2001;7:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 89. | Jonas S, Mittler J, Pascher A, Schumacher G, Theruvath T, Benckert C, Rudolph B, Neuhaus P. Living donor liver transplantation of the right lobe for hepatocellular carcinoma in cirrhosis in a European center. Liver Transpl. 2007;13:896-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 90. | Toso C, Trotter J, Wei A, Bigam DL, Shah S, Lancaster J, Grant DR, Greig PD, Shapiro AM, Kneteman NM. Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2008;14:1107-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 91. | Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: Tokyo University series. Dig Dis. 2007;25:310-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 92. | Volk ML, Vijan S, Marrero JA. A novel model measuring the harm of transplanting hepatocellular carcinoma exceeding Milan criteria. Am J Transplant. 2008;8:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 93. | Duffy JP, Vardanian A, Benjamin E, Watson M, Farmer DG, Ghobrial RM, Lipshutz G, Yersiz H, Lu DS, Lassman C. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg. 2007;246:502-59; discussion 502-59;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 347] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 94. | Wiesner RH, Freeman RB, Mulligan DC. Liver transplantation for hepatocellular cancer: the impact of the MELD allocation policy. Gastroenterology. 2004;127:S261-S267. [PubMed] |
| 95. | Martin AP, Bartels M, Hauss J, Fangmann J. Overview of the MELD score and the UNOS adult liver allocation system. Transplant Proc. 2007;39:3169-3174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 96. | Vibert E, Azoulay D, Hoti E, Iacopinelli S, Samuel D, Salloum C, Lemoine A, Bismuth H, Castaing D, Adam R. Progression of alphafetoprotein before liver transplantation for hepatocellular carcinoma in cirrhotic patients: a critical factor. Am J Transplant. 2010;10:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 97. | Majno P, Giostra E, Mentha G. Management of hepatocellular carcinoma on the waiting list before liver transplantation: time for controlled trials? Liver Transpl. 2007;13:S27-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 98. | Mazzaferro V, Battiston C, Perrone S, Pulvirenti A, Regalia E, Romito R, Sarli D, Schiavo M, Garbagnati F, Marchianò A. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg. 2004;240:900-909. [PubMed] |
| 99. | Graziadei IW, Sandmueller H, Waldenberger P, Koenigsrainer A, Nachbaur K, Jaschke W, Margreiter R, Vogel W. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl. 2003;9:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 336] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 100. | Yao FY, Kerlan RK, Hirose R, Davern TJ, Bass NM, Feng S, Peters M, Terrault N, Freise CE, Ascher NL. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 409] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 101. | Ravaioli M, Grazi GL, Piscaglia F, Trevisani F, Cescon M, Ercolani G, Vivarelli M, Golfieri R, D’Errico Grigioni A, Panzini I. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8:2547-2557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 299] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 102. | Bhoori S, Sposito C, Germini A, Coppa J, Mazzaferro V. The challenges of liver transplantation for hepatocellular carcinoma on cirrhosis. Transpl Int. 2010;23:712-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 103. | Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 785] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 104. | Lo CM, Fan ST, Liu CL, Chan SC, Ng IO, Wong J. Living donor versus deceased donor liver transplantation for early irresectable hepatocellular carcinoma. Br J Surg. 2007;94:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 105. | Fisher RA, Kulik LM, Freise CE, Lok AS, Shearon TH, Brown RS, Ghobrial RM, Fair JH, Olthoff KM, Kam I. Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation. Am J Transplant. 2007;7:1601-1608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 210] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 106. | Prasad KR, Young RS, Burra P, Zheng SS, Mazzaferro V, Moon DB, Freeman RB. Summary of candidate selection and expanded criteria for liver transplantation for hepatocellular carcinoma: a review and consensus statement. Liver Transpl. 2011;17 Suppl 2:S81-S89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 107. | Majno P, Mazzaferro V. Living donor liver transplantation for hepatocellular carcinoma exceeding conventional criteria: questions, answers and demands for a common language. Liver Transpl. 2006;12:896-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 108. | Iwatsuki S, Starzl TE, Sheahan DG, Yokoyama I, Demetris AJ, Todo S, Tzakis AG, Van Thiel DH, Carr B, Selby R. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann Surg. 1991;214:221-28; discussion 228-9. [PubMed] |
| 109. | Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145-151. [PubMed] |
| 110. | Roayaie S, Haim MB, Emre S, Fishbein TM, Sheiner PA, Miller CM, Schwartz ME. Comparison of surgical outcomes for hepatocellular carcinoma in patients with hepatitis B versus hepatitis C: a western experience. Ann Surg Oncol. 2000;7:764-770. [PubMed] |
| 111. | Hemming AW, Nelson DR, Reed AI. Liver transplantation for hepatocellular carcinoma. Minerva Chir. 2002;57:575-585. [PubMed] |
| 112. | D’Amico F, Schwartz M, Vitale A, Tabrizian P, Roayaie S, Thung S, Guido M, del Rio Martin J, Schiano T, Cillo U. Predicting recurrence after liver transplantation in patients with hepatocellular carcinoma exceeding the up-to-seven criteria. Liver Transpl. 2009;15:1278-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 113. | Bozorgzadeh A, Orloff M, Abt P, Tsoulfas G, Younan D, Kashyap R, Jain A, Mantry P, Maliakkal B, Khorana A. Survival outcomes in liver transplantation for hepatocellular carcinoma, comparing impact of hepatitis C versus other etiology of cirrhosis. Liver Transpl. 2007;13:807-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 114. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4508] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 115. | Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, Krieger NR, Schwartz ME. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 346] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 116. | Schwartz M, Roayaie S, Llovet J. How should patients with hepatocellular carcinoma recurrence after liver transplantation be treated? J Hepatol. 2005;43:584-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 117. | Taketomi A, Fukuhara T, Morita K, Kayashima H, Ninomiya M, Yamashita Y, Ikegami T, Uchiyama H, Yoshizumi T, Soejima Y. Improved results of a surgical resection for the recurrence of hepatocellular carcinoma after living donor liver transplantation. Ann Surg Oncol. 2010;17:2283-2289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 118. | Zhou B, Shan H, Zhu KS, Jiang ZB, Guan SH, Meng XC, Zeng XC. Chemoembolization with lobaplatin mixed with iodized oil for unresectable recurrent hepatocellular carcinoma after orthotopic liver transplantation. J Vasc Interv Radiol. 2010;21:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 119. | Kita Y, Klintmalm G, Kobayashi S, Yanaga K. Retransplantation for de novo hepatocellular carcinoma in a liver allograft with recurrent hepatitis B cirrhosis 14 years after primary liver transplantation. Dig Dis Sci. 2007;52:3392-3393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 120. | Chen GH, Fu BS, Yang Y, Cai CJ, Lu MQ, Li H, Wang GS, Yi SH, Xu C, Zhang JF. Early liver retransplantation versus late liver retransplantation: analysis of a single-center experience. Chin Med J (Engl). 2008;121:1992-1996. [PubMed] |
| 121. | Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 407] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 122. | Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122-130. [PubMed] |
| 123. | Lencioni R, Crocetti L. A critical appraisal of the literature on local ablative therapies for hepatocellular carcinoma. Clin Liver Dis. 2005;9:301-14, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 124. | Rhim H. Complications of radiofrequency ablation in hepatocellular carcinoma. Abdom Imaging. 2005;30:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 125. | Lencioni R, Crocetti L. Image-guided thermal ablation of hepatocellular carcinoma. Crit Rev Oncol Hematol. 2008;66:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 126. | Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pagès M, Ayuso C. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 529] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 127. | Lu DS, Yu NC, Raman SS, Limanond P, Lassman C, Murray K, Tong MJ, Amado RG, Busuttil RW. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234:954-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 295] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 128. | Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, Frings H, Laubenberger J, Zuber I, Blum HE. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 674] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 129. | Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma & lt; or =4 cm. Gastroenterology. 2004;127:1714-1723. [PubMed] |
| 130. | Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 431] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 131. | Brunello F, Veltri A, Carucci P, Pagano E, Ciccone G, Moretto P, Sacchetto P, Gandini G, Rizzetto M. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: A randomized controlled trial. Scand J Gastroenterol. 2008;43:727-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 199] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 132. | Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, Bartolozzi C. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 628] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 133. | Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 642] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 134. | Hasegawa K, Kokudo N, Makuuchi M, Izumi N, Ichida T, Kudo M, Ku Y, Sakamoto M, Nakashima O, Matsui O. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol. 2013;58:724-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 301] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 135. | Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 825] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 136. | Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, Konishi J. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 391] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 137. | Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo) embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev. 2011;CD004787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 138. | Molinari M, Kachura JR, Dixon E, Rajan DK, Hayeems EB, Asch MR, Benjamin MS, Sherman M, Gallinger S, Burnett B. Transarterial chemoembolisation for advanced hepatocellular carcinoma: results from a North American cancer centre. Clin Oncol (R Coll Radiol). 2006;18:684-692. [PubMed] |
| 139. | Lladó L, Virgili J, Figueras J, Valls C, Dominguez J, Rafecas A, Torras J, Fabregat J, Guardiola J, Jaurrieta E. A prognostic index of the survival of patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. Cancer. 2000;88:50-57. [PubMed] |
| 140. | Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1063] [Cited by in RCA: 1208] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 141. | Lin DY, Liaw YF, Lee TY, Lai CM. Hepatic arterial embolization in patients with unresectable hepatocellular carcinoma--a randomized controlled trial. Gastroenterology. 1988;94:453-456. [PubMed] |
| 142. | Pelletier G, Roche A, Ink O, Anciaux ML, Derhy S, Rougier P, Lenoir C, Attali P, Etienne JP. A randomized trial of hepatic arterial chemoembolization in patients with unresectable hepatocellular carcinoma. J Hepatol. 1990;11:181-184. [PubMed] |
| 143. | Bruix J, Llovet JM, Castells A, Montañá X, Brú C, Ayuso MC, Vilana R, Rodés J. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology. 1998;27:1578-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 391] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 144. | Nicolini A, Martinetti L, Crespi S, Maggioni M, Sangiovanni A. Transarterial chemoembolization with epirubicin-eluting beads versus transarterial embolization before liver transplantation for hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 145. | Kwon JH, Bae SH, Kim JY, Choi BO, Jang HS, Jang JW, Choi JY, Yoon SK, Chung KW. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer. 2010;10:475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 146. | Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, Johnstone PA, Cardenes HR. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e447-e453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 321] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 147. | Huang WY, Jen YM, Lee MS, Chang LP, Chen CM, Ko KH, Lin KT, Lin JC, Chao HL, Lin CS. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;84:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 148. | Kang JK, Kim MS, Cho CK, Yang KM, Yoo HJ, Kim JH, Bae SH, Jung da H, Kim KB, Lee DH. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. 2012;118:5424-5431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 245] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 149. | Facciuto ME, Singh MK, Rochon C, Sharma J, Gimenez C, Katta U, Moorthy CR, Bentley-Hibbert S, Rodriguez-Davalos M, Wolf DC. Stereotactic body radiation therapy in hepatocellular carcinoma and cirrhosis: evaluation of radiological and pathological response. J Surg Oncol. 2012;105:692-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 150. | Sangro B, Bilbao JI, Boan J, Martinez-Cuesta A, Benito A, Rodriguez J, Panizo A, Gil B, Inarrairaegui M, Herrero I. Radioembolization using 90Y-resin microspheres for patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2006;66:792-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 151. | Kulik LM, Carr BI, Mulcahy MF, Lewandowski RJ, Atassi B, Ryu RK, Sato KT, Benson A, Nemcek AA, Gates VL. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 457] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 152. | Hilgard P, Hamami M, Fouly AE, Scherag A, Müller S, Ertle J, Heusner T, Cicinnati VR, Paul A, Bockisch A. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52:1741-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 353] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 153. | Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 781] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 154. | Abou-Alfa GK. Selection of patients with hepatocellular carcinoma for sorafenib. J Natl Compr Canc Netw. 2009;7:397-403. [PubMed] |
| 155. | Abou-Alfa GK, Amadori D, Santoro A, Figer A, De Greve J, Lathia C, Voliotis D, Anderson S, Moscovici M, Ricci S. Safety and Efficacy of Sorafenib in Patients with Hepatocellular Carcinoma (HCC) and Child-Pugh A versus B Cirrhosis. Gastrointest Cancer Res. 2011;4:40-44. [PubMed] |
| 156. | Hollebecque A, Cattan S, Romano O, Sergent G, Mourad A, Louvet A, Dharancy S, Boleslawski E, Truant S, Pruvot FR. Safety and efficacy of sorafenib in hepatocellular carcinoma: the impact of the Child-Pugh score. Aliment Pharmacol Ther. 2011;34:1193-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 157. | Kim JE, Ryoo BY, Ryu MH, Chang HM, Suh DJ, Lee HC, Lim YS, Kim KM, Kang YK. Sorafenib for hepatocellular carcinoma according to Child-Pugh class of liver function. Cancer Chemother Pharmacol. 2011;68:1285-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 158. | Toh HC, Chen PJ, Carr BI, Knox JJ, Gill S, Ansell P, McKeegan EM, Dowell B, Pedersen M, Qin Q. Phase 2 trial of linifanib (ABT-869) in patients with unresectable or metastatic hepatocellular carcinoma. Cancer. 2013;119:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 159. | Hsu CH, Shen YC, Lin ZZ, Chen PJ, Shao YY, Ding YH, Hsu C, Cheng AL. Phase II study of combining sorafenib with metronomic tegafur/uracil for advanced hepatocellular carcinoma. J Hepatol. 2010;53:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 160. | Thomas MB, Chadha R, Glover K, Wang X, Morris J, Brown T, Rashid A, Dancey J, Abbruzzese JL. Phase 2 study of erlotinib in patients with unresectable hepatocellular carcinoma. Cancer. 2007;110:1059-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 234] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 161. | Thomas MB, Morris JS, Chadha R, Iwasaki M, Kaur H, Lin E, Kaseb A, Glover K, Davila M, Abbruzzese J. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol. 2009;27:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 243] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 162. | Faivre S, Raymond E, Boucher E, Douillard J, Lim HY, Kim JS, Zappa M, Lanzalone S, Lin X, Deprimo S. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicentre, phase II study. Lancet Oncol. 2009;10:794-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 222] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 163. | Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011;140:1410-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 369] [Article Influence: 26.4] [Reference Citation Analysis (0)] |