Published online Jan 21, 2014. doi: 10.3748/wjg.v20.i3.822
Revised: November 6, 2013
Accepted: December 3, 2013
Published online: January 21, 2014
Processing time: 199 Days and 16.7 Hours
AIM: To investigate dysfunctions in esophageal peristalsis and sensation in patients with Barrett’s esophagus following acid infusion using endoscopy-based testing.
METHODS: First, physiological saline was infused into the esophagus of five healthy subjects, at a rate of 10 mL/min for 10 min, followed by infusion of HCl. Esophageal contractions were analyzed to determine whether the contractions observed by endoscopy and ultrasonography corresponded to the esophageal peristaltic waves diagnosed by manometry. Next, using nasal endoscopy, esophageal sensations and contractions were investigated in patients with, as well as controls without, Barrett’s esophagus using the same infusion protocol.
RESULTS: All except one of the propulsive contractions identified endoscopically were recorded as secondary peristaltic waves by manometry. Patients with long segment Barrett’s esophagus (LSBE) tended to have a shorter lag time than the control group, although the difference did not reach statistical significance (88 ± 54 s vs 162 ± 150 s respectively, P = 0.14). Furthermore, patients with LSBE had significantly fewer secondary contractions following the infusion of both saline and HCl than did either the control group or patients with short segment Barrett’s esophagus (4.1 ± 1.2 vs 8.0 ± 2.8, P < 0.001 and 7.3 ± 3.2, P < 0.01, respectively, following saline infusion; 5.3 ± 1.2 vs 8.4 ± 2.4 and 8.1 ± 2.9 respectively, P < 0.01 for both, following infusion of HCl).
CONCLUSION: Using nasal endoscopy and a simple acid-perfusion study, we were able to demonstrate disorders in secondary peristalsis in patients with LSBE.
Core tip: We have developed a simple technique for esophageal examination based on transnasal endoscopy in unsedated patients. First, manometric waves and esophageal contractions were evaluated using three different modalities following the infusion of acid into the lower esophagus. Next, using nasal endoscopy, esophageal contractions and sensations were investigated in patients with Barrett’s esophagus. It was possible to observe secondary peristalsis endoscopically, using nasal endoscopy and a simple acid-perfusion study, we were able to demonstrate disorders in secondary peristalsis in patients with long segment Barrett’s esophagus.
- Citation: Kobayashi G, Kaise M, Arakawa H, Tajiri H. Impairment of secondary peristalsis in Barrett’s esophagus by transnasal endoscopy-based testing. World J Gastroenterol 2014; 20(3): 822-828
- URL: https://www.wjgnet.com/1007-9327/full/v20/i3/822.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i3.822
Because of its increasing prevalence in Asian countries[1,2], the clinical impact of gastroesophageal reflux disease (GERD) is also increasing. Furthermore, some of the patients with GERD will go on to develop Barrett’s esophagus, which itself can progress to adenocarcinoma[3]. Both GERD and Barrett’s esophagus result from chronic injury following long-term exposure of the squamous mucosa to gastric acid or bile. Pathogenic exposure to refluxate may be due to disturbances in anti-reflux barriers or delayed luminal acid clearance because of abnormalities in esophageal motility or the sensory system. However, the precise causative dysfunction varies between patients; thus, esophageal function tests are needed to determine the cause to enable cause-specific treatment. However, such examinations, including manometry[4-7], sensory testing[8-10], and pH monitoring[11-14], are not routinely used in clinical practice because they are tedious, complicated, and invasive.
We have developed a simple and versatile technique for esophageal examination based on transnasal endoscopy in unsedated patients. The test can simultaneously evaluate either structural abnormalities of the lumen and anti-reflux barriers or dysfunctions in esophageal peristalsis and sensation induced by acid infusion. Using this endoscopy-based test in the present study, we examined patients with Barrett’s esophagus and healthy controls to identify abnormalities in esophageal function related to Barrett’s esophagus.
The present study was approved by the Ethics Committee of The Jikei University School of Medicine (Tokyo, Japan) and was conducted at Jikei University Hospital.
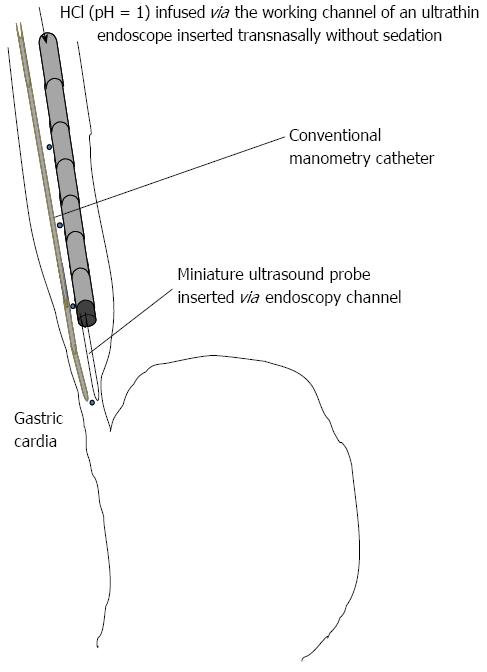
Five healthy subjects without obvious GERD symptoms [all men; age (mean ± SD) 29 ± 3 years] were recruited for the preliminary study. All subjects provided written informed consent before participating in the study. Simultaneous manometry and endoscopy-based testing were performed in these subjects to evaluate physiological saline and acid infusion-induced contractions of the esophagus (Figure 1). An ultrathin endoscope (XP 260 N; Olympus Medical Systems, Tokyo, Japan) was inserted transnasally, without sedation, with the tip of the scope located in the lower esophagus, approximately 5 cm oral from the esophagogastric junction (EGJ). Endoscopic ultrasonography was performed using a radial-type miniature probe (Model UM-S20-17S, 20 MHz; Olympus Medical Systems). The probe was inserted via the endoscope channel and positioned in the lower esophagus approximately 2 cm oral from the EGJ. Conventional manometry was performed using a POLYGRAF ID (Alpinebiomed, Los Angeles, CA, United States) and an infusion pressure four-channel catheter (4.5 mm outside diameter), with an aperture and pressure converter in the 5-cm space (Zinetics, Salt Lake City, UT, United States). To evaluate contractions elicited by the infusion of physiological saline and acid, indigo carmine was used to color both the physiological saline and acid solution. The colored physiological saline was infused initially into the esophagus at a rate of 10 mL/min for 10 min via the working channel of the scope using an autoinfusion pump (TE-171; TERUMO, Tokyo, Japan). Subsequently, and without alerting the healthy subject to the change, the colored HCl (pH = 1) was infused for another 10 min at the same rate[15]. he manometric waves and ultrasonographic and endoscopic views were displayed on the same monitor using a screen separation device (MV-410RGB; HOUEI, Tokyo, Japan).
Esophageal contractions induced by the infusion of physiological saline and acid were analyzed simultaneously by all three modalities, namely conventional manometry, endoscopic ultrasonography, and endoscopic observation. This was done to determine whether the esophageal peristaltic waves diagnosed by manometry corresponded to the propulsive contractions identified by endoscopy. In the present study, primary and secondary peristalsis were defined as successful if a pressure wave > 12 mmHg at the two proximal esophageal recording sites [12 and 17 cm above the lower esophageal sphincter (LES)] and > 25 mmHg at the middle and distal esophageal recording sites progressively traversed all the esophageal recording sites[16]. Peristaltic progression was defined as a peristaltic velocity of ≤ 6 cm/s[17]. Failed peristalsis was defined as either failure to generate a pressure wave > 12 mmHg at the two proximal esophageal recording sites and > 25 mmHg at the middle and distal esophageal recording sites, a failure of the wave to traverse each of the esophageal recording sites, or a peristaltic velocity > 6 cm/s between the recording sites 2 and 17 cm above the LES[16]. Esophageal contractions were determined to be propulsive if a full contraction observed by endoscopic sonography propelled the indigo carmine-stained acid retained in the esophagus into the stomach. Esophageal contractions were determined to be non-propulsive if an incomplete contraction did not propel the acid into the stomach. Peristaltic waves and propulsive contractions were identified as primary or secondary on the basis of the presence or absence of deglutition, respectively.
After we confirmed the concordance of esophageal peristalsis as assessed by manometry and propulsive contraction determined endoscopically, we performed the primary study using endoscopy-based testing without manometry. The ultrathin endoscope was inserted transnasally in unsedated patients to allow for endoscopic observation of the esophagus and cardia. Then, esophageal propulsive contractions and esophageal sensations were assessed simultaneously during infusion of physiological saline and HCl (pH = 1) (10 min each; 10 mL/min) via the endoscope channel. A 5-min interval was allowed between the acid and saline infusions.
The endpoints used to assess esophageal sensations following infusion were lag time, intensity rate, and the acid perfusion sensitivity score (APSS). Lag time was defined as the time (in seconds) to the initial perception of typical symptoms, such as heartburn. The intensity of symptoms associated with acid perfusion was evaluated using a previously validated verbal descriptor scale, with symptoms scored on a scale of 0-10, where 0 means no symptoms and 10 means strong symptoms.The APSS was calculated from the duration of typical symptom perception, expressed in seconds, and the sensory intensity rating at the end of the acid perfusion. The APSS was divided by 100 for convenience[15].
This primary study was conducted in patients with Barrett’s esophagus and subjects undergoing a planned endoscopic examination as part of a routine health check. All subjects provided written informed consent. Using endoscopy-based testing, we evaluated propulsive contractions and acid-induced sensations in these subjects to identify any abnormalities related to Barrett’s esophagus. Furthermore, sera from all subjects were tested for the presence of Helicobacter pylori antibody. Subjects were asked to stop any acid inhibitory drugs or prokinetics 2 weeks before the examination. If, for any reason, subjects could not stop their medication, they were not included in the study. Furthermore, subjects with a previous history of upper gastrointestinal surgery were excluded from the study.
Barrett’s esophagus was diagnosed on the basis of endoscopic detection of columnar epithelium extending continuously from the EGJ into the esophagus, without obtaining histological confirmation of the presence of intestinal metaplasia[18]. The EGJ was defined as the end of the palisading vessels of the lower esophagus[18]. Short segment Barrett’s esophagus (SSBE) was defined as the presence of a columnar epithelium covering < 3 cm of at least one segment from the EGJ. Long segment Barrett’s esophagus (LSBE) was defined as the presence of columnar epithelium > 3 cm from the EGJ and always covering the entire circumference[19].
All data are presented as the mean ± SD. The significance of differences among the control, SSBE and LSBE groups was assessed using the Mann-Whitney U test. P < 0.05 was considered significant.
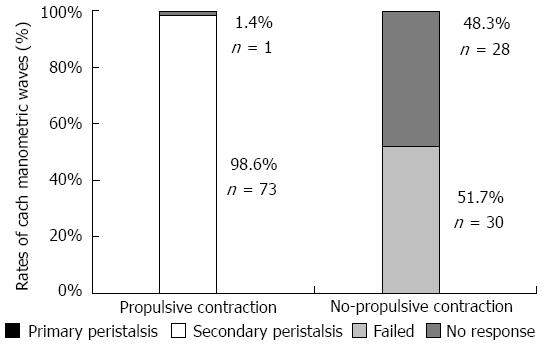
Physiological saline- and acid infusion-induced esophageal contractions were successfully recorded in five subjects using three different modalities simultaneously. Endoscopy revealed a total of 132 esophageal contractions without deglutition during physiological saline and acid infusion. Of these contractions, 74 (56%) and 58 (44%) contractions were determined to be propulsive and non-propulsive, respectively. All but one of the propulsive contractions assessed by endoscopy were recorded as secondary peristaltic waves by manometry (Figure 2). Primary peristalsis by deglutition was not observed. It is likely that some sort of mechanical failure was responsible for the one propulsive wave showing no marked wave on manometry. Of the 58 non-propulsive contractions assessed by endoscopy, manometry recorded 51.7% and 48.3% as failed and no response, respectively. The high concordance between esophageal peristalsis assessed by manometry and propulsive contractions assessed by endoscopy indicates that endoscopic observation of propulsive contractions is a satisfactory method for evaluating secondary peristalsis induced by physiological saline and acid infusion.
Twenty-six patients with Barrett’s esophagus (mean age 40 ± 15 years) and 25 subjects undergoing endoscopic surveillance as part of their health check up (mean age 32 ± 7 years) were enrolled in the primary study. Of the 26 patients with Barrett’s esophagus, 17 and nine were determined to have SSBE and LSBE, respectively. None of the 25 individuals undergoing the health check had Barrett’s esophagus and so were used as the non-Barrett’s esophagus control group. Two of the 26 patients with Barrett’s esophagus (one in each of the LSBE and SSBE groups) were excluded from the study because there was unsatisfactory accumulation of solution following infusion in the esophagus, probably due to a large hiatal opening. The characteristics of the remaining subjects in each group are given in Table 1. Subjects in the non-Barrett’s esophagus control group were younger, with a higher proportion of women, than patients with Barrett’s esophagus. The rate of erosive reflux esophagitis was significantly higher in patients with Barrett’s esophagus than in the control group.
| Control (n = 25) | SSBE (n = 16) | LSBE (n = 8) | |
| Sex (male/female) | 19/6 | 15/1b | 8/0b |
| Age (yr) | 32 ± 7 | 38.4 ± 14.4 | 38.0 ± 14.0 |
| BMI (kg/m2) | 22 ± 2.8 | 22.7 ± 3.1 | 22.2 ± 3.5 |
| No. smokers | 7 (28) | 4 (25) | 4 (50)bd |
| No. drinkers | 20 (80) | 14 (87.6) | 7 (87.5) |
| No. Helicobacter pylori antibody positive | 4 (18.2) | 2 (12.5) | 0 (0)bd |
| No. with erosive reflux esophagitis | 1 (4) | 7 (43.8)b | 5 (62.5)bd |
Intensity rates following acid infusion were comparable between non-Barrett’s esophagus controls and patients with Barrett’s esophagus (Table 2). There were no significant differences between the groups in intensity rate. There was a tendency for lag time to be shorter in the LSBE compared with control group, although the difference did not reach statistical significance (88 ± 54 s vs 162 ± 150 s, respectively, P = 0.14). This may indicate that patients with LSBE were more sensitive to acid perfusion. Furthermore, there were no significant differences in the APSS following acid infusion between the non-Barrett’s esophagus controls and patients with Barrett’s esophagus. These results suggest that acid-induced esophageal sensations did not differ between patients with and without Barrett’s esophagus.
| Lag time (s) | Intensity rate | APSS | |
| Control group | 6.1 ± 2.3 | 162 ± 150 | 9.0 ± 8.7 |
| Barrett’s esophagus | 5.8 ± 2.2 | 162 ± 164 | 7.7 ± 6.1 |
| SSBE | 5.7 ± 2.3 | 199 ± 189 | 8.8 ± 6.9 |
| LSBE | 6.1 ± 2.0 | 88 ± 54 | 5.4 ± 3.3 |
There were no significant differences among the groups in the frequency of primary contractions (Table 3) or in the frequency of secondary contractions in the esophagus following 10-min infusion of physiological saline or acid. However, when patients with Barrett’s esophagus were divided into those with LSBE and SSBE, patients in the LSBE group exhibited significantly fewer secondary contractions following infusion of physiological saline than did the non-Barrett’s esophagus controls and patients in the SSBE group (4.1 ± 1.2 vs 8.0 ± 2.8, P < 0.001 and 7.3 ± 3.2, P < 0.01, respectively; Table 3). Similarly, patients in the LSBE group exhibited a lower frequency of acid-induced secondary contractions than did either the control group or patients in the SSBE group (5.3 ± 1.2 vs 8.4 ± 2.4 and 8.1 ± 2.9, respectively, P < 0.01 for both). Although there was a tendency for acid infusion to induce a higher frequency of secondary contractions than infusion of physiological saline, the differences did not reach statistical significance.
Endoscopy is essential for the diagnosis of mucosal and structural abnormalities of the upper gastrointestinal tract in individuals with GERD symptoms. The primary aim of endoscopic examination is to exclude malignant dysphagia and to detect Barrett’s esophagus, with its associated cancer risk. In addition, Kawai et al[20] and we (present study) have demonstrated that transnasal endoscopy in unsedated patients can be used to evaluate esophageal function. Because esophageal propulsive contractions assessed by endoscopy corresponded well to esophageal peristalsis recorded by manometry, endoscopy-based testing, as described herein, may prove to be an adequate method with which to evaluate the frequency of acid perfusion-induced secondary peristalsis. Because this testing simultaneously assessed esophageal sensory status induced by acid infusion, we were able to obtain endoscopic findings and functional information for the esophagus in one examination.
Long-term exposure of the squamous mucosa of the lower esophagus to gastric acid or bile can lead to the development of Barrett’s esophagus. Pathogenic exposure to refluxate can result from disturbances in anti-reflux barriers or delayed luminal acid clearance due to abnormalities in esophageal motility or the sensory system. Using endoscopy-based testing, we were able to demonstrate impairments in acid clearance due to a reduction in secondary peristalsis in patients with LSBE compared with patients with SSBE and the non-Barrett’s esophagus controls. The impairment in secondary peristalsis revealed in the present study is comparable with the findings of a previous study, in which LSBE was characterized by a greater impairment in primary peristaltic wave amplitude than SSBE[21]. Primary peristalsis is the initial response to acid reflux in individuals when in the upright position, whereas secondary peristalsis is the initial clearing event when individuals are supine and asleep. Therefore, combined impairment in both primary and secondary esophageal peristalsis in patients with LSBE may elicit longer acid exposure. Indeed, it is known that the total percentage of time of esophageal acid exposure over 24 h is significantly greater in patients with LSBE compared with SSBE[22].
Impairment of secondary esophageal peristalsis has been reported in patients with various esophageal disorders other than Barrett’s esophagus. For example, defective triggering of secondary peristalsis has been reported in patients with non-erosive reflux disease[23], whereas impaired esophageal bolus transit and clearance by secondary peristalsis was demonstrated in patients with non-obstructive dysphagia[24]. However, the mechanisms underlying defective secondary peristalsis remain unknown. Because secondary peristalsis is a reflex response to esophageal distention, the defect may lie in the esophageal motor nerves or muscles, esophageal sensation, the central integrative mechanisms, or a combination of these[16]. Iwakiri et al suggest that the primary defect lies in esophageal sensation because the triggering of secondary peristalsis was abnormal in all patients with normal primary peristalsis and, when triggered, the wave amplitude and velocity of the secondary peristaltic response were normal. Studies using esophageal balloon distention have reported decreased sensitivity to this stimulus in patients with Barrett’s esophagus, and this could contribute to the impaired esophageal motility in Barrett’s esophagus[25]. As indicated in Table 3, there was no difference in the frequency of secondary contractions induced by acid and saline. A possible explanation for this observation is that the effects of volume stimulation are greater than those of chemical stimulation in triggering secondary peristalsis, but the precise mechanisms involved remain unknown.
Previous studies have demonstrated that patients with Barrett’s esophagus have impaired sensitivity to esophageal distention as well as visceral sensitivity to acid perfusion[25,26]. Using endoscopy-based testing in the present study, we tested visceral sensitivity to acid perfusion and showed that acid infusion-induced sensations in patients with LSBE were comparable to those in patients with SSBE and in the control group. This finding is not compatible with most previous studies that reported that patients with Barrett’s esophagus have impaired visceral sensitivity to acid perfusion. It is possible that this discrepancy between the findings of the present and previous studies may be due to type I error, especially because of the small number of patients with LSBE in the present study. If we compared the lag time or APSS of patients with LSBE and the pooled values for each of these parameters in the control plus SSBE groups, the P values are 0.11 and 0.18, respectively. The mechanism underlying esophageal hyposensitivity in Barrett’s esophagus has not been elucidated, but it has been proposed that Barrett’s mucosa is less sensitive to chemical stimuli. For example, Fass et al[27] demonstrated increased sensitivity to acid in patients with Barrett’s esophagus after complete reversal using multipolar electrocoagulation and suggested that chemoreceptor sensitization was possibly induced by the electrocoagulation technique. However, a recent study showed a significant decrease in esophageal chemoreceptor sensitivity to acid and alkaline in patients with successful reversal of Barrett’s esophagus using argon plasma coagulation. Further studies are needed to evaluate esophageal hypersensitivities in Barrett’s esophagus.
There are some limitations to the present study. First, the controls enrolled in the present study were younger than the patients with Barrett’s esophagus, and the proportion of women in the control group was higher than that in the Barrett’s esophagus group. To overcome this issue, we excluded women from the control and SSBE groups before analyzing the data. Although, after exclusion, the mean age of the control group remained lower than that of the Barrett’s esophagus group (30.1 ± 5.2 years vs 38.1 ± 13.6 years, respectively), the results were very similar to those obtained using the original population. Aging may influence secondary esophageal propulsive contractions, and the older LSBE group may exhibit impaired peristalsis compared with the control group. However, differences in secondary esophageal propulsive contractions were seen between age-matched SSBE and LSBE groups, which suggests that, regardless of age, patients with LSBE have impaired secondary esophageal peristalsis at least in comparison with patients with SSBE.
The endoscopy-based testing used in the present study can evaluate the frequency of esophageal peristalsis but cannot provide detailed information regarding motility parameters, such as esophageal pressures; for detailed evaluation of esophageal dysmotility, examinations such as high-resolution manometry are necessary. Nevertheless, the endoscopy-based testing described in the present study can be used in clinical practice because of its simplicity and versatility.
In conclusion, observation of secondary peristalsis was possible using an endoscopic-based technique. Performing a simple acid perfusion study in unsedated LSBE patients, we were able to demonstrate disorders in secondary peristalsis using nasal endoscopy.
Because of its increasing prevalence in Asian countries, the clinical impact of gastroesophageal reflux disease (GERD) is also increasing. Furthermore, some of the patients with GERD will go on to develop Barrett’s esophagus, which itself can progress to adenocarcinoma. The precise causative dysfunction varies between patients; thus, esophageal function tests are needed to determine the cause to enable cause-specific treatment. However, such examinations, including manometry, sensory testing and pH monitoring, are not routinely used in clinical practice because they are tedious, complicated, and invasive.
The authors have developed a simple and versatile technique for esophageal examination based on transnasal endoscopy in unsedated patients. The test can simultaneously evaluate either structural abnormalities of the lumen and anti-reflux barriers or dysfunctions in esophageal peristalsis and sensation induced by acid infusion. Using this endoscopy-based test in the present study, the authors examined patients with Barrett’s esophagus and healthy controls to identify abnormalities in esophageal function related to Barrett’s esophagus.
First, manometric waves and esophageal contractions were evaluated in healthy subjects using three different modalities. An transnasal endoscopy, endoscopic ultrasonography and manometry was performed into the lower esophagus. Physiological saline was infused initially into the esophagus at a rate of 10 mL/min for 10 min. Subsequently, HCl was infused for 10 min at a similar rate. Esophageal contractions were analyzed. Next, using nasal endoscopy, esophageal sensations and contractions were investigated in patients with and controls without Barrett’s esophagus following the same infusion method. The endoscopy-based testing used in the present study can evaluate the frequency of esophageal peristalsis. The endoscopy-based testing described in the present study can be used in clinical practice because of its simplicity and versatility.
The observation of secondary peristalsis was possible using an endoscopic-based technique. Performing a simple acid perfusion study in unsedated long segment Barrett’s esophagus (LSBE) patients, we were able to demonstrate disorders in secondary peristalsis using nasal endoscopy.
Primary and secondary peristalsis were defined as successful if a pressure wave > 12 mmHg at the two proximal esophageal recording sites above the lower esophageal sphincter and > 25 mmHg at the middle and distal esophageal recording sites progressively traversed all the esophageal recording sites. Peristaltic progression was defined as a peristaltic velocity of ≤ 6 cm/s. Barrett’s esophagus was diagnosed on the basis of endoscopic detection of columnar epithelium extending continuously from the esophagogastric junction (EGJ) into the esophagus, without obtaining histological confirmation of the presence of intestinal metaplasia. The EGJ was defined as the end of the palisading vessels of the lower esophagus.
This is a good descriptive study in which the authors analyzed dysfunctions in esophageal peristalsis and sensation in patients with Barrett’s esophagus following acid infusion using endoscopy-based testing. The results are interesting and suggest that disorders in secondary peristalsis in patients with LSBE.
P- Reviewers: Cao WB, Koike T S- Editor: Zhai HH L- Editor: A E- Editor: Wu HL
| 1. | Kang JY, Ho KY. Different prevalences of reflux oesophagitis and hiatus hernia among dyspeptic patients in England and Singapore. Eur J Gastroenterol Hepatol. 1999;11:845-850. [PubMed] |
| 2. | Furukawa N, Iwakiri R, Koyama T, Okamoto K, Yoshida T, Kashiwagi Y, Ohyama T, Noda T, Sakata H, Fujimoto K. Proportion of reflux esophagitis in 6010 Japanese adults: prospective evaluation by endoscopy. J Gastroenterol. 1999;34:441-444. [PubMed] |
| 3. | Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2028] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 4. | Jacob P, Kahrilas PJ, Vanagunas A. Peristaltic dysfunction associated with nonobstructive dysphagia in reflux disease. Dig Dis Sci. 1990;35:939-942. [PubMed] |
| 5. | Pai CG. Secondary oesophageal peristalsis in gastro-oesophageal reflux disease. J Gastroenterol Hepatol. 2000;15:30-34. [PubMed] |
| 6. | Iwakiri K, Sugiura T, Hayashi Y, Kotoyori M, Kawakami A, Makino H, Nomura T, Miyashita M, Takubo K, Sakamoto C. Esophageal motility in Japanese patients with Barrett’s esophagus. J Gastroenterol. 2003;38:1036-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Aben-Athar CG, Dantas RO. Primary and secondary esophageal contractions in patients with gastroesophageal reflux disease. Braz J Med Biol Res. 2006;39:1027-1031. [PubMed] |
| 8. | Johnson DA, Winters C, Spurling TJ, Chobanian SJ, Cattau EL. Esophageal acid sensitivity in Barrett’s esophagus. J Clin Gastroenterol. 1987;9:23-27. [PubMed] |
| 9. | Marrero JM, de Caestecker JS, Maxwell JD. Effect of famotidine on oesophageal sensitivity in gastro-oesophageal reflux disease. Gut. 1994;35:447-450. [PubMed] |
| 10. | Nagahara A, Miwa H, Minoo T, Hojo M, Kawabe M, Osada T, Kurosawa A, Asaoka D, Terai T, Ohkusa T. Increased esophageal sensitivity to acid and saline in patients with nonerosive gastro-esophageal reflux disease. J Clin Gastroenterol. 2006;40:891-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Vandenplas Y, Helven R, Goyvaerts H. Comparative study of glass and antimony electrodes for continuous oesophageal pH monitoring. Gut. 1991;32:708-712. [PubMed] |
| 12. | Jamieson JR, Stein HJ, DeMeester TR, Bonavina L, Schwizer W, Hinder RA, Albertucci M. Ambulatory 24-h esophageal pH monitoring: normal values, optimal thresholds, specificity, sensitivity, and reproducibility. Am J Gastroenterol. 1992;87:1102-1111. [PubMed] |
| 13. | Kasapidis P, Xynos E, Mantides A, Chrysos E, Demonakou M, Nikolopoulos N, Vassilakis JS. Differences in manometry and 24-H ambulatory pH-metry between patients with and without endoscopic or histological esophagitis in gastroesophageal reflux disease. Am J Gastroenterol. 1993;88:1893-1899. [PubMed] |
| 14. | Loughney T, Maydonovitch CL, Wong RK. Esophageal manometry and ambulatory 24-hour pH monitoring in patients with short and long segment Barrett’s esophagus. Am J Gastroenterol. 1998;93:916-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Fass R, Naliboff BD, Fass SS, Peleg N, Wendel C, Malagon IB, Mayer EA. The effect of auditory stress on perception of intraesophageal acid in patients with gastroesophageal reflux disease. Gastroenterology. 2008;134:696-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Schoeman MN, Holloway RH. Stimulation and characteristics of secondary oesophageal peristalsis in normal subjects. Gut. 1994;35:152-158. [PubMed] |
| 17. | Hewson EG, Ott DJ, Dalton CB, Chen YM, Wu WC, Richter JE. Manometry and radiology. Complementary studies in the assessment of esophageal motility disorders. Gastroenterology. 1990;98:626-632. [PubMed] |
| 18. | Manabe N, Haruma K, Imamura H, Kamada T, Kusunoki H, Inoue K, Shiotani A, Hata J. Does short-segment columnar-lined esophagus elongate during a mean follow-up period of 5.7 years? Dig Endosc. 2011;23:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Amano Y, Kinoshita Y. Barrett esophagus: perspectives on its diagnosis and management in asian populations. Gastroenterol Hepatol (N Y). 2008;4:45-53. [PubMed] |
| 20. | Kawai T, Yamagishi T, Yagi K, Kataoka M, Kawakami K, Sofuni A, Itoi T, Sakai Y, Moriyasu F, Osaka Y. Impact of transnasal ultrathin esophagogastroduodenoscopy (UT-EGD) in the evaluation of esophageal peristaltic function. J Gastroenterol Hepatol. 2008;23 Suppl 2:S181-S185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Zentilin P, Conio M, Mele MR, Mansi C, Pandolfo N, Dulbecco P, Gambaro C, Tessieri L, Iiritano E, Bilardi C. Comparison of the main oesophageal pathophysiological characteristics between short- and long-segment Barrett’s oesophagus. Aliment Pharmacol Ther. 2002;16:893-898. [PubMed] |
| 22. | Frazzoni M, Manno M, De Micheli E, Savarino V. Pathophysiological characteristics of the various forms of gastro-oesophageal reflux disease. Spectrum disease or distinct phenotypic presentations? Dig Liver Dis. 2006;38:643-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Iwakiri K, Hayashi Y, Kotoyori M, Tanaka Y, Kawami N, Sano H, Takubo K, Sakamoto C, Holloway RH. Defective triggering of secondary peristalsis in patients with non-erosive reflux disease. J Gastroenterol Hepatol. 2007;22:2208-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Chen CL, Szczesniak MM, Cook IJ. Identification of impaired oesophageal bolus transit and clearance by secondary peristalsis in patients with non-obstructive dysphagia. Neurogastroenterol Motil. 2008;20:980-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Trimble KC, Pryde A, Heading RC. Lowered oesophageal sensory thresholds in patients with symptomatic but not excess gastro-oesophageal reflux: evidence for a spectrum of visceral sensitivity in GORD. Gut. 1995;37:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 195] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Fass R, Yalam JM, Camargo L, Johnson C, Garewal HS, Sampliner RE. Increased esophageal chemoreceptor sensitivity to acid in patients after successful reversal of Barrett’s esophagus. Dig Dis Sci. 1997;42:1853-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Fass R, Sampliner RE, Malagon IB, Hayden CW, Camargo L, Wendel CS, Garewal HS. Failure of oesophageal acid control in candidates for Barrett’s oesophagus reversal on a very high dose of proton pump inhibitor. Aliment Pharmacol Ther. 2000;14:597-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |