Published online Jan 21, 2014. doi: 10.3748/wjg.v20.i3.774
Revised: October 22, 2013
Accepted: November 1, 2013
Published online: January 21, 2014
Processing time: 175 Days and 5.9 Hours
AIM: To evaluate the effect of the shunting branch of the portal vein (PV) (left or right) and the initial stent position (optimal or suboptimal) of a transjugular intrahepatic portosystemic shunt (TIPS).
METHODS: We retrospectively reviewed 307 consecutive cirrhotic patients who underwent TIPS placement for variceal bleeding from March 2001 to July 2010 at our center. The left PV was used in 221 patients and the right PV in the remaining 86 patients. And, 224 and 83 patients have optimal stent position and sub-optimal stent positions, respectively. The patients were followed until October 2011 or their death. Hepatic encephalopathy, shunt dysfunction, and survival were evaluated as outcomes. The difference between the groups was compared by Kaplan-Meier analysis. A Cox regression model was employed to evaluate the predictors.
RESULTS: Among the patients who underwent TIPS to the left PV, the risk of hepatic encephalopathy (P = 0.002) and mortality were lower (P < 0.001) compared to those to the right PV. Patients who underwent TIPS with optimal initial stent position had a higher primary patency (P < 0.001) and better survival (P = 0.006) than those with suboptimal initial stent position. The shunting branch of the portal vein and the initial stent position were independent predictors of hepatic encephalopathy and shunt dysfunction after TIPS, respectively. And, both were independent predictors of survival.
CONCLUSION: TIPS placed to the left portal vein with optimal stent position may reduce the risk of hepatic encephalopathy and improve the primary patency rates, thereby prolonging survival.
Core tip: This study reported the long-term follow-up results of a large cohort of cirrhotic patients who underwent transjugular intrahepatic portosystemic shunt (TIPS) for variceal bleeding. The results demonstrated that the use of the left portal vein (PV) during the TIPS procedure could reduce post-TIPS hepatic encephalopathy risk and improve patient survival when compared to the use of the right PV, and that the deployment of a stent with optimal stent position could reduce the incidence of shunt dysfunction and benefit patient survival when compared to the deployment of a stent with suboptimal stent position.
- Citation: Bai M, He CY, Qi XS, Yin ZX, Wang JH, Guo WG, Niu J, Xia JL, Zhang ZL, Larson AC, Wu KC, Fan DM, Han GH. Shunting branch of portal vein and stent position predict survival after transjugular intrahepatic portosystemic shunt. World J Gastroenterol 2014; 20(3): 774-785
- URL: https://www.wjgnet.com/1007-9327/full/v20/i3/774.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i3.774
Transjugular intrahepatic portosystemic shunt (TIPS) placement is accepted worldwide as a means to decompress portal hypertension and to alleviate variceal hemorrhage[1-6]. However, this procedure has two major risks, which include hepatic encephalopathy (HE) (which is seen in somewhere between 21%-77% of patients per year)[1,2,7,8] and shunt dysfunction (which occurs in anywhere from 14%-82% of patients per year)[9-15].
Previous studies have reported that creation of a TIPS to the left portal vein (PV) instead of the right PV can decrease the risk of HE[16,17]. However, the sample sizes of these studies were relevantly small (the largest study included only 80 patients)[17]. One of these two studies evaluated the survival of patients who underwent left vs right PV TIPS and demonstrated that there was no difference in the survival between these two groups of patients[16].
In addition, several studies have implied that TIPS stents, when extended to the hepato-caval junction [rather than terminating in the hepatic vein (HV)], have a decreased incidence of shunt dysfunction[13,18,19]. However, these studies have not addressed the effect of stent position upon patient survival. Furthermore, to our knowledge, no study has evaluated the effect of the shunting branch of the PV and stent position concurrently.
This study aims to evaluate the effect of the shunting branch of the PV and the initial stent position on patient prognosis, particularly patient survival, in a large series of cirrhotic patients who underwent TIPS for variceal bleeding.
Between March 2001 and July 2010, all consecutive patients with cirrhosis who underwent a TIPS procedure for an indication of variceal bleeding at our center were retrospectively analyzed in the present study (both in the elective and emergency settings). The exclusion criteria are as follows: (1) PV thrombosis; (2) hepatocellular carcinoma; (3) other malignant diseases; (4) sepsis; (5) renal failure (serum creatinine > 265 μmol/L); (6) heart failure; and (7) age < 18 years. Local ethical committee approved the study protocol. Written informed consent for TIPS procedure was obtained from every patient.
Liver cirrhosis was diagnosed according to a history of liver disease, decreased liver function, portal hypertension and characteristic imaging features suggesting cirrhosis. A biopsy was performed when hepatocellular carcinoma was suspected. The diagnosis of PV thrombosis was established mainly based on color Doppler ultrasound (CDUS) and computed tomography as our previous description[20]. The definition of technical success was successful creation of a TIPS between the HV and PV and reduction of the portosystemic gradient (PSG) to < 12 mmHg or by > 25%[21].
HE was diagnosed and classified according to the West Haven criteria[22]. Grade III and IV were considered to represent severe HE. Recurrent (at least three episodes of HE in the last 3 mo) and/or persistent HE (continuously detectable altered mental state with further episodic deteriorations) despite protein restriction and active medical treatment were considered to be refractory HE[23].
Shunt dysfunction was suggested when any one of the following events was observed: (1) variceal bleeding; (2) occurrence of severe ascites; or (3) a maximum flow velocity < 50 cm/s or the absence of flow within the shunt as demonstrated by CDUS. Suspected shunt dysfunction was confirmed by portography and a pressure measurement that showed a PSG > 15 mmHg[20]. The duration of time from the TIPS procedure to the first shunt dysfunction was defined as the primary patency.
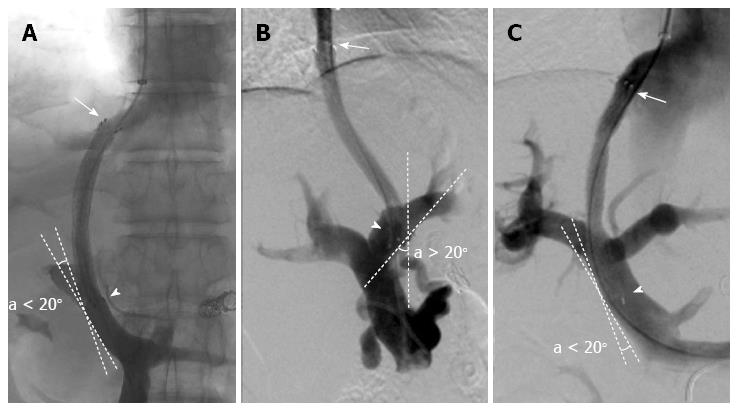
Optimal initial stent position (O-SP) (Figure 1) was defined as a stent position that satisfied the following two criteria: (1) the cephalic end of the stent extended to the hepato-caval junction[18]; and (2) the caudal end of the stent was parallel to the vascular wall of the PV (the angle between the tangent line of the caudal end of the stent and the vascular wall of the PV was less than 20°). Otherwise, the stent position was considered to be suboptimal. We reviewed the stent position by anteroposterior and lateral imaging. If the position was identified as suboptimal by either imaging view, the stent was diagnosed as a suboptimal initial stent position (sub-O-SP). The sub-O-SP diagnosis comprised of the following three categories: (1) suboptimal in the HV; (2) suboptimal in the PV; and (3) suboptimal in both the HV and PV. Based on the predefined criteria, the stent position of each patient was classified by two interventional radiologists with ten (C. H) or nine (W. G) years of experience, who were blinded to each other's classifications and to the patients’ outcomes.
The technique for creating a TIPS has been described previously[13,24,25]. After indirect portography (mesenteric artery angiography) was performed, the HV (commonly, the right HV) was reached using a TIPS set (RUPS-100, Cook, Cook Inc., Bloomington, IL, United States), and the PV was punctured under the guiding of digital subtraction angiography. The following four steps were employed to puncture the PV: (1) the anatomical position between HV and PV was estimated by CT imaging and indirect portography; (2) the metal cannula of the RUPS-100 was bended to an appropriate angle (usually 30o-70o for the left and 20o-50o for the right PV, respectively) according to the estimated anatomical position; (3) the RUPS-100 was introduced into the right HV; and (4) the end of the RUPS-100 was turned to a point at the left/right PV and then the targeted PV could be successfully punctured. For an experienced expert, either the left or right PV can be selected and successfully punctured, which have been proved in previous studies[16,17]. Which PV branch was punctured was determined by the interventional radiologists and recorded at the time of the TIPS procedure. A 10-mm stent was used for TIPS creation before October 2006 and an 8-mm stent (BARD, Luminexx, Voisins le Bretonneux, France) was used thereafter to avoid excessive portosystemic shunting. Furthermore, additional dilations were performed whenever the PSG was > 12 mmHg or the reduction in the PSG was < 25%[21]. Markedly enlarged gastroesophageal collateral vessels observed during the TIPS procedure were embolized with coils (Cook Incorporated, 750 Daniels Way Bloomington, IN). For the patients included in this study, covered stents could not be employed because the State Food and Drug Administration had not approved these stents at the time of the TIPS procedures.
After the TIPS procedure, intravenous heparin (8000-12000 u/d) was given for 5-7 d and then warfarin for 6 mo and lifelong aspirin were prescribed at dosages to achieve an international normalized ratio (INR) of up to two times the upper limit of normal to prevent shunt dysfunction[20]. The antithrombotic therapy strategy was made according to the practice guidelines of American College of Chest Physicians[26]. A TIPS revision was planned whenever shunt dysfunction was recognized.
The patients were followed until October 2011 or their death. Variceal bleeding, ascites, HE, and survival were assessed at one, three, six and 12 mo and then yearly. Blood tests, coagulation function tests (prothrombin time, INR) and CDUS (diameter, flow velocity and direction of flow in the PV and shunt), if possible, were obtained at the follow-up time points and any time when symptoms recurred (hematemesis, melena or large volume ascites).
Numerical variables are expressed as mean value ± standard deviation. Normal continuous variables were compared using the Student’s t test. Non-normal continuous variables were compared using the Mann-Whitney rank-sum test. Nominal variables are expressed as frequencies and compared using the χ2 test. Accumulated proportions were assessed using Kaplan-Meier curves and compared using the log-rank test. The following items were included in univariate analyses: age, gender, etiology, procedure type (elective/emergency), ascites, previous HE, splenectomy, platelet count, serum albumin, bilirubin, creatinine, sodium concentrations, INR, Child-Pugh score, MELD score, PSG, stent diameter, shunting branch of the PV, initial stent position, HE within six months, shunt dysfunction within six months, and TIPS date. A Cox proportional regression hazards model was used to assess the prognostic value of the significant variables found in the univariate analyses. Patients who were lost to follow-up or underwent liver transplantation were censored at the last follow-up date and the date of transplantation, respectively. All of the statistical analyses were performed with SPSS 17.0 (SPSS, Chicago, IL), and a two-tailed P value < 0.05 was considered statistically significant.
Figure 2 presents the patient selection flowchart. The median follow-up time was 2.5 years (range, 0.1-10.9 years). Patient demographics of our cohort of 307 patients prior to TIPS creation are presented in Table 1. Of these patients, 258 underwent TIPS creation in elective settings and 49 in emergency settings. TIPS creation was technically successful in all of the included patients. The left PV was used in 221 patients and the right PV in the remaining 86 patients. For these two groups of patients, no significant difference was observed in the baseline clinical and laboratory characteristics (Table 1). In three of the included patients, intra-abdominal bleeding caused by an extrahepatic PV puncture was successfully treated by rapid decompression of the portal system with stent deployment. Other procedure-related complications included one patient with a bile duct injury and one patient with a liver capsule hematoma, both of whom are alive at the time of follow-up for this study.
| Characteristic | Total | Shunting branch of the PV | Initial stent position | ||
| (n = 307) | Left (n = 221) | Right (n = 86) | Optimal (n = 224) | Sub-optimal (n = 83) | |
| Age (yr) | 50.7 ± 12.8 | 50.0 ± 12.9 | 52.5 ± 12.3 | 50.8 ± 12.6 | 50.4 ± 13.3 |
| Gender (male/female) | 209/98 | 151/70 | 58/28 | 147/77 | 62/21 |
| TIPS procedure (elective/emergency) | 258/49 | 190/31 | 68/18 | 190/34 | 68/15 |
| Etiology (viral/not viral1) | 275/32 | 199/22 | 76/10 | 197/27 | 78/5 |
| Ascites (no/yes) | 94/213 | 74/147 | 20/66 | 69/155 | 25/58 |
| Previous HE (no/yes) | 298/9 | 214/7 | 84/2 | 218/6 | 80/3 |
| Previous splenectomy (no/yes) | 252/55 | 179/42 | 73/13 | 184/40 | 68/15 |
| Platelet count (109/L) | 82.3 ± 65.4 | 82.3 ± 68.9 | 82.6 ± 55.8 | 85.5 ± 68.4 | 73.9 ± 56.2 |
| Albumin (g/L) | 33.3 ± 5.2 | 33.4 ± 5.0 | 32.9 ± 5.9 | 33.1 ± 5.2 | 33.8 ± 5.2 |
| Total bilirubin (μmol/L) | 24.7 ± 19.2 | 24.3 ± 18.1 | 25.7 ± 21.7 | 24.0 ± 17.8 | 26.4 ± 22.4 |
| INR | 1.3 ± 0.3 | 1.3 ± 0.3 | 1.3 ± 0.3 | 1.3 ± 0.3 | 1.3 ± 0.3 |
| Serum creatinine (μmol/L) | 82.4 ± 21.8 | 82.3 ± 21.9 | 82.6 ± 21.6 | 81.0 ± 21.2 | 86.2 ± 22.9 |
| Sodium (mmol/L) | 138.9 ± 4.7 | 139.0 ± 4.3 | 138.4 ± 5.6 | 138.6 ± 5.0 | 139.6 ± 3.6 |
| Child-Pugh score | 7.1 ± 1.6 | 7.1 ± 1.6 | 7.3 ± 1.7 | 7.1 ± 1.7 | 7.1 ± 1.5 |
| Child-Pugh classification (A/B/C) | 126/157/24 | 94/110/17 | 32/47/7 | 93/111/20 | 33/46/4 |
| MELD score | 11.1 ± 3.3 | 11.1 ± 3.3 | 11.1 ± 3.5 | 11.2 ± 3.5 | 10.8 ± 2.7 |
| Pre-TIPS PSG (mmHg) | 22.3 ± 4.5 | 22.5 ± 4.3 | 21.7 ± 4.9 | 22.4 ± 4.6 | 22.0 ± 4.2 |
| Reduction ratio of PSG (%) | 45.8 ± 17.4 | 46.3 ± 17.4 | 44.5 ± 17.3 | 45.7 ± 17.4 | 45.9 ± 17.5 |
| Stent diameter (8-/10-mm) | 206/101 | 150/71 | 56/30 | 150/74 | 56/27 |
| Shunting branch of the PV (right/left) | 86/221 | - | - | 57/164 | 26/60 |
| Initial stent position (optimal/suboptimal) | 224/83 | 164/57 | 60/26 | - | - |
| TIPS date2 (before 2006/after 2006) | 118/189 | 75/146 | 43/433 | 83/141 | 35/48 |
| Lost to follow-up | 19 | 12 | 7 | 15 | 4 |
The position of the stent was classified as O-SP in 224 patients, sub-O-SP in 83 patients, including sub-O-SP in the HV in 63 patients, PV in 17 patients and both HV and PV in the remaining three patients. Table 1 demonstrates that patients with O-SP and those with sub-O-SP also had comparable baseline characteristics. A high inter-observer agreement was demonstrated between the two interventional radiologists who classified the initial stent position (κ = 0.98, P < 0.001). Disagreements for the three patients in whom a consensus was not reached were resolved through discussion.
In total, 209 episodes of overt HE were observed in 128 patients. The majority (n = 71) of the first episodes of HE were grade II. During the follow-up, 72 patients had one episodes of HE, and the rest had two or more episodes HE. HE were successfully controlled by medical treatments in all patients except the three who showed refractory HE and required a reduction of the stent size three, 10, and 17 mo after TIPS implantation. The proportions of patients remaining free of HE were 74.5% in three months, 68.1% in one year, and 56.8% in three years.
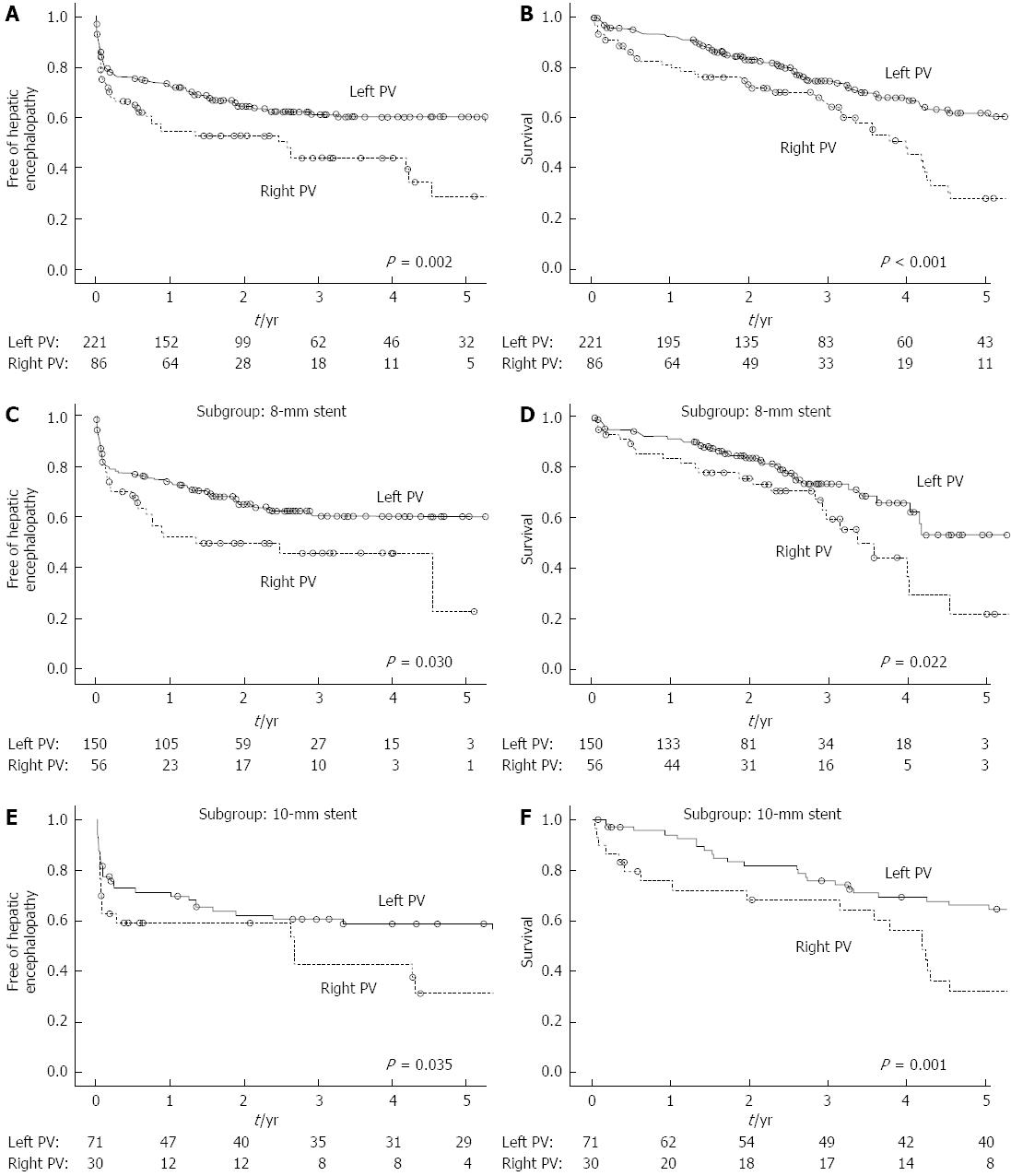
The proportion of patients free of HE after one and three years were 54.3% and 44.0% for the patients with a right PV TIPS vs 73.4% and 61.3% for the patients with a left PV TIPS (Figure 3A, log-rank test: P = 0.002). In the 8-mm stent (Figure 3C, log-rank test: P = 0.030) and 10-mm stent subgroups (Figure 3E, log-rank test: P = 0.035), the patients with a left PV TIPS had a lower rate of post-procedure HE. Severe HE was observed in 21 (9.5%) patients in the left PV group and in 13 (15.1%) patients in the right PV group (P = 0.159). Of the three patients who presented refractory HE, two underwent a left and one underwent a right PV TIPS.
After univariate analysis (Table 2) and multivariate analysis, age (P = 0.001), INR (P = 0.014), Child-Pugh score (P = 0.004), MELD score (P = 0.021), reduction ratio in PSG (P = 0.009) and shunting branch of PV (P = 0.014) were identified as independent predictors of HE (Table 3).
| Variable | Hepatic encephalopathy | Shunt dysfunction | Survival | ||||||
| HR | 95%CI | P value | HR | 95%CI | P value | HR | 95%CI | P value | |
| Age (yr) | 1.03 | 1.01-1.04 | < 0.001 | 0.99 | 0.97-1.01 | NS | 1.01 | 0.99-1.03 | NS |
| Gender (male/female) | 1.04 | 0.72-1.50 | NS | 1.62 | 1.07-2.45 | 0.023 | 1.41 | 0.92-2.16 | NS |
| TIPS procedure (elective/emergency) | 1.37 | 0.88-2.13 | NS | 0.66 | 0.38-1.14 | NS | 1.57 | 1.02-2.41 | 0.041 |
| Ascites (yes/no) | 1.55 | 1.04-2.30 | 0.031 | 0.90 | 0.62-1.33 | NS | 2.22 | 1.38-3.57 | 0.001 |
| Previous splenectomy (yes/no) | 0.97 | 0.61-1.54 | NS | 1.65 | 1.09-2.50 | 0.018 | 0.61 | 0.36-1.02 | NS |
| Platelet count (109/L) | 1.00 | 0.99-1.01 | NS | 1.00 | 0.99-1.01 | NS | 0.99 | 0.99-1.00 | 0.021 |
| Albumin (g/L) | 0.96 | 0.93-0.99 | 0.022 | 0.98 | 0.95-1.02 | NS | 0.96 | 0.92-0.99 | 0.035 |
| Total bilirubin (μmol/L) | 1.00 | 0.99-1.01 | NS | 1.00 | 0.99-1.01 | NS | 1.01 | 1.00-1.02 | 0.002 |
| INR | 2.09 | 1.20-3.64 | 0.009 | 0.82 | 0.41-1.63 | NS | 3.12 | 1.86-5.23 | < 0.001 |
| Child-Pugh score | 1.17 | 1.06-1.29 | 0.003 | 1.01 | 0.90-1.13 | NS | 1.29 | 1.15-1.44 | < 0.001 |
| MELD score | 1.05 | 1.01-1.11 | 0.032 | 0.97 | 0.91-1.03 | NS | 1.09 | 1.04-1.14 | < 0.001 |
| Reduction ratio of PSG (%) | 1.02 | 1.01-1.03 | 0.004 | 0.99 | 0.98-1.01 | NS | 1.01 | 0.99-1.02 | NS |
| Shunting branch of the PV (right /left) | 1.75 | 1.22-2.52 | 0.003 | 0.81 | 0.53-1.23 | NS | 2.10 | 1.43-3.07 | < 0.001 |
| Initial stent position (sub-optimal/optimal) | 0.75 | 0.49-1.15 | NS | 2.24 | 1.54-3.25 | < 0.001 | 1.88 | 1.44-2.47 | < 0.001 |
| HE within 6 mo (yes/no) | - | - | - | - | - | - | 1.60 | 1.07-2.38 | 0.021 |
| Shunt dysfunction within 6 mo (yes/no) | - | - | - | - | - | - | 1.87 | 1.15-3.04 | 0.011 |
| Variable | Multivariate analysis | ||
| HR | 95%CI | P value | |
| Hepatic encephalopathy | |||
| Age | 1.02 | 1.01-1.04 | 0.001 |
| INR | 2.01 | 1.15-3.50 | 0.014 |
| Child-Pugh score | 1.17 | 1.05-1.31 | 0.004 |
| MELD | 1.06 | 1.01-1.11 | 0.021 |
| Shunting branch of the PV (right/left) | 1.59 | 1.10-2.30 | 0.014 |
| Reduction ratio of PSG (%) | 1.01 | 1.00-1.03 | 0.009 |
| Shunt dysfunction | |||
| Previous splenectomy (yes/no) | 1.76 | 1.16-2.67 | 0.008 |
| Initial stent position (sub-optimal/optimal) | 2.30 | 1.57-3.35 | < 0.001 |
| Survival | |||
| Ascites (yes/no) | 1.87 | 1.13-3.08 | 0.014 |
| INR | 2.31 | 1.34-3.98 | 0.003 |
| Shunting branch of the PV (right/left) | 1.92 | 1.30-2.84 | 0.001 |
| Initial stent position (sub-optimal/optimal) | 1.68 | 1.12-2.50 | 0.011 |
| Child-Pugh score | 1.27 | 1.13-1.43 | < 0.001 |
| MELD | 1.09 | 1.03-1.14 | 0.003 |
| HE within six months (yes/no) | 1.54 | 1.02-2.35 | 0.043 |
| Shunt dysfunction within 6 mo (yes/no) | 2.48 | 1.47-4.19 | 0.001 |
During the follow-up, 118 patients had at least one episode of shunt dysfunction. Of these 118 patients, 80 underwent 108 TIPS revisions, 25 died due to the first post-TIPS variceal bleeding, five underwent endoscopic therapies and two underwent surgical therapies for variceal rebleeding. Six patients required large volume paracentesis due to severe ascites. The primary patency rates of our cohort after one, three, and five years were 79.9%, 58.7%, and 46.0%, respectively.
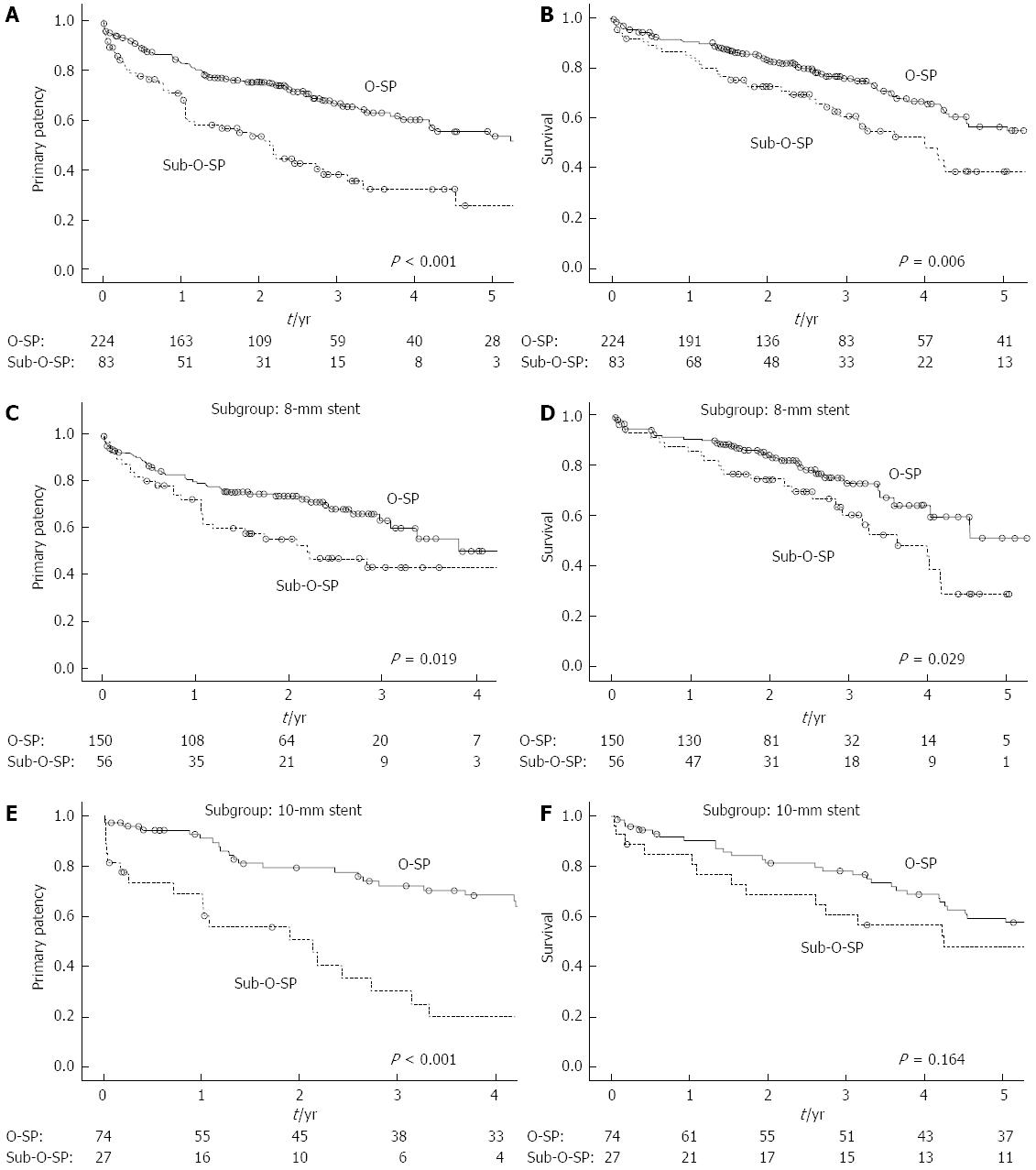
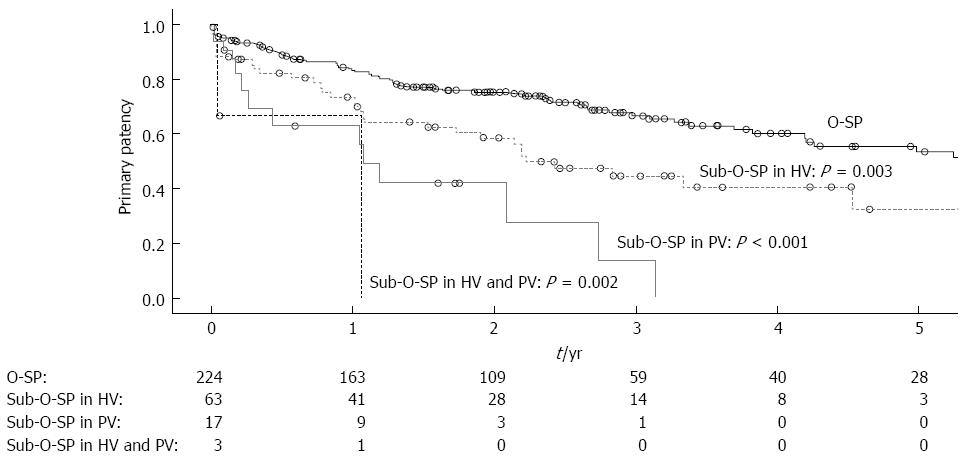
The 1-, 3- and 5-year primary patency rates were 83.3%, 66.7% and 53.5% for patients with O-SP and 70.9%, 38.2% and 25.8% for patients with sub-O-SP, respectively (Figure 4A, log-rank test: P < 0.001). Moreover, patients with each of the three types of sub-O-SP had a significantly higher risk of shunt dysfunction than patients with O-SP (Figure 5). In the 8-mm stent (Figure 4C, log-rank test: P = 0.019) and 10-mm stent subgroups (Figure 4E, log-rank test: P < 0.001), the primary patency rates in the patients with O-SP were significantly higher compared to those with sub-O-SP.
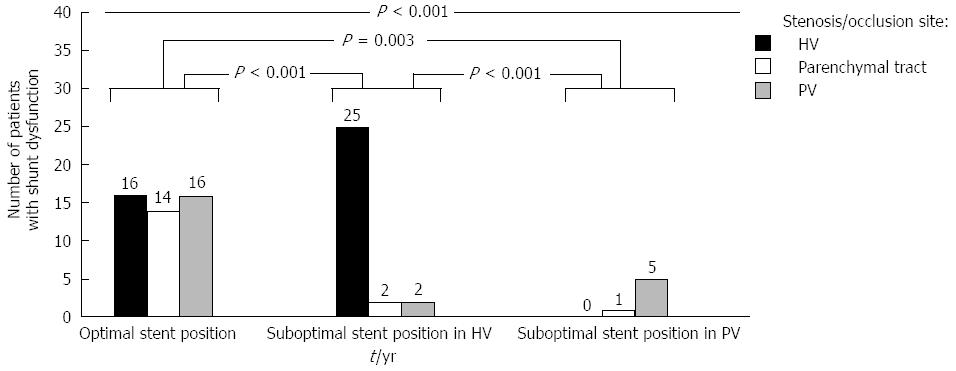
Among patients with shunt dysfunction and portography, those with sub-O-SP in the HV usually had stenosis or occlusion in the HV (86.2%), and those with sub-O-SP in the PV had stenosis or occlusion in the PV (83.3%). Patients with O-SP experienced stenosis or occlusion in the PV at a rate of 33.3% and in the HV at a rate of 33.3%. The differences among the three groups and between any two of these three groups were statistically significant (Figure 6).
Three variables were evaluated as potential risk factors for shunt dysfunction in univariate analyses (Table 2). The multivariate analysis revealed that previous splenectomy (P = 0.008) and initial stent position (P < 0.001) were independent predictors of primary patency.
Among the 110 deaths, underlying etiologies included liver failure in 56, variceal rebleeding in 42, hepatocellular carcinoma in four, and other causes in eight (which included cerebral hemorrhage, heart failure, myocardial infarction, car accident, encephalitis, diabetes, esophageal cancer and pancreatic cancer). The overt survival rates for the included patients were 89.0% at one year, 71.4% at three years, and 51.0% at five years with a median survival time of 5.6 years (95%CI: 4.3-6.9).
The median survival time was 8.4 years (95%CI: 5.2-11.5) for patients with a left PV TIPS and 3.9 years (95%CI: 3.3-4.7) for patients with a right PV TIPS (Figure 3B: log-rank test: P < 0.001). The patients with a left PV TIPS had a significantly higher survival rate compared to those with a right PV TIPS in both the 8-mm (Figure 3D, log-rank test: P = 0.022) and 10-mm stent subgroups (Figure 3F, log-rank test: P = 0.001).
For the patients with O-SP and those with sub-O-SP, the survival rates after one, three and five years were 90.4%, 75.8% and 56.6%, and 85.3%, 60.7% and 38.9%, respectively (Figure 4B: log-rank test: P = 0.006). In the 8-mm stent subgroup, the survival rate for the patients with O-SP was significantly higher compared to that for the patients with sub-O-SP (Figure 4D, log-rank test: P = 0.029). In the 10-mm stent subgroup, the 3-year survival rates were 78.2% and 60.6% for the patients with O-SP and those with sub-O-SP, respectively (Figure 4F, log-rank test: P = 0.164).
Thirteen variables were identified as potential prognostic factors of survival in the univariate analyses (Table 2). A multivariate analysis showed that ascites (P < 0.015), INR (P = 0.002), Child-Pugh score (P < 0.001), MELD score (P = 0.002), the shunting branch of the PV (P = 0.001), the initial stent position (P = 0.010), HE within 6 mo (P = 0.043) and shunt dysfunction within 6 mo (P = 0.001) were independent predictors (Table 3).
This study of TIPS creation for variceal bleeding in a large cohort of cirrhotic patients verified that shunting the left PV may decrease the risk of HE and that the deployment of a stent with O-SP could reduce the incidence of shunt dysfunction. Most importantly, we revealed that the shunting branch of the PV and the initial stent position were independent prognostic factors of patient survival.
Recently, a decreased risk of HE after left PV TIPS creation was observed in a study of 72 patients[16] and consequently confirmed by a study with 80 patients[17]. However, the former study found no significant difference in the overall survival of patients with a TIPS placed in the left vs right PV[16], and the latter study did not address the survival of these two groups of patients[17]. The authors of the former study considered that the negative results were attributed to the short observation periods and the relatively small sample size of the study, as that they did not employ survival as the primary endpoint[16]. We confirmed that patients with a TIPS placed through the left PV have a lower risk of HE in a large cohort of patients. In our multivariate analysis, patients with HE within six months and the use of the right PV were found to be two of the independent risk factors for mortality. These results suggest that TIPS to the left PV may improve patient survival by lowering the risk of HE to a great degree. Survival is usually considered to be the strongest endpoint for evaluating the effectiveness of a therapy. Thus, our study presents important evidence for using the left PV in cirrhotic patients who underwent TIPS creation for variceal bleeding.
It is reported that most HE neurotoxins, such as ammonia, are derived from the intestine[27], which may be directed predominantly to the right PV[28,29]. Thus, TIPS placement through the right PV may theoretically lead to increased neurotoxins in the systemic circulation, in which the effect will result in an increased incidence of HE and decreased survival[16,17].
One previous study demonstrated that patients with the cephalic end of the stent extended to the hepato-caval junction had a longer patency lifespan compared to those with a stent terminating in the HV; however, the authors did not assess the effect of the spatial relationship between the caudal end of the stent and the vascular wall of the PV on shunt patency[18]. Stenosis in the PV was also reported as an important cause of shunt dysfunction, especially in patients with covered stents[13,19,30-32]. In our 13 years’ experience, we have the impression that failure of the caudal end of the stent to be parallel to the vascular wall of the PV probably increases the risk of stenosis in the PV. In order to validate our impression, this suboptimal spatial relationship (the caudal end of the stent is unparallel to the vascular wall of the PV, Figure 1B) was defined as sub-O-SP in the PV and its effect on shunt dysfunction was studied in this study.
The high inter-observer agreement on the stent position classifications indicated that our classification criteria were reliable and valid. In this study, the patients with sub-O-SP in the HV and those with sub-O-SP in the PV had significantly lower primary patency rates compared to those with O-SP, and the outcomes of the three patients with sub-O-SP in both the HV and PV were notably worse. These results suggest that the deployment of a stent with the cephalic end extending to the hepato-caval junction and the caudal end parallel to the vascular wall of the PV is favorable in a TIPS procedure to decrease the risk of shunt dysfunction.
Additionally, the stenosis or occlusion sites in the patients with shunt dysfunction correlated well with their initial suboptimal stent sites. It has been reported that an uncovered HV is more susceptible to pseudointimal hyperplasia and more predisposed to shunt thrombosis caused by turbulence and the shear stress of high-velocity blood flow[33]. Moreover, in the patients with a stent terminating in the HV, uncovered outflow segments of the parenchymal tract are more likely to be formed by stent migration caused by organ movement[34]. For the patients with the caudal end of the stent failing to be parallel to the vascular wall of the PV, the chronic trauma to the PV intima caused by the end of the stent was reported to be responsible for the stenosis or occlusion in the PV[13,30].
Furthermore, our data demonstrate that patients with sub-O-SP had a significantly lower survival rate than those with O-SP. Moreover, the O-SP was revealed as an independent predictor of improved survival in the multivariate analysis. These results indicate that initial stent position plays an role in patient survival[13,18,19]. Subsequently, shunt dysfunction within six months was identified as one of the independent risk factors for survival, which suggests that the increased incidence of shunt dysfunction in patients with sub-O-SP may be partially responsible for the decreased survival rates. The higher shunt dysfunction rate determines many of the relevant outcome parameters such as rebleeding, recurrence of ascites, hepatorenal syndrome, spontaneous bacterial peritonitis, hospitalization, and cost, most of which are also closely related to survival[35].
Compared to historical data in randomized studies, our patients demonstrated higher patency rates. This was also observed in our previous study on TIPS for PV thrombosis in cirrhosis[20]. It was reported in a randomized controlled trial that the routine administration of anticoagulants has a considerable effect on the improved patency rate[36]. Thus, the higher patency rate of our patients was partially attributed to the routine use of anticoagulants. To some degree, our study validated the effectiveness of anticoagulation on the prevention of shunt dysfunction. Another possible reason for our higher patency rate is that patients included in a randomized trial are more closely followed than in a retrospective study.
The retrospective design and long study duration are some of the limitations of this study. Modifications in the technical aspects of the TIPS procedure and improvements in the supportive care most likely occurred during a long-term study. However, in our study, the TIPS date was not identified as a risk factor for HE, shunt dysfunction, and mortality (Table 2), which means the patient outcome was relatively stable during the study period. Another limitation is the use of two different sized stents, which adds another confounding variable. However, the results of the subgroup analyses according to the diameter of the stent are consistent with the results of the analyses in the total patient population. These results suggest that the shunting branch of PV and the initial stent position are important when stent in either diameter is used. The use of an uncovered stent is also a limitation of this study because of the worldwide popularity of covered stents. Certainly, TIPS with a covered stent faces challenge of shunting either the left or right PV, and with the risk of having a sub-O-SP as well. To our knowledge, the effectiveness of the shunting branch of the PV and initial stent position on survival has yet not been studied in covered stents. Thus, the results of uncovered stents for TIPS creation will most likely have important reference value for the clinical practice and research of TIPS with covered stents.
In conclusion, this study of TIPS for variceal bleeding in cirrhotic patients found the following: (1) placement of a left PV TIPS decreases the risk of HE; (2) deployment of a stent with O-SP reduces the risk of shunt dysfunction; and (3) both of these factors may improve patient survival. The shunting of the left PV and the deployment of a stent with O-SP should be recommended for TIPS creation. Further prospective studies are needed to confirm these results.
The authors thank all the patients who are involved in this study. There is no financial support for this work. The authors thank Ziwei Liu, Zhengyu Wang and Peng Liu for their contributions to the data collection.
Transjugular intrahepatic portosystemic shunt (TIPS) is used worldwide for the prevention of variceal bleeding. Hepatic encephalopathy (HE) and shunt dysfunction are the major drawbacks of this procedure. Previous studies demonstrated that creation of a TIPS to the left portal vein (PV) instead of the right PV could decrease the risk of HE and that stent position is related to the occurrence of shunt dysfunction. However, the effects of the shunting branch of the portal vein and the initial stent position on patient survival were confused.
More and more patients underwent the TIPS procedure for the complications of portal hypertension. For the use of TIPS procedure, the research hotspot is how to reduce the procedure-related complications and improve the patient survival by devising new devices, polishing the procedure technique, and bettering the patient selection.
Previous studies have found that patients with a TIPS placed to the left PV had lower risk of HE than those with a TIPS placed to the right PV. However, it is unclear whether the use of the left PV could improve patient survival. The results of our study validated the effect of creation of a TIPS to the left PV on the reduction of HE risk. Furthermore, we found that patients with a TIPS placed to the left PV had significantly better survival. Previously, several studies have assessed the effect of stent position on shunt patency rate. They demonstrated that patients with the cephalic end of the stent extended to the hepato-caval junction had a longer patency lifespan. All of these studies did not consider the positions of cephalic and caudal ends of the stent at the same time. In the present study, we defined the stent position as optimal initial stent position (O-SP) or non-O-SP by considering the positions of both the cephalic and caudal ends of the stent. The results demonstrated that patients with O-SP had better patency rate and long term survival.
The results of the present study suggest that further TIPS creation should be placed to the left PV with O-SP in both the cephalic and caudal ends.
TIPS is an interventional procedure which created a shunt (the shunt is maintained with a metal stent) within the liver between the portal vein and hepatic vein to decompress the portal pressure. O-SP is a stent position that satisfied the following two criteria: (1) the cephalic end of the stent extended to the hepato-caval junction; and (2) the caudal end of the stent was parallel to the vascular wall of the PV (the angle between the tangent line of the caudal end of the stent and the vascular wall of the PV was less than 20°).
This is an interesting study which retrospectively analyzed more than 300 patients who underwent TIPS procedure for variceal bleeding. The results are useful and suggest that further TIPS should be placed to the left PV with O-SP to improve the patient outcome.
P- Reviewers: Balaban YH, Yoshida H S- Editor: Cui XM L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Rössle M, Deibert P, Haag K, Ochs A, Olschewski M, Siegerstetter V, Hauenstein KH, Geiger R, Stiepak C, Keller W. Randomised trial of transjugular-intrahepatic-portosystemic shunt versus endoscopy plus propranolol for prevention of variceal rebleeding. Lancet. 1997;349:1043-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 171] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, Abraldes JG, Nevens F, Vinel JP, Mössner J, Bosch J. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 842] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 3. | Boyer TD, Haskal ZJ. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology. 2010;51:306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 405] [Article Influence: 27.0] [Reference Citation Analysis (1)] |
| 4. | European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1131] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 5. | Cello JP, Ring EJ, Olcott EW, Koch J, Gordon R, Sandhu J, Morgan DR, Ostroff JW, Rockey DC, Bacchetti P. Endoscopic sclerotherapy compared with percutaneous transjugular intrahepatic portosystemic shunt after initial sclerotherapy in patients with acute variceal hemorrhage. A randomized, controlled trial. Ann Intern Med. 1997;126:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 143] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Pomier-Layrargues G, Villeneuve JP, Deschênes M, Bui B, Perreault P, Fenyves D, Willems B, Marleau D, Bilodeau M, Lafortune M. Transjugular intrahepatic portosystemic shunt (TIPS) versus endoscopic variceal ligation in the prevention of variceal rebleeding in patients with cirrhosis: a randomised trial. Gut. 2001;48:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Sanyal AJ, Freedman AM, Luketic VA, Purdum PP, Shiffman ML, Tisnado J, Cole PE. Transjugular intrahepatic portosystemic shunts for patients with active variceal hemorrhage unresponsive to sclerotherapy. Gastroenterology. 1996;111:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | García-Villarreal L, Martínez-Lagares F, Sierra A, Guevara C, Marrero JM, Jiménez E, Monescillo A, Hernández-Cabrero T, Alonso JM, Fuentes R. Transjugular intrahepatic portosystemic shunt versus endoscopic sclerotherapy for the prevention of variceal rebleeding after recent variceal hemorrhage. Hepatology. 1999;29:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 97] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Somberg KA, Riegler JL, LaBerge JM, Doherty-Simor MM, Bachetti P, Roberts JP, Lake JR. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunts: incidence and risk factors. Am J Gastroenterol. 1995;90:549-555. [PubMed] |
| 10. | Lind CD, Malisch TW, Chong WK, Richards WO, Pinson CW, Meranze SG, Mazer M. Incidence of shunt occlusion or stenosis following transjugular intrahepatic portosystemic shunt placement. Gastroenterology. 1994;106:1277-1283. [PubMed] |
| 11. | Jalan R, Elton RA, Redhead DN, Finlayson ND, Hayes PC. Analysis of prognostic variables in the prediction of mortality, shunt failure, variceal rebleeding and encephalopathy following the transjugular intrahepatic portosystemic stent-shunt for variceal haemorrhage. J Hepatol. 1995;23:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Bureau C, Garcia-Pagan JC, Otal P, Pomier-Layrargues G, Chabbert V, Cortez C, Perreault P, Péron JM, Abraldes JG, Bouchard L, Bilbao JI, Bosch J, Rousseau H, Vinel JP. Improved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: results of a randomized study. Gastroenterology. 2004;126:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 338] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 13. | Rossi P, Salvatori FM, Fanelli F, Bezzi M, Rossi M, Marcelli G, Pepino D, Riggio O, Passariello R. Polytetrafluoroethylene-covered nitinol stent-graft for transjugular intrahepatic portosystemic shunt creation: 3-year experience. Radiology. 2004;231:820-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Yang Z, Han G, Wu Q, Ye X, Jin Z, Yin Z, Qi X, Bai M, Wu K, Fan D. Patency and clinical outcomes of transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stents versus bare stents: a meta-analysis. J Gastroenterol Hepatol. 2010;25:1718-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Vignali C, Bargellini I, Grosso M, Passalacqua G, Maglione F, Pedrazzini F, Filauri P, Niola R, Cioni R, Petruzzi P. TIPS with expanded polytetrafluoroethylene-covered stent: results of an Italian multicenter study. AJR Am J Roentgenol. 2005;185:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Chen L, Xiao T, Chen W, Long Q, Li R, Fang D, Wang R. Outcomes of transjugular intrahepatic portosystemic shunt through the left branch vs. the right branch of the portal vein in advanced cirrhosis: a randomized trial. Liver Int. 2009;29:1101-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Xue H, Yuan J, Chao-Li Y, Palikhe M, Wang J, Shan-Lv L, Qiao W. Follow-up study of transjugular intrahepatic portosystemic shunt in the treatment of portal hypertension. Dig Dis Sci. 2011;56:3350-3356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Clark TW, Agarwal R, Haskal ZJ, Stavropoulos SW. The effect of initial shunt outflow position on patency of transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 2004;15:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Angeloni S, Merli M, Salvatori FM, De Santis A, Fanelli F, Pepino D, Attili AF, Rossi P, Riggio O. Polytetrafluoroethylene-covered stent grafts for TIPS procedure: 1-year patency and clinical results. Am J Gastroenterol. 2004;99:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Han G, Qi X, He C, Yin Z, Wang J, Xia J, Yang Z, Bai M, Meng X, Niu J. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with symptomatic portal hypertension in liver cirrhosis. J Hepatol. 2011;54:78-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 187] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 21. | Rössle M, Siegerstetter V, Olschewski M, Ochs A, Berger E, Haag K. How much reduction in portal pressure is necessary to prevent variceal rebleeding? A longitudinal study in 225 patients with transjugular intrahepatic portosystemic shunts. Am J Gastroenterol. 2001;96:3379-3383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1410] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 23. | Riggio O, Angeloni S, Salvatori FM, De Santis A, Cerini F, Farcomeni A, Attili AF, Merli M. Incidence, natural history, and risk factors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent grafts. Am J Gastroenterol. 2008;103:2738-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 205] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 24. | Rössle M, Haag K, Ochs A, Sellinger M, Nöldge G, Perarnau JM, Berger E, Blum U, Gabelmann A, Hauenstein K. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. N Engl J Med. 1994;330:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 474] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | LaBerge JM, Somberg KA, Lake JR, Gordon RL, Kerlan RK, Ascher NL, Roberts JP, Simor MM, Doherty CA, Hahn J. Two-year outcome following transjugular intrahepatic portosystemic shunt for variceal bleeding: results in 90 patients. Gastroenterology. 1995;108:1143-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 214] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Popma JJ, Weitz J, Bittl JA, Ohman EM, Kuntz RE, Lansky AJ, King SB. Antithrombotic therapy in patients undergoing coronary angioplasty. Chest. 1998;114:728S-741S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Plauth M, Roske AE, Romaniuk P, Roth E, Ziebig R, Lochs H. Post-feeding hyperammonaemia in patients with transjugular intrahepatic portosystemic shunt and liver cirrhosis: role of small intestinal ammonia release and route of nutrient administration. Gut. 2000;46:849-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Sherlock S. Portal circulation and portal hypertension. Gut. 1978;19:70-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Groszmann RJ, Kotelanski B, Cohn JN. Hepatic lobar distribution of splenic and mesenteric blood flow in man. Gastroenterology. 1971;60:1047-1052. [PubMed] |
| 30. | Cura M, Cura A, Suri R, El-Merhi F, Lopera J, Kroma G. Causes of TIPS dysfunction. AJR Am J Roentgenol. 2008;191:1751-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Freedman AM, Sanyal AJ, Tisnado J, Cole PE, Shiffman ML, Luketic VA, Purdum PP, Darcy MD, Posner MP. Complications of transjugular intrahepatic portosystemic shunt: a comprehensive review. Radiographics. 1993;13:1185-1210. [PubMed] |
| 32. | Sterling KM, Darcy MD. Stenosis of transjugular intrahepatic portosystemic shunts: presentation and management. AJR Am J Roentgenol. 1997;168:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Ducoin H, El-Khoury J, Rousseau H, Barange K, Peron JM, Pierragi MT, Rumeau JL, Pascal JP, Vinel JP, Joffre F. Histopathologic analysis of transjugular intrahepatic portosystemic shunts. Hepatology. 1997;25:1064-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Tesdal IK, Jaschke W, Bühler M, Adamus R, Filser T, Holm E, Georgi M. Transjugular intrahepatic portosystemic shunting (TIPS) with balloon-expandable and self-expanding stents: technical and clinical aspects after 3 1/2 years’ experience. Cardiovasc Intervent Radiol. 1997;20:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Angermayr B, Cejna M, Koenig F, Karnel F, Hackl F, Gangl A, Peck-Radosavljevic M. Survival in patients undergoing transjugular intrahepatic portosystemic shunt: ePTFE-covered stentgrafts versus bare stents. Hepatology. 2003;38:1043-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Sauer P, Theilmann L, Herrmann S, Bruckner T, Roeren T, Richter G, Stremmel W, Stiehl A. Phenprocoumon for prevention of shunt occlusion after transjugular intrahepatic portosystemic stent shunt: a randomized trial. Hepatology. 1996;24:1433-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |