Published online Jan 21, 2014. doi: 10.3748/wjg.v20.i3.706
Revised: November 7, 2013
Accepted: December 3, 2013
Published online: January 21, 2014
Processing time: 160 Days and 23.4 Hours
Since the discovery of Helicobacter pylori (H. pylori) infection in the stomach, the bacteria infection and non-steroidal anti-inflammatory drugs (NSAIDs) use had been considered to be the 2 main causes of peptic ulcers. However, there have been recent reports of an increase in the proportion of peptic ulcers without these known risk factors; these are termed idiopathic peptic ulcers. Such trend was firstly indicated in 1990s from some reports in North America. In Asia, numerous studies reported that idiopathic ulcers accounted for a small percentage of all ulcers in the 1990s, but in the 2000s, multiple studies reported that the proportion of idiopathic ulcers had reached 10%-30%, indicating that the incidence of idiopathic ulcers in Asia has also been rising in recent years. While a decline in H. pylori infection rates of general population in Asia is seen as the main reason for the increased incidence of idiopathic ulcers, it is also possible that the absolute number of idiopathic ulcer cases has increased. Advanced age, serious systemic complication, and psychological stress are considered to be the potential risk factors for idiopathic ulcers. Management of idiopathic ulcers is challenging, at present, because there is no effective preventative measure against recurrence in contrast with cases of H. pylori-positive ulcers and NSAIDs-induced ulcers. As it is expected that H. pylori infection rates in Asia will decline further in the future, measures to treat idiopathic ulcers will also likely become more important.
Core tip: In Asia, numerous studies reported that idiopathic ulcers accounted for a small percentage of all ulcers in the 1990s, but in the 2000s, multiple studies reported that the proportion of idiopathic ulcers had reached 10%-30%, indicating that the incidence of idiopathic ulcers in Asia has also been rising in recent years. As it is expected that Helicobacter pylori infection rates in Asia will decline further in the future, measures to treat idiopathic ulcers will also likely become more important.
-
Citation: Iijima K, Kanno T, Koike T, Shimosegawa T.
Helicobacter pylori -negative, non-steroidal anti-inflammatory drug: Negative idiopathic ulcers in Asia. World J Gastroenterol 2014; 20(3): 706-713 - URL: https://www.wjgnet.com/1007-9327/full/v20/i3/706.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i3.706
After Helicobacter pylori (H. pylori) was discovered in 1982, it was considered to be the cause of a large number of peptic ulcers (90%-95% of duodenal and 70%-90% of gastric ulcers)[1]. H. pylori, aspirin, and other non-steroidal anti-inflammatory drugs (NSAIDs) comprise the causes of a large proportion of peptic ulcers. A subsequent rise in global NSAID use and a relative increase in the proportion of NSAID-caused ulcers[2,3] led to wide acceptance that H. pylori infection and NSAID use are the 2 main causes of peptic ulcers[4]. Other causes include Zollinger-Ellison syndrome, Crohn’s disease, and viral infections such as cytomegalovirus and herpes.
However, there have been recent reports of an increase in the proportion of peptic ulcers without these known risk factors; these are termed idiopathic peptic ulcers. By the 1990s, several studies reported that idiopathic ulcers comprised 20%-40% of all peptic ulcers in North America[5-7]. In this region, 20% of H. pylori-positive ulcers recurred after bacterial elimination[8], a markedly higher percentage than that reported in other areas. This finding indicated that a large number of “bystander” cases existed, wherein a patient is positive for H. pylori but the bacteria are not directly involved in causing the ulcer. In the 2000s, increases in the proportion of idiopathic ulcers were also reported in Europe and Asia (Table 1). However, a few studies still report low idiopathic ulcer proportions (4%), including a recent report from Italy[6].
| Ref. | Year | Country | Sampling period | Sampling style | Subjects disease | Subjects number | Prevalence of IPU (%) | Diagnostic method for H. pylori |
| North America | ||||||||
| Perterson et al[5] | 1996 | United States | Not mentioned | RCT | DU | 185 | 26 | Hist/RUT/culture |
| Jyotheeswaran et al[6] | 1998 | United States | 1993-1996 | Retrospective | PUD | 305 | 39 | Hist/RUT |
| Ciociola et al[7] | 1999 | United States | 1991-1995 | 6 RCT | DU | 2910 | 27 | Hist/RUT/culture |
| Europe | ||||||||
| McColl et al[9] | 1993 | United Kingdom | Past 5 yr | Cross-sectional | DU | 400 | 1.5 | Hist/RUT/UBT/antibody |
| Gisbert et al[10] | 1999 | Spain | Not mentioned | Prospective | DU | 774 | 0.8 | RUT/culture/UBT |
| Meucci et al[11] | 2000 | Italy | 1995-1996 | Prospective | PUD | 409 | 4.4 | Hist/RUT |
| Bytzer et al[12] | 2001 | Denmark | 1993-1995 | RCT | DU | 276 | 8 | Hist/culture/antibody |
| Konturek et al[13] | 2003 | Poland | 1996-2000 | Prospective | PUD | 1898 | 18.7 | UBT |
| Arents et al[14] | 2004 | Netherland | 1991-1998 | Retrospective | PUD | 405 | 5 | Hist/RUT/culture |
| Arroyo et al[15] | 2004 | Spain | Not mentioned | Prospective | PUD | 830 | 4.1 | Hist/RUT/UBT |
| Sbrozzi-Vanni et al[16] | 2010 | Italy | 2005-2007 | Retrospective | PUD | 300 | 4 | Hist/UBT |
| Musumba et al[3] | 2012 | United Kingdom | 2005-2010 | Retrospective and prospective | PUD | 386 | 12 | Hist/RUT/antibody |
| Australia | ||||||||
| Borody et al[17] | 1991 | Australia | Not mentioned | Prospective | DU | 302 | 0.3 | Hist/culture |
| Xia[18] | 2000 | Australia | Not mentioned | Prospective | PUD | 48 | 40 | Hist/RUT/culture |
| Asia | ||||||||
| Tsuji et al[19] | 1999 | Japan | 1995-1997 | Prospective | DU and GU | DU: 120, GU: 215 | GU:2.3, DU: 0.9 | Hist/RUT/culture/antibody |
| Higuchi et al[20] | 1999 | Japan | Not mentioned | Cross-sectional | DU | 338 | 2.4 | Hist/RUT/culture/UBT/antibody |
| Aoyama et al[21] | 2000 | Japan | 1995-1998 | Cross-sectional | DU and GU | 302 | 2.6 | Hist/culture/antibody |
| Nishikawa et al[22] | 2000 | Japan | 1992-1997 | Cross-sectional | PUD | 398 | 1.3 | Hist/RUT/antibody |
| Chan et al[23] | 2001 | Hong Kong | 1997-1998 | Prospective | hPUD | 977 | 4.1 | Hist/RUT |
| Xia et al[24] | 2001 | Hong Kong | 1997-1999 | Prospective | DU | 599 | 17.4 | Hist/RUT/UBT |
| Kamada et al[25] | 2003 | Japan | Past 8 yr | Cross-sectional | DU | 464 | 1.3 | Hist//UBT/antibody |
| Chu et al[26] | 2005 | Hong Kong | 1996-2002 | Prospective | DU | 1343 | 23 | Hist/RUT |
| Yakoob et al[27] | 2005 | Pakistan | 1999-2000 | Retrospective | DU | 217 | 29 | Hist/RUT |
| Hung et al[28] | 2005 | Hong Kong | 2000 | Prospective | hPUD | 638 | 18.8 | Hist/RUT |
| Ong et al[29] | 2006 | Singapore | 2002-2004 | Prospective | PUD | 600 | 8 | Hist/RUT |
| Ootani et al[30] | 2006 | Japan | 2000-2002 | Prospective | hPUD | 116 | 1.7 | RUT/UBT/antibody |
| Jang et al[31] | 2008 | South Korea | 2004-2005 | Prospective | PUD | 895 | 22.2 | Hist/RUT |
| Chen et al[32] | 2010 | Taiwan | 2003-2004 | Prospective | DU | 731 | 8 | RUT/UBT |
| Goenka et al[33] | 2011 | India | 2008-2009 | Prospective | PUD | 142 | 36.6 | RUT/UBT |
| Chang et al[34] | 2011 | Taiwan | 2007-2008 | Prospective | PUD | 204 | 17.2 | Hist/RUT/UBT |
| Wong et al[35] | 2012 | Hong Kong | 2002-2009 | Prospective | hPUD | 4827 | 13.8 | Hist/RUT |
| Kang et al[36] | 2012 | South Korea | 2006-2008 | Prospective | PUD | 173 | 16.2 | Hist/RUT/culture/antibody |
Detailed reviews of idiopathic ulcers were published in 2002[37] and 2008[38]. Here, we examine more recent trends in the incidence of idiopathic ulcers, particularly in Asia.
When diagnosing idiopathic ulcers, H. pylori infection and history of NSAID use, the 2 main causes of peptic ulcers, must be completely ruled out. Otherwise, one will naturally find a high proportion of idiopathic ulcers.
Various methods are used to diagnose H. pylori infection, none of which is 100% accurate alone. Thus, the absence of H. pylori can be determined only when several tests are combined and the results of all are found to be negative[38]. Diagnostic methods that use endoscopic biopsy specimens-histology, culture, and rapid urease tests (RUT)-can show false negatives due to sampling errors caused by non-uniform distribution of H. pylori inside the stomach[39]. Therefore, biopsies must be obtained from several locations. Further, these methods can show false negatives immediately after acute upper digestive tract hemorrhage[40]. In these situations, other diagnostic methods must be used in combination. The urea breath test (UBT) examines urease activity throughout the stomach due to H. pylori, and is used to compensate for sampling errors in tests using biopsy specimens. However, care is needed when using UBT, as false negatives can result when bacterial concentrations decrease during administration of proton pump inhibitors (PPIs)[41], similar to other tests using biopsies. The serum antibody method is not affected by PPI administration or acute upper digestive tract hemorrhage; therefore, it is useful for diagnosing H. pylori infection in these situations. However, this method continues to show positive results for some time after bacterial elimination[42], making it difficult to differentiate between current and past infections. Nevertheless, it is a useful test because it provides a precise diagnosis of idiopathic ulcer (as few false negatives as possible). There are several tests for even more precise diagnosis of H. pylori-negative patients; although these tests do not directly confirm the presence of H. pylori, they eliminate the diagnosis of idiopathic ulcer based on tissue findings associated with infection, such as neutrophil infiltration into the gastric mucosa or atrophy of the gastric mucosa[28,30].
Another important factor in diagnosing idiopathic ulcers is careful elimination of NSAID users. Aspirin and other NSAIDs can be purchased without a doctor’s prescription in many countries. A large number of surreptitious NSAID users likely exist; therefore, patients must be questioned scrupulously about their drug history. Indeed, several previous studies have clarified the existence of surreptitious NSAIDs users. Lanas et al[43] examined patients with gastrointestinal perforation using platelet cyclooxygenase activity in the blood as an objective marker of aspirin usage. They identified 13% more aspirin users with this method than were found through investigation of medical history. Moreover, based on measurements of blood salicylic acid concentrations, Hirschowitz et al[44] reported that 50% of intractable peptic ulcer patients who denied using aspirin were in fact aspirin users. These findings underscore the necessity of carefully eliminating NSAID users when diagnosing idiopathic ulcers. Retrospective studies, which can only determine NSAID usage from past medical records, would be inevitably too lenient in eliminating NSAID users. The result would be a tendency to report higher rates of idiopathic ulcers; therefore, care must be taken when interpreting such data.
Six reports of the proportion of idiopathic ulcers in Asia were published in 1999-2003 based on patient data from the 1990s[19-24], 5 of which reported low rates of 1.3%-4.1%[19-23]. In 9 studies from 2005-2006 based on patient data from the 2000s[28-36], almost all reported that the proportion of idiopathic ulcers was 10%-30%, indicating that the proportion of these ulcers among all peptic ulcers in Asia is increasing. A decline in H. pylori infection rates among background healthy individuals is likely a cause of this increase. This trend is common among Asian countries[45], and indicates that recent improvements in sanitation and the increased use of H. pylori elimination therapies have decreased the H. pylori infection rates in the region. Even if the increase in idiopathic ulcers is merely a relative rise accompanying a decline in H. pylori-positive ulcers, or if H. pylori has only been coexisting as a bystander in many cases, the decline in H. pylori infection rates among the overall population has given prominence to the issue of idiopathic ulcers. Further, it has been reported that not only the proportion of idiopathic ulcers has increased but also the actual number of cases has been increasing annually[28]. This trend suggests the existence of not just a relative cause but also some other direct factor that is contributing to the incidence of idiopathic ulcers. Next, we will examine the incidence of idiopathic ulcers in various Asian countries in detail.
Hong Kong has been the most active region in conducting clinical studies on idiopathic ulcers; these studies have provided valuable data for understanding trends in idiopathic ulcer incidence. Two studies on the proportion of idiopathic ulcers based on patient data from the 1990s reported somewhat scattered results of 4% and 17%[23,24]. However, 3 studies based on data from the 2000s reported relatively high and consistent values of 14%-23%[26,28,34]. In particular, a recent major study on approximately 5000 peptic ulcer patients from 2002-2009 reported a 13.8% proportion of idiopathic ulcers[34], again showing that these ulcers are not rare in Hong Kong. A study on yearly changes in the proportion of idiopathic ulcers in the same institution reported a rise from 1997-1998 to 2000 of 4.1% to 18.8%[28], with other reports also showed an increase in the proportion of idiopathic ulcers over time[26]. While this rise in idiopathic ulcer rates reflects a relative increase accompanying a decline in H. pylori-positive ulcers due to the increased use of bacterial elimination therapy or decline of H. pylori infection rate among background healthy population[46], yearly data also suggest that the actual number of idiopathic ulcer patients is increasing[28].
Five studies of the frequency of idiopathic ulcers in Japan based on 1990s patient data reported very low rates of 0.9%-2.6%[19-22,24]. In addition, a 2000-2002 investigation of hemorrhagic ulcer patients found that 11% were H. pylori-negative and NSAID-negative[30]. However, after eliminating cases with a possible history of H. pylori infection based on histological atrophy of the gastric mucosa, the final proportion of cases with idiopathic ulcers was 1.7%[30]. There have since been no further studies on the frequency of idiopathic ulcers in Japan. Recently, there has been a marked decline in H. pylori infection rates among the general Japanese population[47], and it is possible there has been an accompanying rise in the proportion of idiopathic ulcers, as has occurred in other Asian countries. Sugiyama et al[48] found a similarly low proportion of idiopathic ulcers in Japanese ulcer patients divided into 2 age groups, which showed major differences in H. pylori infection rates. Based on this finding, they surmised that the proportion of idiopathic ulcers does not vary according to H. pylori infection rates. Considering the extremely low proportion of idiopathic ulcers in Japan in the 1990s, information on recent trends would be of great interest.
There are two reports from South Korea on the frequency of idiopathic ulcers based on patient data from the 2000s. One of them, based on 2004-2005 data, reported a high (22%) proportion of idiopathic ulcers[31]. However, the diagnosis of H. pylori infection in this study was based on only a single RUT and histological examination. Therefore, it is unclear whether the diagnosis of H. pylori negativity was sufficiently precise. The more recent study, based on 2006-2008 data, employed a strict definition of H. pylori negativity using a urease test, histological examination, culture, and the serum antibody method. This report confirmed a high proportion of idiopathic ulcers (16.2%)[36]. No reports from South Korea are based on patient data from the 1990s; therefore, comparisons with past data cannot be performed. However, it can be surmised that idiopathic ulcers are not rare in South Korea, similar to Europe and the United States. The recent decline in H. pylori infection rates among the general South Korean population[49,50] can be considered as the cause of the increase in the proportion of idiopathic ulcers unrelated to H. pylori.
A study from Taiwan based on data from 2003-2004 reported an idiopathic ulcer frequency of 8%[32]. Another report based on 2007-2008 data reported a 17% frequency[34], indicating that the proportion of idiopathic ulcers has been rising in recent years. The cause of this trend is also thought to be a decline in H. pylori infection rates in Taiwan[33].
A study of 1999-2000 patient data from Pakistan reported that 29% of ulcers were idiopathic[27]. Although this developing country was thought to have a high H. pylori infection rate at the time[51], the study reported a large proportion of idiopathic ulcers. However, problems with the methods used to diagnose H. pylori infection and issues with the retrospective study design, which did not allow a precise idiopathic ulcer diagnosis, possibly led to this apparently high proportion.
A study of 2002-2004 patient data from Singapore reported an 11% proportion of idiopathic ulcers[29]. However, data on serum thromboxane B2 concentrations suggested that 1/3 of these cases were surreptitious NSAID users, resulting in a true proportion of idiopathic ulcers of 8%[29]. Singapore is a multi-ethnic country and is known to have relatively lower rates of H. pylori infection than other Asian nations[52]. Nevertheless, this final proportion of idiopathic ulcers is relatively low. This study showed the importance of eliminating surreptitious NSAID users through blood tests, as well as through interviews.
Patient data from India from 2008-2009 showed an extremely high proportion of idiopathic ulcers at 37%[33], on par with rates in North America. This study diagnosed H. pylori using RUT and UBT, thus having a certain level of precision. Although the H. pylori infection rates in India have traditionally been high[53,54], recent studies have reported a decline in infection rates[55], and the high frequency of idiopathic ulcers may be a reflection of this. However, since this study involved only a single institution and a relatively small number of ulcer patients, further investigation is needed.
Although a clear cause of idiopathic ulcers hasn’t yet to be shown, several factors related to the condition have been proposed.
Numerous studies in Europe, the United States, and Asia have shown that idiopathic ulcer patients are significantly older than those with simple H. pylori ulcers or H. pylori/NSAID ulcers[11,23,24,28,29,56], although older age may be a mere confounder of the following risk factors such as systemic complications or psychological stress. Aging has been shown to be accompanied by a decline in the defense functions of the gastric mucosa owing to a variety of mechanisms, particularly a lower prostaglandin concentration in the gastric mucosa as a person ages[57], which may be a potential cause for gastric ulcers but not for duodenal ulcers. Prostaglandin plays a central role in the gastric mucosa defense structure by increasing gastric mucus secretions, bicarbonate secretions, and blood flow[58]. Thus, the diminished prostaglandin concentration in elderly individuals renders the gastric mucosa more fragile, creating an environment in which ulcers are more likely to occur.
Many studies, primarily from Asia, have reported that a wide variety[22,24,26,29] of serious[35] systemic complications are risk factors for idiopathic ulcers. This finding is possibly related to reports stating that peptic ulcers that occur in intensive care units or against a background of serious underlying disease are unrelated to H. pylori infection[59,60]. Physical or psychological (see below) stress caused by an underlying disease might be related to ulcer incidence.
Several recent studies have reported the relationship between hepatocirrhosis and hemorrhagic peptic ulcers[61-63]. In particular, decompensated hepatocirrhosis was demonstrated to be a risk factor for hemorrhagic peptic ulcer, independent of H. pylori infection[61-63]. Thus, hepatocirrhosis is an important cause of idiopathic ulcers. Although the pathology by which hepatocirrhosis leads to a peptic ulcer is complex, portal hypertension is likely involved. Visceral congestion from portal hypertension might be linked to ulcer incidence, as it damages the gastroduodenal mucosal blood flow and inhibits the process of mucosal repair[63-65]. In addition, decreased gastric prostaglandin synthesis observed in hepatocirrhosis patients may be involved in the hepatocirrhosis-related gastric mucosal injury[62,63].
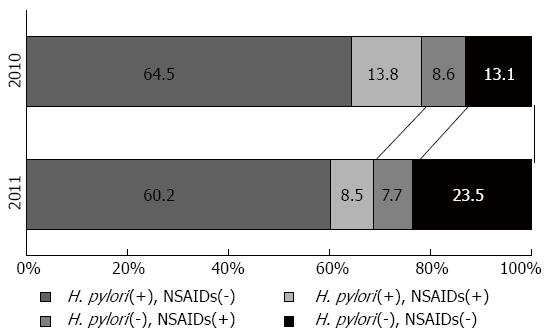
A link between psychological stress and peptic ulcers has long been suggested[65], but once H. pylori infection and damage from NSAID use were identified as the 2 main causes of peptic ulcers, it became unclear whether psychological stress should be considered an independent cause of peptic ulcer[4,21]. However, there have been reports of ulcer formation in the victims of the Great East Japan Earthquake that struck in 2011[67,68]. Kanno et al[67] compared the ulcer incidence 3 mo after the disaster with the ulcer incidence in the same period during the previous year; they found a 1.5-fold rise in ulcer cases after the disaster. Further, the proportion of H. pylori- and NSAID-negative (that is, idiopathic) ulcers rose significantly from 13% in 2010 to 24% after the disaster in 2011 (Figure 1)[67]. This study excluded cases complicated by severe trauma due to the disaster, showing indirectly that psychological stress can be an independent cause of peptic ulcers among disaster victims. Interestingly, the H. pylori-, NSAID-negative ulcers that arose after the disaster were in patients who were significantly older than those with other ulcer types[67]; this finding is consistent with the characteristic of idiopathic ulcers described above.
Bacterial elimination therapy is often effective for preventing the recurrence of H. pylori-positive ulcers and does not require subsequent maintenance therapy with acid-suppressive agents[4]. In NSAID-induced ulcers, changing therapy to COX-2-selective NSAIDs or other alternative medications that do not cause as much damage to the gastrointestinal mucosa can be expected to suppress recurrences[4]. However, for idiopathic ulcers, although acid-suppressive agents can produce temporary relief, they are not an effective preventative measure against recurrence. It has been shown that recurrence rates are high when patients remain unmedicated after such temporary cures. A study from Denmark observed 32 unmedicated patients with H. pylori-negative duodenal ulcers for 2 years, and reported a 35% recurrence rate[12]. Additionally, a prospective study from Hong Kong that observed unmedicated patients with idiopathic, hemorrhagic gastroduodenal ulcers for 7 years found that 42% experienced a relapse of ulcer hemorrhage. This is 4 times the percentage found while observing patients with H. pylori-positive ulcers who were unmedicated after bacterial elimination[69]. Further, a recent report from South Korea found that compared to H. pylori-positive ulcers and NSAID-induced ulcers, idiopathic ulcers showed more recurrences, which led to increased medical costs[36].
Although it has been shown that recurrences can easily occur when patients with idiopathic ulcer are not treated, there is no consensus on whether maintenance therapy with acid-suppressive agents can effectively prevent these recurrences. In the aforementioned study from Denmark, PPI administration was found to have an effect on preventing the recurrence of H. pylori-negative duodenal ulcers[12]. However, a recent major follow-up study from Hong Kong that examined 663 patients with idiopathic hemorrhagic gastroduodenal ulcers did not find H2-blocker or PPI administration to be effective in preventing ulcer hemorrhage relapse[35]. Around 50% of the cases in the Hong Kong study were gastric ulcers[35], and it was found that gastric ulcers, even idiopathic cases, might show different reactivity to acid-suppressive agents than duodenal ulcers. Until it is determined which medications can effectively prevent the recurrence of idiopathic ulcers, the most realistic choice appears to be continuous PPI therapy.
Numerous studies reported that idiopathic ulcers in Asia accounted for a small percentage of all ulcers in the 1990s, but in the 2000s, multiple studies reported that the proportion of idiopathic ulcers had reached 10%-30%. Despite limitations such as difficulties in precise diagnosis of H. pylori infection and identification of surreptitious NSAID users, it is clear that the incidence of idiopathic ulcers in Asia has been rising in recent years. While a decline in H. pylori infection rates in Asia is seen as the main reason for the increased incidence of idiopathic ulcers, it is also possible that the absolute number of idiopathic ulcer cases has increased. As it is expected that H. pylori infection rates in Asia will decline further in the future, measures to treat idiopathic ulcers will also likely become more important. Further multicenter studies from many different countries, with the same protocol, are necessary to investigate the real incidence of idiopathic ulcers and the real causes.
P- Reviewers: Abulezz T, Savopoulos CGG, Yasuda H S- Editor: Song XX L- Editor: A E- Editor: Ma S
| 1. | Kuipers EJ, Thijs JC, Festen HP. The prevalence of Helicobacter pylori in peptic ulcer disease. Aliment Pharmacol Ther. 1995;9 Suppl 2:59-69. [PubMed] |
| 2. | Lassen A, Hallas J, Schaffalitzky de Muckadell OB. Complicated and uncomplicated peptic ulcers in a Danish county 1993-2002: a population-based cohort study. Am J Gastroenterol. 2006;101:945-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 3. | Musumba C, Jorgensen A, Sutton L, Van Eker D, Moorcroft J, Hopkins M, Pritchard DM, Pirmohamed M. The relative contribution of NSAIDs and Helicobacter pylori to the aetiology of endoscopically-diagnosed peptic ulcer disease: observations from a tertiary referral hospital in the UK between 2005 and 2010. Aliment Pharmacol Ther. 2012;36:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009;374:1449-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 535] [Article Influence: 33.4] [Reference Citation Analysis (1)] |
| 5. | Peterson WL, Ciociola AA, Sykes DL, McSorley DJ, Webb DD. Ranitidine bismuth citrate plus clarithromycin is effective for healing duodenal ulcers, eradicating H. pylori and reducing ulcer recurrence. RBC H. pylori Study Group. Aliment Pharmacol Ther. 1996;10:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Jyotheeswaran S, Shah AN, Jin HO, Potter GD, Ona FV, Chey WY. Prevalence of Helicobacter pylori in peptic ulcer patients in greater Rochester, NY: is empirical triple therapy justified? Am J Gastroenterol. 1998;93:574-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Ciociola AA, McSorley DJ, Turner K, Sykes D, Palmer JB. Helicobacter pylori infection rates in duodenal ulcer patients in the United States may be lower than previously estimated. Am J Gastroenterol. 1999;94:1834-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 137] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Laine L, Hopkins RJ, Girardi LS. Has the impact of Helicobacter pylori therapy on ulcer recurrence in the United States been overstated? A meta-analysis of rigorously designed trials. Am J Gastroenterol. 1998;93:1409-1415. [PubMed] |
| 9. | McColl KE, el-Nujumi AM, Chittajallu RS, Dahill SW, Dorrian CA, el-Omar E, Penman I, Fitzsimons EJ, Drain J, Graham H. A study of the pathogenesis of Helicobacter pylori negative chronic duodenal ulceration. Gut. 1993;34:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 86] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Gisbert JP, Blanco M, Mateos JM, Fernández-Salazar L, Fernández-Bermejo M, Cantero J, Pajares JM. H. pylori-negative duodenal ulcer prevalence and causes in 774 patients. Dig Dis Sci. 1999;44:2295-2302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Meucci G, Di Battista R, Abbiati C, Benassi R, Bierti L, Bortoli A, Colombo E, Ferrara A, Prada A, Spinzi G. Prevalence and risk factors of Helicobacter pylori-negative peptic ulcer: a multicenter study. J Clin Gastroenterol. 2000;31:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Bytzer P, Teglbjaerg PS. Helicobacter pylori-negative duodenal ulcers: prevalence, clinical characteristics, and prognosis--results from a randomized trial with 2-year follow-up. Am J Gastroenterol. 2001;96:1409-1416. [PubMed] |
| 13. | Konturek SJ, Bielański W, Płonka M, Pawlik T, Pepera J, Konturek PC, Czarnecki J, Penar A, Jedrychowski W. Helicobacter pylori, non-steroidal anti-inflammatory drugs and smoking in risk pattern of gastroduodenal ulcers. Scand J Gastroenterol. 2003;38:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Arents NL, Thijs JC, van Zwet AA, Kleibeuker JH. Does the declining prevalence of Helicobacter pylori unmask patients with idiopathic peptic ulcer disease? Trends over an 8 year period. Eur J Gastroenterol Hepatol. 2004;16:779-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Arroyo MT, Forne M, de Argila CM, Feu F, Arenas J, de la Vega J, Garrigues V, Mora F, Castro M, Bujanda L. The prevalence of peptic ulcer not related to Helicobacter pylori or non-steroidal anti-inflammatory drug use is negligible in southern Europe. Helicobacter. 2004;9:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Sbrozzi-Vanni A, Zullo A, Di Giulio E, Hassan C, Corleto VD, Lahner E, Annibale B. Low prevalence of idiopathic peptic ulcer disease: an Italian endoscopic survey. Dig Liver Dis. 2010;42:773-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Borody TJ, George LL, Brandl S, Andrews P, Ostapowicz N, Hyland L, Devine M. Helicobacter pylori-negative duodenal ulcer. Am J Gastroenterol. 1991;86:1154-1157. [PubMed] |
| 18. | Xia HH, Kalantar JS, Mitchell HM, Talley NJ. Can helicobacter pylori serology still be applied as a surrogate marker to identify peptic ulcer disease in dyspepsia? Aliment Pharmacol Ther. 2000;14:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Tsuji H, Kohli Y, Fukumitsu S, Morita K, Kaneko H, Ohkawara T, Minami M, Ueda K, Sawa Y, Matsuzaki H. Helicobacter pylori-negative gastric and duodenal ulcers. J Gastroenterol. 1999;34:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Higuchi K, Arakawa T, Fujiwara Y, Uchida T, Tominaga K, Watanabe T, Kuroki T. Is Helicobacter pylori-negative duodenal ulcer masked by the high prevalence of H. pylori infection in the general population? Am J Gastroenterol. 1999;94:3083-3084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Aoyama N, Shinoda Y, Matsushima Y, Shirasaka D, Kinoshita Y, Kasuga M, Chiba T. Helicobacter pylori-negative peptic ulcer in Japan: which contributes most to peptic ulcer development, Helicobacter pylori, NSAIDS or stress? J Gastroenterol. 2000;35 Suppl 12:33-37. [PubMed] |
| 22. | Nishikawa K, Sugiyama T, Kato M, Ishizuka J, Komatsu Y, Kagaya H, Katagiri M, Nishikawa S, Hokari K, Takeda H. Non-Helicobacter pylori and non-NSAID peptic ulcer disease in the Japanese population. Eur J Gastroenterol Hepatol. 2000;12:635-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Chan HL, Wu JC, Chan FK, Choi CL, Ching JY, Lee YT, Leung WK, Lau JY, Chung SC, Sung JJ. Is non-Helicobacter pylori, non-NSAID peptic ulcer a common cause of upper GI bleeding? A prospective study of 977 patients. Gastrointest Endosc. 2001;53:438-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Xia HH, Wong BC, Wong KW, Wong SY, Wong WM, Lai KC, Hu WH, Chan CK, Lam SK. Clinical and endoscopic characteristics of non-Helicobacter pylori, non-NSAID duodenal ulcers: a long-term prospective study. Aliment Pharmacol Ther. 2001;15:1875-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Kamada T, Haruma K, Kusunoki H, Miyamoto M, Ito M, Kitadai Y, Yoshihara M, Chayama K, Tahara K, Kawamura Y. Significance of an exaggerated meal-stimulated gastrin response in pathogenesis of Helicobacter pylori-negative duodenal ulcer. Dig Dis Sci. 2003;48:644-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Chu KM, Kwok KF, Law S, Wong KH. Patients with Helicobacter pylori positive and negative duodenal ulcers have distinct clinical characteristics. World J Gastroenterol. 2005;11:3518-3522. [PubMed] |
| 27. | Yakoob J, Jafri W, Jafri N, Islam M, Abid S, Hamid S, AliShah H, Shaikh H. Prevalence of non-Helicobacter pylori duodenal ulcer in Karachi, Pakistan. World J Gastroenterol. 2005;11:3562-3565. [PubMed] |
| 28. | Hung LC, Ching JY, Sung JJ, To KF, Hui AJ, Wong VW, Leong RW, Chan HL, Wu JC, Leung WK. Long-term outcome of Helicobacter pylori-negative idiopathic bleeding ulcers: a prospective cohort study. Gastroenterology. 2005;128:1845-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Ong TZ, Hawkey CJ, Ho KY. Nonsteroidal anti-inflammatory drug use is a significant cause of peptic ulcer disease in a tertiary hospital in Singapore: a prospective study. J Clin Gastroenterol. 2006;40:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Ootani H, Iwakiri R, Shimoda R, Nakahara S, Amemori S, Fujise T, Kikkawa A, Tsunada S, Sakata H, Fujimoto K. Role of Helicobacter pylori infection and nonsteroidal anti-inflammatory drug use in bleeding peptic ulcers in Japan. J Gastroenterol. 2006;41:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Jang HJ, Choi MH, Shin WG, Kim KH, Chung YW, Kim KO, Park CH, Baek IH, Baik KH, Kae SH. Has peptic ulcer disease changed during the past ten years in Korea? A prospective multi-center study. Dig Dis Sci. 2008;53:1527-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Chen TS, Luo JC, Chang FY. Prevalence of Helicobacter pylori infection in duodenal ulcer and gastro-duodenal ulcer diseases in Taiwan. J Gastroenterol Hepatol. 2010;25:919-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Goenka MK, Majumder S, Sethy PK, Chakraborty M. Helicobacter pylori negative, non-steroidal anti-inflammatory drug-negative peptic ulcers in India. Indian J Gastroenterol. 2011;30:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Chang CY, Wu MS, Lee CT, Hwang JC, Tai CM, Perng DS, Lin CW, Wang WL, Wang JD, Lin JT. Prospective survey for the etiology and outcome of peptic ulcer bleeding: a community based study in southern Taiwan. J Formos Med Assoc. 2011;110:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Wong GL, Au KW, Lo AO, Tse YK, Ching JY, To KF, Chan FK. Gastroprotective therapy does not improve outcomes of patients with Helicobacter pylori-negative idiopathic bleeding ulcers. Clin Gastroenterol Hepatol. 2012;10:1124-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Kang JM, Seo PJ, Kim N, Lee BH, Kwon J, Lee DH, Jung HC. Analysis of direct medical care costs of peptic ulcer disease in a Korean tertiary medical center. Scand J Gastroenterol. 2012;47:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Quan C, Talley NJ. Management of peptic ulcer disease not related to Helicobacter pylori or NSAIDs. Am J Gastroenterol. 2002;97:2950-2961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Gisbert JP, Calvet X. Review article: Helicobacter pylori-negative duodenal ulcer disease. Aliment Pharmacol Ther. 2009;30:791-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3553] [Article Influence: 122.5] [Reference Citation Analysis (3)] |
| 40. | Gisbert JP, Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:848-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 41. | Laine L, Estrada R, Trujillo M, Knigge K, Fennerty MB. Effect of proton-pump inhibitor therapy on diagnostic testing for Helicobacter pylori. Ann Intern Med. 1998;129:547-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 126] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Luthra GK, DiNuzzo AR, Gourley WK, Crowe SE. Comparison of biopsy and serological methods of diagnosis of Helicobacter pylori infection and the potential role of antibiotics. Am J Gastroenterol. 1998;93:1291-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Lanas A, Serrano P, Bajador E, Esteva F, Benito R, Sáinz R. Evidence of aspirin use in both upper and lower gastrointestinal perforation. Gastroenterology. 1997;112:683-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 125] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Hirschowitz BI, Lanas A. Intractable upper gastrointestinal ulceration due to aspirin in patients who have undergone surgery for peptic ulcer. Gastroenterology. 1998;114:883-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Tan HJ, Goh KL. Changing epidemiology of Helicobacter pylori in Asia. J Dig Dis. 2008;9:186-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Xia B, Xia HH, Ma CW, Wong KW, Fung FM, Hui CK, Chan CK, Chan AO, Lai KC, Yuen MF. Trends in the prevalence of peptic ulcer disease and Helicobacter pylori infection in family physician-referred uninvestigated dyspeptic patients in Hong Kong. Aliment Pharmacol Ther. 2005;22:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Fujisawa T, Kumagai T, Akamatsu T, Kiyosawa K, Matsunaga Y. Changes in seroepidemiological pattern of Helicobacter pylori and hepatitis A virus over the last 20 years in Japan. Am J Gastroenterol. 1999;94:2094-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Sugiyama T, Nishikawa K, Komatsu Y, Ishizuka J, Mizushima T, Kumagai A, Kato M, Saito N, Takeda H, Asaka M. Attributable risk of H. pylori in peptic ulcer disease: does declining prevalence of infection in general population explain increasing frequency of non-H. pylori ulcers? Dig Dis Sci. 2001;46:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Yim JY, Kim N, Choi SH, Kim YS, Cho KR, Kim SS, Seo GS, Kim HU, Baik GH, Sin CS. Seroprevalence of Helicobacter pylori in South Korea. Helicobacter. 2007;12:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 50. | Lim SH, Kwon JW, Kim N, Kim GH, Kang JM, Park MJ, Yim JY, Kim HU, Baik GH, Seo GS. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol. 2013;13:104. [PubMed] |
| 51. | Abbas Z, Jafri W, Khan AH, Shah MA. Prevalence of Helicobacter pylori antibodies in endoscopy personnel and non-medical volunteers of Karachi. J Pak Med Assoc. 1998;48:201-203. [PubMed] |
| 52. | Ho KY, Chan YH, Kang JY. Increasing trend of reflux esophagitis and decreasing trend of Helicobacter pylori infection in patients from a multiethnic Asian country. Am J Gastroenterol. 2005;100:1923-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 53. | Katelaris PH, Tippett GH, Norbu P, Lowe DG, Brennan R, Farthing MJ. Dyspepsia, Helicobacter pylori, and peptic ulcer in a randomly selected population in India. Gut. 1992;33:1462-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Graham DY, Adam E, Reddy GT, Agarwal JP, Agarwal R, Evans DJ, Malaty HM, Evans DG. Seroepidemiology of Helicobacter pylori infection in India. Comparison of developing and developed countries. Dig Dis Sci. 1991;36:1084-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 250] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 55. | Singh V, Trikha B, Nain CK, Singh K, Vaiphei K. Epidemiology of Helicobacter pylori and peptic ulcer in India. J Gastroenterol Hepatol. 2002;17:659-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Kemppainen H, Räihä I, Sourander L. Clinical presentation of peptic ulcer in the elderly. Gerontology. 1997;43:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 57. | Cryer B, Redfern JS, Goldschmiedt M, Lee E, Feldman M. Effect of aging on gastric and duodenal mucosal prostaglandin concentrations in humans. Gastroenterology. 1992;102:1118-1123. [PubMed] |
| 58. | Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn’t the stomach digest itself? Physiol Rev. 2008;88:1547-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 435] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 59. | Schilling D, Haisch G, Sloot N, Jakobs R, Saggau W, Riemann JF. Low seroprevalence of Helicobacter pylori infection in patients with stress ulcer bleeding--a prospective evaluation of patients on a cardiosurgical intensive care unit. Intensive Care Med. 2000;26:1832-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Halm U, Halm F, Thein D, Mohr FW, Mössner J. Helicobacter pylori infection: a risk factor for upper gastrointestinal bleeding after cardiac surgery? Crit Care Med. 2000;28:110-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Luo JC, Leu HB, Hou MC, Huang CC, Lin HC, Lee FY, Chang FY, Chan WL, Lin SJ, Chen JW. Cirrhotic patients at increased risk of peptic ulcer bleeding: a nationwide population-based cohort study. Aliment Pharmacol Ther. 2012;36:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 62. | Chang SS, Hu HY. Helicobacter pylori is not the predominant etiology for liver cirrhosis patients with peptic ulcer disease. Eur J Gastroenterol Hepatol. 2013;25:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Lo GH, Yu HC, Chan YC, Chen WC, Hsu PI, Lin CK, Lai KH. The effects of eradication of Helicobacter pylori on the recurrence of duodenal ulcers in patients with cirrhosis. Gastrointest Endosc. 2005;62:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 64. | McCormack TT, Sims J, Eyre-Brook I, Kennedy H, Goepel J, Johnson AG, Triger DR. Gastric lesions in portal hypertension: inflammatory gastritis or congestive gastropathy? Gut. 1985;26:1226-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 380] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 65. | Iwao T, Toyonaga A, Ikegami M, Shigemori H, Oho K, Sumino M, Tanikawa K. Gastric mucus generation in cirrhotic patients with portal hypertension. Effects of tetraprenylacetone. Dig Dis Sci. 1996;41:1727-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 66. | Szabo S. Hans Selye and the development of the stress concept. Special reference to gastroduodenal ulcerogenesis. Ann N Y Acad Sci. 1998;851:19-27. [PubMed] |
| 67. | Kanno T, Iijima K, Abe Y, Koike T, Shimada N, Hoshi T, Sano N, Ohyauchi M, Ito H, Atsumi T. Peptic ulcers after the Great East Japan earthquake and tsunami: possible existence of psychosocial stress ulcers in humans. J Gastroenterol. 2013;48:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 68. | Kanno T, Iijima K, Abe Y, Koike T, Shimada N, Hoshi T, Sano N, Ohyauchi M, Ito H, Atsumi T. Hemorrhagic ulcers after Great East Japan Earthquake and Tsunami: features of post-disaster hemorrhagic ulcers. Digestion. 2013;87:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 69. | Wong GL, Wong VW, Chan Y, Ching JY, Au K, Hui AJ, Lai LH, Chow DK, Siu DK, Lui YN. High incidence of mortality and recurrent bleeding in patients with Helicobacter pylori-negative idiopathic bleeding ulcers. Gastroenterology. 2009;137:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |