Published online Aug 7, 2014. doi: 10.3748/wjg.v20.i29.9665
Revised: April 6, 2014
Accepted: April 30, 2014
Published online: August 7, 2014
Processing time: 311 Days and 14.4 Hours
Restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) has become the surgical treatment of choice for many patients with medically refractory ulcerative colitis (UC) and familial adenomatous polyposis (FAP). UC patients with IPAA (UC-IPAA) are, nevertheless, susceptible to inflammatory and noninflammatory sequelae such as pouchitis, which is only rarely noted in FAP patients with IPAA. Pouchitis is the most frequent long-term complication of UC-IPAA patients, with a cumulative prevalence of up to 50%. Although the aetiology of pouchitis remains unclear, accumulating evidence suggests that a dysbiosis of the pouch microbiota and an abnormal mucosal immune response are implicated in its pathogenesis. Studies using culture and molecular techniques have detected a dysbiosis of the pouch microbiota in patients with pouchitis. Risk factors, genetic associations, and serological markers suggest that interactions between the host immune response and the pouch microbiota underlie the aetiology of this idiopathic inflammatory condition. This systematic review focuses on the dysbiosis of the microbiota that inhabit the pouch in UC and FAP patients and its interaction with the mucosal immune system. A meta-analysis was not attempted due to the highly heterogeneous microbiota composition and the different detection methods used by the various studies. Although no specific bacterial species, genus, or family has as yet been identified as pathogenic, there is evidence that a dysbiosis characterized by decreased gut microbiota diversity in UC-IPAA patients may, in genetically predisposed subjects, lead to aberrant mucosal immune regulation triggering an inflammatory process.
Core tip: This is a systemic review assessing the relationship between the microbiota that inhabit the ileal-anal pouch following restorative proctocolectomy in ulcerative colitis and familial adenomatous polyposis patients and the inflammatory response that can occur. A meta-analysis was not attempted in view of the highly heterogeneous microbiota composition and the different detection methods utilized. Although no specific bacterial species, genus, or family has as yet been identified as pathogenic, there is evidence that dysbiosis and reduced bacterial diversity of the microbiota found in ulcerative colitis patients who have undergone restorative proctocolectomy may, in genetically predisposed subjects, lead to aberrant mucosal immune regulation triggering an inflammatory process.
- Citation: Angriman I, Scarpa M, Castagliuolo I. Relationship between pouch microbiota and pouchitis following restorative proctocolectomy for ulcerative colitis. World J Gastroenterol 2014; 20(29): 9665-9674
- URL: https://www.wjgnet.com/1007-9327/full/v20/i29/9665.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i29.9665
About 20%-25% of patients with ulcerative colitis (UC) require a colectomy at some point in their lives, and in most cases the operative surgical procedure chosen is a restorative proctocolectomy (RPC) with ileal pouch-anal anastomosis (IPAA)[1,2]. Diseased colonic/rectal tissue is removed during the procedure, and transanal fecal continence is maintained by creating an ileal pouch. Although effective, inflammation of the ileal pouch (pouchitis) is a common complication, with almost 50% of patients experiencing an acute episode within 5 years and about 5% of those going on to develop chronic inflammation[1-5]. The cumulative incidence of pouchitis in patients who undergo an IPAA for familial adenomatous polyposis (FAP) is much lower, ranging from 0% to 10%[6]. Reasons for the higher frequency of pouchitis in UC remain unknown.
Common clinical symptoms in patients with pouchitis include increased bowel movements, abdominal pain/cramping, urgency, incontinence, generalized fatigue/malaise, fever, and, occasionally, bloody stools. The diagnosis of pouchitis is based on assorted clinical, endoscopic, and histologic findings, including endoscopic and microscopic evidence of inflammation of the ileal pouch[7]. In the absence of evident signs of inflammation of the terminal ileum, fecal lactoferrin, or calprotectin (mucosal inflammation markers) can help to distinguish pouchitis from irritable pouch syndrome[8]. Once diagnosed, disease activity can be quantified using the pouchitis disease activity index (PDAI), which takes into consideration clinical findings, histology, and laboratory parameters[9].
Histological features of pouchitis can also be non-specific, including acute inflammation with polymorphonuclear leukocyte infiltration, crypt abscesses, and ulceration in association with a chronic inflammatory infiltrate[7,10]. A discrepancy between endoscopic and histologic findings, possibly linked to sampling errors, has been noted in patients with pouchitis[11,12]. Morphological alterations of the epithelium lining the ileal pouch, characterized by flattening and a reduced number or complete disappearance of the villi leading to villous atrophy (colonic metaplasia), normally develop within 12-18 mo after ileostomy closure[10,12]. Although a causal association has not been proven, once colonic metaplasia has developed in the pouch, pouchitis may occur[13].
In accordance with Mahadevan and Sandborn’s definition, the pattern of pouchitis is classified as infrequent (1 or 2 acute episodes), relapsing (3 acute episodes), or continuous. Recurrent pouchitis is defined, according to this classification, as relapsing (more than two episodes) or chronic[13]. Chronic pouchitis is usually refractory to medical therapy and/or requires maintenance therapy, and may lead to pouch excision and permanent terminal ileostomy[13]. Risk factors linked to pouchitis include extensive UC[2,14], backwash ileitis[14], extraintestinal manifestations, chiefly primary sclerosing cholangitis[15-17], being a non-smoker, and regular use of nonsteroidal anti-inflammatory drugs[18,19].
Genetic polymorphisms in interleukin-1 receptor antagonist (IL-1Ra) or the presence of perinuclear neutrophil cytoplasmic antibodies[20] have also been associated with pouchitis. Although definitive proof is still lacking[21], indirect evidence from clinical practice supports the hypothesis that the pouch microbiota (i.e., a microbial imbalance) plays an important role in the pathogenesis of pouchitis[22,23]. It has been seen, in fact, that mucosal inflammation is localized in the area of the gut characterized by the highest bacterial concentrations[24] and is limited to the mucosal surface[25]. Short-term, oral antibiotic therapy (i.e., metronidazole or ciprofloxacin)[4,26] has been reported to be an effective treatment for both pouchitis and pre-pouch ileitis in up to 87.5% of patients[27,28]. Probiotics have also been shown to reduce disease relapse and the risk of disease onset[29]. Findings on risk factors, genetic associations, and the serological markers of pouchitis all seem to point to the conclusion that host immune responses and pouch microbiota interactions trigger this idiopathic inflammatory condition[30].
Studies focusing on the analysis of the gut microbiota following IPAA in patients with UC or FAP were eligible for inclusion. Studies examining pouch disease were also included, with microbiota analysis being carried out in at least 10 patients. Studies or case reports dealing exclusively with operative or postoperative management or referring to fewer than 10 patients following IPAA were excluded. Studies reporting on partial analysis of the microbiota or on patients with anastomosis for Crohn’s disease (CD) were also excluded. Only studies providing information on analysis of the entire bacterial microbiota were included. Whenever publications reporting on overlapping patient data were being considered, only the most complete and recent set of data were included.
With the assistance of a clinical librarian, two researchers (Angriman I and Scarpa M) consulted Medline, the Embase Medical Database, the Cochrane Database of Systematic Reviews, and the Cochrane Central Register of Controlled Trials (CENTRAL) for studies concerning ileal pouches carried out between January 1978 (publication date of the first manuscripts on RPC) and October 2013. The keywords and Medical Subject Headings (MeSH) used were “pouchitis OR chronic pouchitis OR acute pouchitis OR inflammation of the ileal pouch AND microbiota OR bacteria OR microbiome”. Only clinical studies in English, Dutch, Spanish, German, French, and/or Italian were considered. A manual cross-reference search for qualified papers was also carried out to identify additional relevant articles, and the resulting studies that were compatible with the inclusion criteria were evaluated independently. Unpublished data or findings published in abstracts were not taken into consideration. A negotiated agreement was reached between the two researchers whenever there was discordance regarding study inclusion.
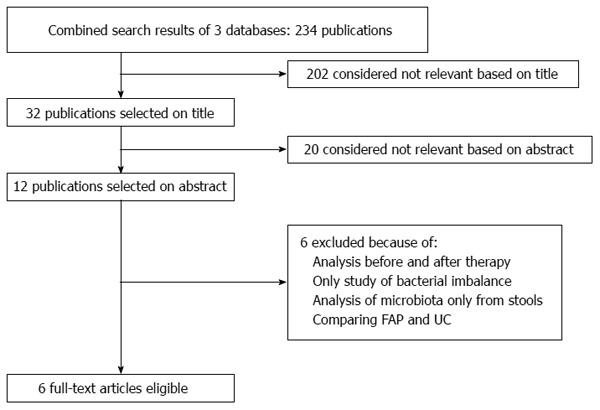
Only data from original articles were extracted using a preformatted sheet with spaces for the following parameters: demographic data, pouchitis symptoms, presence of GI bleeding, frequency of bowel movements, abdominal pain, PDAI score, and endoscopic and histological diagnoses (Figure 1).
Review Manager 4.2 software (The Cochrane Collaboration, Copenhagen: The Nordic Cochrane Centre, 2003) was used for all statistical analyses. A meta-analysis was not attempted due to the highly heterogeneous microbiota composition and the different detection methods used.
The composition of the bacterial microbiota in the terminal ileum differs significantly from that found in the colon[31,32], and there are likewise significant differences in the microbial composition of the luminal and mucosal compartments of the gut[33]. Moreover, it is known that the gut microbiota profoundly influences the intestinal mucosa and gut-associated lymphoid tissue[34]. Although the microbiota that colonizes the mucosal surface usually exercises beneficial trophic, immunomodulatory, and anti-inflammatory effects, an “imbalanced” microbiota can damage the mucosa by producing toxins or triggering abnormal immune responses[35]. Alterations in the intestinal mucosal-associated microbiota have, in fact, been linked to the pathogenesis of several gastrointestinal inflammatory disorders.
Many of the studies concerning the microbiota after IPAA that were carried out prior to the formulation of PDAI in 1994 show inconsistencies in their definition and diagnosis criteria, as well as in the distinction between acute and chronic pouchitis[9]. In addition, the majority of studies focusing on the pouch microbiota also used culture methods, despite 60%-80% of gut bacterial species being unculturable[36,37]. Variability in the use of fecal or mucosal samples further contributed to the discrepancies in findings noted in many of these studies.
It is well-established that, within the first year after ileostomy closure, the overall composition of microbiota shows similarities with that of the colon[38,39]. A number of studies using fecal cultures to evaluate the microbiota of pouches in UC and FAP patients[40,41] produced conflicting results with regard to the ratio of anaerobic bacteria to aerobic bacteria, total bacterial counts, and sulfate-reducing bacteria[42] in the pouchitis and in non-pouchitis patients. The high grade of variability in these kinds of studies may be due to the daily variability of stool composition in relation to diet. Moreover, the high frequency of bowel movement in IPAA patients may enhance this variability.
The mucosal-adherent microbiota, which is in close contact with the gut mucosa, has recently been shown[37,43] to be distinct from the luminal and fecal ones, which are made up of free-living or particle-attached cells. The differences in community structure are probably linked to a number of factors, such as differential substrate availability (mucus vs undigested dietary residues), oxygen levels, and host-microbe interactions. In particular, mucosa adherent microbiota may be influenced by drugs. The close proximity of the mucosal-adherent microbiota to the gut epithelium suggests that these bacteria may be more relevant than the luminal microbiota in the pathogenesis of inflammatory bowel disease (IBD), since they, as well as their excreted products, probably have direct contact with the host[37]. Moreover, since they live in a mucous environment their populations are more protect, and thus more constant.
The ideal microbiota analysis probably would examine both the fecal and mucosal-adherent microbiota. In fact, fecal microbiota may give a rough but more complete idea of whole bowel microbiota, while mucosa adherent bacteria directly cross-talk with the host and are more likely to be involved in the pathogenesis of pouchitis.
Knowledge about the complexity and diversity of the gut microbiota underwent a radical revision when 16S rRNA techniques were introduced[31,32], with 16S rRNA gene sequencing results representing gene copy number rather than true bacterial counts. Possibly biased by differential DNA extraction and PCR amplification rates, the methodology represents, nevertheless, the best available option, and is considered the “gold standard” for the analysis of gut-associated complex microbial communities[44]. Some studies used molecular techniques to show that microbiota that inhabit the ileal-anal pouch differ from that of the normal large intestine. UC pouches with or without pouchitis appear to harbor particularly more unusual microbes. Proteobacteria, which normally account for only a small proportion of the microbiota in the healthy colon[31] and were found to make up 20% in IBD patients[32], comprised up to 90% (median = 66.6%) in UC-IPAA patients in one study[45]. That same study also showed lower than normal proportions of Bacteroidetes, Lachnospiraceae and Ruminococcaceae. A comparison of the two study cohorts revealed that the UC-IPAA patients had increased proportions of the phylum Proteobacteria and decreased levels of Bacteroidetes with respect to the FAP-IPAA patients.
Consistent with a reduced bacterial diversity observed in both CD[46] and UC[47,48], another study demonstrated that there is a significantly lower bacterial diversity in UC-IPAA patients, with or without pouchitis, with respect to FAP-IPAA patients[49]. However, only minor compositional differences were detected in the microbiota of UC patients with active pouchitis with respect to those with no disease history. The authors of that study hypothesized that dysbiosis predisposes UC patients to pouchitis either by increasing the likelihood of immune system stimulation or by reducing microbiota diversity, which may itself be sufficient to stimulate the immune system, leading to mucosal inflammation. The observation that VSL#3 administration increases bacterial diversity and thus reduces relapse risk[50] supports the second hypothesis. Zella et al[45] demonstrated that the microbial environment in the pouches of UC-associated, healthy UC and FAP patients is distinctly different. Using 16S ribosomal gene-based Terminal Restriction Fragment Length Polymorphism (TRFLP) data, those investigators identified significant differences in fecal and mucosal bacterial communities in the three patient cohorts. Broad differences in TRFLP profiles were further analyzed using DNA sequencing, which revealed multiple significant variations in specific bacterial genera in the pooled fecal bacterial DNA samples in the UC and FAP groups.
Tannock et al[51] demonstrated that bacteria uncommonly present in the stools of humans in general and in FAP patients in particular comprised about 50% of the microbiota of patients with pouchitis when antibiotic-treated (CP-off = asymptomatic), and that antibiotic treatment reduced the proportion of the unknown bacteria in their feces. Chronic or recurrent pouchitis was therefore found to be associated with microbiota containing bacteria not commonly associated with human feces or FAP pouches. The uncommon bacteria constituting a large proportion of the CP-off but not of the untreated CP-on (symptomatic) microbiota were found to be members of the Caulobacteraceae, Sphingomonadaceae, Comamonadaceae, Peptostreptococcaceae and Clostridiaceae. At least some of these groups could theoretically be linked to the pathogenesis of pouchitis. The investigators also noted a wide diversity in the clostridial operational taxonomic units in the CP-off microbiota, presumably denoting particularly favorable conditions in the pouch for the expansion of clostridial populations. The authors concluded that C. perfringens may play a role in the etiology of pouchitis in some patients.
Although it is not clear if reduced biodiversity causes, perpetuates, or is a result of IBD, other studies have also demonstrated a quantitative and qualitative (biodiversity) reduction in the representation of the Firmicutes phylum, particularly clostridial cluster IV members, in the feces of CD patients. This phylogenetic group contains several butyrate-producing bacteria, such as Faecalibacterium prausnitzii (F. prausnitzii). Butyrate and other short chain fatty acids are considered important energy sources for colonic epithelial cells, have anti-inflammatory properties, and improve the intestinal barrier function of epithelial cells. A reduction in butyrate-producing bacteria in the colon or pouch might then have an overall detrimental effect on the gut mucosa[52-56]. Indeed, although F. prausnitzii was not detected in chronic pouchitis stool microbiota, low levels were found in FAP and normal pouch feces. Members of the Lachnospiraceae, some of which produce butyric acid, were depleted in chronic pouchitis microbiota in FAP and normal pouches (average 33.61% and 21.86%, respectively). The authors reported that normal and FAP pouches showed similar proportions of Lachnospiraceae and clostridial cluster IV, indicating that measures of these bacterial groups in the microbiota could be useful biomarkers of pouch health.
In a recent study, we reported that the Enterobacteriaceae, Streptococcaceae, Enterococcaceae, and Bacteroidaceae were the most frequent strains of cultivable bacteria adherent to the pouch mucosa, while Lachnospiraceae, Fusobacteiaceae, Veillonella, Staphylococcaceae, Bifidobacteriaceae, Eubacteriaceae, Bacillaceae, Moraxellaceae, Burkholderiaceae and Corynebacteriaceae were the least frequent ones. Although their incidence in the pouch mucosa was similar in the chronic pouchitis and normal pouch groups, we detected a significantly higher incidence of Clostridiaceae spp. in the chronic/recurrent pouchitis group compared to that of the normal pouch. The presence of Clostridiaceae spp. was found to be an independent predictor of chronic/relapsing pouchitis[57] at multiple logistic regression analysis. In another study, we found that patients with a clinical diagnosis of pouchitis had a significantly reduced level of Enterococcaceae spp. (including bacteria strictly adherent to the pouch mucosa and in the pouch mucous) vs those without a clinical diagnosis. Total Enterobacteriaceae spp. and Streptococcaceae spp. counts in the mucosa were also decreased in patients with clinical pouchitis compared with patients with ‘‘healthy’’ pouches. Our findings indicate that Bacteroidaceae spp. and Clostridiaceae spp. are more frequently associated with microscopic inflammation of the pouch mucosa and may therefore play a pathogenic role in pouchitis. Enterococcaceae spp., as well as possibly Enterobacteriaceae spp. and Streptococcaceae spp., may instead play an active role in maintaining immunologic homeostasis within the pouch mucosa, with low levels feasibly favoring the development of pouchitis[58].
Zella et al[45] similarly noted an overall increase in fecal Clostridium spp. in UC-associated pouchitis with respect to that in FAP patients. They also noted a reduction in Bacteroides spp. in the inflamed pouch with respect to FAP pouches, confirming that a mucosal and luminal dysbiosis exists in pouchitis not only when compared to the healthy UC pouch but also to a non-IBD one.
The overall decrease in the Bacteroidetes phylum in the inflamed pouch is consistent with several studies that included data on the molecular analysis of intestinal microbiota in both CD and UC[47,59,60]. In line with previous reports, those studies also found that healthy UC pouches differed significantly from FAP ones, leading to the hypothesis that an alteration in the ileal pouch microbiota may be exclusive to the UC disease state, with or without inflammation. In particular, since Bacteroidetes may play a key role in maintaining gut health, a relative reduction in this population may favor inflammation[45]. In addition, those authors found a significant increase in clostridia, namely among the Clostridium, Lachnospiraceae, and Roseburia genera, in the inflamed pouch. Roseburia are flagellated commensal inhabitants of the colon. Flagellin has been shown to induce proinflammatory gene expression by activating toll-like receptor 5 (TLR5); subjects with TLR5 polymorphisms and low levels of anti-flagellin antibodies may be protected from developing CD. Present in 50% of CD and 6% of UC patients, high levels of anti-flagellin antibodies, specifically anti-CBir1 antibodies to flagellin of Clostridium spp., may be associated with the development of pouchitis[61,62]. Additionally, the mucin-degrading Akkermansia genus of the Verrucomicrobia phylum was significantly more prevalent in pouchitis. There are several theories regarding the role of mucin in protecting the intestinal epithelium in IBD, with mutations, alterations, and degradation of mucins being associated with CD and UC[63-66].
In conclusion, studies using molecular techniques to analyze the microbiota have confirmed that there is dysbiosis of the pouches of UC-IPAA patients; there are, however, conflicting results with regard to differences in the abundance of particular species and phylotypes associated with pouchitis and in the degree of community diversity of the pouch microbiota (Table 1). As dysbiosis of the gut microbiota could be a pathogenic prerequisite for the idiopathic development of gut inflammation, this would explain the higher frequency of pouchitis in UC-IPAA with respect to FAP-IPAA patients. Although dysbiosis is found in the UC pouch, it may not be the single cause leading to inflammation, but rather a predisposing factor.
| Ref. | Year | Microbiota identification | Sampling andanalysis | Normal pouch (n) | Pouchitis(n) | Bacteria normalpouch | Bacteria pouchitis | Taxonomy level | Methods | Database |
| Komanduri et al[89] | 2007 | 16S rRNA | Mucosa adherent microbiota | 15 | 5 | Clostridium paraputrificum | Fusobacterium varium | Genus and species | LH-PCR | PCO plot |
| Technique (LH–PCR) | E. coli/Shigella ssp. | Ruminococcus obeum | ||||||||
| Lim et al[21] | 2009 | 16S rDNA PCR products | Stool microbiota | 15 | 5 | No differences | Genus and species | T-RFLP | BLAST | |
| McLaughlin et al[49] | 2010 | 16S rDNA PCR products | Mucosal adherent microbiota | 8 | 8 | No differences | Phylum , family and species | PCR | Mega BLAST | |
| Zella et al[45] | 2011 | 16S rRNA PCR products | Stool microbiota | 3 | 9 | Bacteroidetes (vs FAP) | Clostridium | Phylum, Family Genus, species | RFLP | BLAST |
| Eubacterium | ||||||||||
| Firmicutes (vs FAP) | ||||||||||
| Scarpa et al[58] | 2011 | 16S rDNA PCR products | Mucosa adherent microbiota culture | 22 | 10 | Enterobacteriaceae | Clostridiaceae | Family | GAPDH, qRT-PCR | BLAST |
| Enterococcaceae | Bacteroidaceae | |||||||||
| Tyler et al[90] | 2013 | 16S rDNA PCR products | Mucosa adherent microbiota | 19 | 15 | Bacteroides | Proteobacteria | Phylum, genus | qPCR | Mothur |
| Sutterella | Firmicutes | |||||||||
| Blautia | Roseburia spp. | |||||||||
| Moryella | Eubacterium rectale | |||||||||
| Dorea | ||||||||||
| Parabacteroides |
While some investigations have failed to identify a specific microbe as a causative agent[23], others have stated that they believe sulfate-reducing bacteria, such as Clostridiaceae spp., Enterobacteriaceae spp. and Bacteroidaceae spp., might be implicated in the pathogenesis of pouchitis[67-71]. Our group recently observed that Clostridiaceae spp. adherent to pouch mucosa are associated with chronic/relapsing pouchitis while Enterococcaceae spp. and possibly Enterobacteriaceae spp. and Streptococcaceae spp. may play a role in maintaining immunologic homeostasis within the pouch mucosa, with low levels of these bacteria favoring the development of acute pouchitis[57,58]. Deregulated mucosal cell immunity toward the microbiota also seems to be implicated in the pathogenesis of pouchitis. Epithelial and immune cells within the intestinal mucosa recognize conserved microbial structures, known as pathogen-associated molecular patterns (PAMPs), through membrane-bound and cytoplasmic receptors [so-called pattern recognition receptors (PRRs)], which, when activated, trigger intracellular signaling pathways to elicit a variety of stereotyped (so-called innate) immune responses[72].
Fragments of bacterial peptidoglycan (the main component of the bacterial cell wall) can, for example, serve as PAMPs that bind to the PRR toll-like receptor 2 (TLR2), while bacterial lipopolysaccharides (LPS; found in the outer membrane of gram-negative bacteria) can serve as PAMPs that bind to TLR4. Binding of these conserved bacterial structures to TLRs triggers a number of intracellular signaling cascades which, through NF-κB and protein kinase activation, lead to the transcription of a series of genes[73]. In macrophages this activation results in the expression of several pro-inflammatory cytokines [including interleukin (IL)-1β, tumor-necrosis factor (TNF)-α, and IL-6], chemokines, adhesion molecules, leukotrienes, nitric oxide, and production of reactive oxygen species, while in dendritic cells it enhances major histocompatibility complex class II and co-stimulatory molecule expression favoring subsequent antigen-specific, T-helper cell activation[74,75]. Microbiota associated PAMPs modulating the release of a number of biologically active molecules profoundly influence the function and integrity of the intestinal mucosa.
Experimental findings indicate that these mechanisms are activated in pouchitis. In a recent study, Toiyama et al[76] reported that TLR-2 and TLR-4 were greatly up-regulated in the mucosa of active pouchitis, while TLR-3 and TLR-5 expression was barely detectable in the normal ileum and not detectable at all in pouch mucosa with or without inflammation. We confirmed in a recent study that high mucosal TLR-2 and TLR4 mRNA levels are associated with chronic/relapsing pouchitis[57].
Epithelial cells are also equipped with various antimicrobial peptides that act rapidly to kill a wide range of microorganisms. Defensins are an important class of antimicrobial peptides, which are small cationic arginine-rich peptides with a molecular weight of 3-5 kDa expressed exclusively in Paneth cells (α-defensins) or generally in intestinal epithelial cells (β-defensins)[74,75]. They are classified as α- and β-defensins depending on the position of three intramolecular disulfide bridges between cysteine residues. The α-defensins include the human neutrophil peptides 1-4, as well as epithelial human defensin 5 (HD-5) and human defensin 6 (HD-6). α-defensins, which may act as chemokines, have a wide variety of antimicrobial activities[77-79]. HD-5 and HD-6 are the major antimicrobial peptides of the small intestine, and their expression is increased in the colonic mucosa of IBD patients in the form of metaplastic Paneth cells[80-83]. In contrast with enteric α-defensins, human β-defensin 1 (HBD1) and other members of the β-defensin family appear to be expressed by most epithelial cells of the small and large intestines. HBD-1 is expressed constitutively, while HBD-2 and HBD-3 expression require NF-κB activation[84]. Microbes or their products could be involved in defensin deregulation in normal pouches after surgery and in the presence of pouchitis[85]. A recent study reported that defensins are up-regulated in response to bacterial PAMPs or cytokines[86]. The increase in HBD-2 and HBD-3 in pouchitis may thus reflect mucosal damage and infection[85]. The interplay between the microbiota adherent to the pouch mucosa, the mucosal innate immune system, and defensin expression may thus contribute to the pathogenesis of pouchitis.
Some evidence suggests that there is a disruption in the host-gut microbiota homeostasis[67-71] associated to (or even caused by) the deregulation of the innate immunity machinery within the ileal mucosa[73,76]. In fact, in recent studies, we observed that Enterococcaceae spp., Enterobacteriaceae spp. and Streptococcaceae spp. may play an active role in maintaining immunologic homeostasis within the pouch mucosa, as low levels seem to favor the development of acute pouchitis[21]. The presence of Clostridiaceae spp. adherent to the pouch mucosa and the high TLR-2 and TLR-4 mRNA expressions were, moreover, associated with chronic/relapsing pouchitis[57].
Accumulating experimental evidence indicates that dysbiosis of the ileal-anal pouch microbiota and deregulation of the mucosal immune system play an important role in the pathogenesis of pouchitis. While its diversity was reduced, only minor compositional differences were found in the microbiota of UC patients with active pouchitis with respect to that in subjects without disease history. However, there are no studies in the literature which have analyzed the direct impact of pouchitis therapy on pouch microbiota. The most likely hypothesis is that dysbiosis predisposes UC patients to pouchitis by increasing the likelihood of immune system stimulation or by reducing microbiota diversity, which is itself sufficient to induce unbalanced activity of the immune system leading to mucosal inflammation. The failure to identify a particular bacterial species associated with pouchitis concurs with clinical experience indicating that antibiotics with a very different spectrum of antimicrobial activity are equally effective in pouchitis. Some investigators have recently shown that many patients with the form that is refractory to empirical antibiotic treatment have antibiotic resistant coliforms, and microbiological testing has been able to predict an effective antibiotic regime for those patients[87]. Together with other findings, this suggests that antibiotic therapy is effective in pouchitis as it reduces the total gut microbial load, and therefore the stimulus to the immune system rather than eliminating a specific disease-activating bacterial species. Although the connection between dysbiosis of the microbiota and aberrant immune responses remains unclear, alterations in the mucosal immune system support the hypothesis that dysbiosis plays an active role in inducing and maintaining persistent inflammation. The dysbiosis in UC-IPAA patients, characterized by reduced diversity of the microbiota, may lead to aberrant mucosal immune regulation triggering the inflammatory processes in genetically predisposed patients. Secondary effects on the function of the epithelial membrane barrier and defensin overexpression could subsequently worsen dysbiosis and favor the chronic activation of mucosal immune responses[88].
The authors are grateful to Linda Inverso for her assistance in editing the final version of this manuscript.
P- Reviewer: Day AS, Kaymakoglu S, Lakatos PL, Leal RF, O'Riordan JM, Reyes VE, Sipos F, Sorrentino D, van Langenberg DR, Wasserberg V S- Editor: Ma YJ L- Editor: Rutherford A E- Editor: Wang CH
| 1. | Onaitis MW, Mantyh C. Ileal pouch-anal anastomosis for ulcerative colitis and familial adenomatous polyposis: historical development and current status. Ann Surg. 2003;238:S42-S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Fazio VW, Ziv Y, Church JM, Oakley JR, Lavery IC, Milsom JW, Schroeder TK. Ileal pouch-anal anastomoses complications and function in 1005 patients. Ann Surg. 1995;222:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 854] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 3. | Sandborn WJ, Pardi DS. Clinical management of pouchitis. Gastroenterology. 2004;127:1809-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Hurst RD, Molinari M, Chung TP, Rubin M, Michelassi F. Prospective study of the incidence, timing and treatment of pouchitis in 104 consecutive patients after restorative proctocolectomy. Arch Surg. 1996;131:497-500; discussion 501-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 178] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Svaninger G, Nordgren S, Oresland T, Hultén L. Incidence and characteristics of pouchitis in the Kock continent ileostomy and the pelvic pouch. Scand J Gastroenterol. 1993;28:695-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 76] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Lovegrove RE, Tilney HS, Heriot AG, von Roon AC, Athanasiou T, Church J, Fazio VW, Tekkis PP. A comparison of adverse events and functional outcomes after restorative proctocolectomy for familial adenomatous polyposis and ulcerative colitis. Dis Colon Rectum. 2006;49:1293-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Moskowitz RL, Shepherd NA, Nicholls RJ. An assessment of inflammation in the reservoir after restorative proctocolectomy with ileoanal ileal reservoir. Int J Colorectal Dis. 1986;1:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 244] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Parsi MA, Shen B, Achkar JP, Remzi FF, Goldblum JR, Boone J, Lin D, Connor JT, Fazio VW, Lashner BA. Fecal lactoferrin for diagnosis of symptomatic patients with ileal pouch-anal anastomosis. Gastroenterology. 2004;126:1280-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Sandborn WJ, Tremaine WJ, Batts KP, Pemberton JH, Phillips SF. Pouchitis after ileal pouch-anal anastomosis: a Pouchitis Disease Activity Index. Mayo Clin Proc. 1994;69:409-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 520] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 10. | Shepherd NA, Jass JR, Duval I, Moskowitz RL, Nicholls RJ, Morson BC. Restorative proctocolectomy with ileal reservoir: pathological and histochemical study of mucosal biopsy specimens. J Clin Pathol. 1987;40:601-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 245] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Setti Carraro PG, Talbot IC, Nicholls JR. Patterns of distribution of endoscopic and histological changes in the ileal reservoir after restorative proctocolectomy for ulcerative colitis. A long-term follow-up study. Int J Colorectal Dis. 1998;13:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Shepherd NA, Healey CJ, Warren BF, Richman PI, Thomson WH, Wilkinson SP. Distribution of mucosal pathology and an assessment of colonic phenotypic change in the pelvic ileal reservoir. Gut. 1993;34:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 93] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Mahadevan U, Sandborn WJ. Diagnosis and management of pouchitis. Gastroenterology. 2003;124:1636-1650. [PubMed] |
| 14. | Schmidt CM, Lazenby AJ, Hendrickson RJ, Sitzmann JV. Preoperative terminal ileal and colonic resection histopathology predicts risk of pouchitis in patients after ileoanal pull-through procedure. Ann Surg. 1998;227:654-662; discussion 663-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 106] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Penna C, Dozois R, Tremaine W, Sandborn W, LaRusso N, Schleck C, Ilstrup D. Pouchitis after ileal pouch-anal anastomosis for ulcerative colitis occurs with increased frequency in patients with associated primary sclerosing cholangitis. Gut. 1996;38:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 347] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 16. | Shepherd NA, Hultén L, Tytgat GN, Nicholls RJ, Nasmyth DG, Hill MJ, Fernandez F, Gertner DJ, Rampton DS, Hill MJ. Pouchitis. Int J Colorectal Dis. 1989;4:205-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 111] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Lohmuller JL, Pemberton JH, Dozois RR, Ilstrup D, van Heerden J. Pouchitis and extraintestinal manifestations of inflammatory bowel disease after ileal pouch-anal anastomosis. Ann Surg. 1990;211:622-627; discussion 627-629. [PubMed] |
| 18. | Shen B, Fazio VW, Remzi FH, Bennett AE, Lopez R, Lavery IC, Brzezinski A, Sherman KK, Lashner BA. Effect of withdrawal of nonsteroidal anti-inflammatory drug use on ileal pouch disorders. Dig Dis Sci. 2007;52:3321-3328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Achkar JP, Al-Haddad M, Lashner B, Remzi FH, Brzezinski A, Shen B, Khandwala F, Fazio V. Differentiating risk factors for acute and chronic pouchitis. Clin Gastroenterol Hepatol. 2005;3:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Fleshner PR, Vasiliauskas EA, Kam LY, Fleshner NE, Gaiennie J, Abreu-Martin MT, Targan SR. High level perinuclear antineutrophil cytoplasmic antibody (pANCA) in ulcerative colitis patients before colectomy predicts the development of chronic pouchitis after ileal pouch-anal anastomosis. Gut. 2001;49:671-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 171] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Lim M, Sagar P, Finan P, Burke D, Schuster H. Dysbiosis and pouchitis. Br J Surg. 2006;93:1325-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Four-week open-label trial of metronidazole and ciprofloxacin for the treatment of recurrent or refractory pouchitis. Aliment Pharmacol Ther. 2002;16:909-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 133] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1077] [Cited by in RCA: 956] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 24. | Schultz M, Sartor RB. Probiotics and inflammatory bowel diseases. Am J Gastroenterol. 2000;95:S19-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 92] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Bell AJ, Nicholls RJ, Forbes A, Ellis HJ, Ciclitira PJ. Human lymphocyte stimulation with pouchitis flora is greater than with flora from a healthy pouch but is suppressed by metronidazole. Gut. 2004;53:1801-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Shen B, Achkar JP, Lashner BA, Ormsby AH, Remzi FH, Brzezinski A, Bevins CL, Bambrick ML, Seidner DL, Fazio VW. A randomized clinical trial of ciprofloxacin and metronidazole to treat acute pouchitis. Inflamm Bowel Dis. 2001;7:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 232] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 27. | Shen B. Diagnosis and treatment of patients with pouchitis. Drugs. 2003;63:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 611] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 29. | Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 708] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 30. | Landy J, Al-Hassi HO, McLaughlin SD, Knight SC, Ciclitira PJ, Nicholls RJ, Clark SK, Hart AL. Etiology of pouchitis. Inflamm Bowel Dis. 2012;18:1146-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2501] [Cited by in RCA: 2744] [Article Influence: 182.9] [Reference Citation Analysis (1)] |
| 32. | Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780-13785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3731] [Cited by in RCA: 3427] [Article Influence: 190.4] [Reference Citation Analysis (1)] |
| 33. | Swidsinski A, Loening-Baucke V, Lochs H, Hale LP. Spatial organization of bacterial flora in normal and inflamed intestine: a fluorescence in situ hybridization study in mice. World J Gastroenterol. 2005;11:1131-1140. [PubMed] |
| 34. | Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1339] [Cited by in RCA: 1376] [Article Influence: 80.9] [Reference Citation Analysis (1)] |
| 35. | Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis as a prerequisite for IBD. Gut. 2004;53:1057. [PubMed] |
| 36. | Hayashi H, Sakamoto M, Benno Y. Phylogenetic analysis of the human gut microbiota using 16S rDNA clone libraries and strictly anaerobic culture-based methods. Microbiol Immunol. 2002;46:535-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 303] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 37. | Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5700] [Cited by in RCA: 5584] [Article Influence: 279.2] [Reference Citation Analysis (2)] |
| 38. | Falk A, Olsson C, Ahrné S, Molin G, Adawi D, Jeppsson B. Ileal pelvic pouch microbiota from two former ulcerative colitis patients, analysed by DNA-based methods, were unstable over time and showed the presence of Clostridium perfringens. Scand J Gastroenterol. 2007;42:973-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Kohyama A, Ogawa H, Funayama Y, Takahashi K, Benno Y, Nagasawa K, Tomita S, Sasaki I, Fukushima K. Bacterial population moves toward a colon-like community in the pouch after total proctocolectomy. Surgery. 2009;145:435-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Onderdonk AB, Dvorak AM, Cisneros RL, McLeod RS, Antionoli D, Silen W, Blair JE, Monahan-Earley RA, Cullen J, Cohen Z. Microbiologic assessment of tissue biopsy samples from ileal pouch patients. J Clin Microbiol. 1992;30:312-317. [PubMed] |
| 41. | McLeod RS, Antonioli D, Cullen J, Dvorak A, Onderdonk A, Silen W, Blair JE, Monahan-Earley R, Cisneros R, Cohen Z. Histologic and microbiologic features of biopsy samples from patients with normal and inflamed pouches. Dis Colon Rectum. 1994;37:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Duffy M, O’Mahony L, Coffey JC, Collins JK, Shanahan F, Redmond HP, Kirwan WO. Sulfate-reducing bacteria colonize pouches formed for ulcerative colitis but not for familial adenomatous polyposis. Dis Colon Rectum. 2002;45:384-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Zoetendal EG, von Wright A, Vilpponen-Salmela T, Ben-Amor K, Akkermans AD, de Vos WM. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl Environ Microbiol. 2002;68:3401-3407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 594] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 44. | Zoetendal EG, Rajilic-Stojanovic M, de Vos WM. High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut. 2008;57:1605-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 456] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 45. | Zella GC, Hait EJ, Glavan T, Gevers D, Ward DV, Kitts CL, Korzenik JR. Distinct microbiome in pouchitis compared to healthy pouches in ulcerative colitis and familial adenomatous polyposis. Inflamm Bowel Dis. 2011;17:1092-1100. [PubMed] |
| 46. | Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Fölsch UR, Timmis KN, Schreiber S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 875] [Cited by in RCA: 936] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 47. | Andoh A, Sakata S, Koizumi Y, Mitsuyama K, Fujiyama Y, Benno Y. Terminal restriction fragment length polymorphism analysis of the diversity of fecal microbiota in patients with ulcerative colitis. Inflamm Bowel Dis. 2007;13:955-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 48. | Sokol H, Lepage P, Seksik P, Doré J, Marteau P. Temperature gradient gel electrophoresis of fecal 16S rRNA reveals active Escherichia coli in the microbiota of patients with ulcerative colitis. J Clin Microbiol. 2006;44:3172-3177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 49. | McLaughlin SD, Walker AW, Churcher C, Clark SK, Tekkis PP, Johnson MW, Parkhill J, Ciclitira PJ, Dougan G, Nicholls RJ. The bacteriology of pouchitis: a molecular phylogenetic analysis using 16S rRNA gene cloning and sequencing. Ann Surg. 2010;252:90-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 50. | Kühbacher T, Ott SJ, Helwig U, Mimura T, Rizzello F, Kleessen B, Gionchetti P, Blaut M, Campieri M, Fölsch UR. Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchitis. Gut. 2006;55:833-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 51. | Tannock GW, Lawley B, Munro K, Lay C, Taylor C, Daynes C, Baladjay L, Mcleod R, Thompson-Fawcett M. Comprehensive analysis of the bacterial content of stool from patients with chronic pouchitis, normal pouches, or familial adenomatous polyposis pouches. Inflamm Bowel Dis. 2012;18:925-934. [PubMed] |
| 52. | Nasmyth DG, Godwin PG, Dixon MF, Williams NS, Johnston D. Ileal ecology after pouch-anal anastomosis or ileostomy. A study of mucosal morphology, fecal bacteriology, fecal volatile fatty acids, and their interrelationship. Gastroenterology. 1989;96:817-824. [PubMed] |
| 53. | Sagar PM, Taylor BA, Godwin P, Holdsworth PJ, Johnston D, Lewis W, Miller A, Quirke P, Williamson M. Acute pouchitis and deficiencies of fuel. Dis Colon Rectum. 1995;38:488-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Böhmig GA, Krieger PM, Säemann MD, Wenhardt C, Pohanka E, Zlabinger GJ. n-butyrate downregulates the stimulatory function of peripheral blood-derived antigen-presenting cells: a potential mechanism for modulating T-cell responses by short-chain fatty acids. Immunology. 1997;92:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 55. | Segain JP, Raingeard de la Blétière D, Bourreille A, Leray V, Gervois N, Rosales C, Ferrier L, Bonnet C, Blottière HM, Galmiche JP. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut. 2000;47:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 973] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 56. | Thibault R, Blachier F, Darcy-Vrillon B, de Coppet P, Bourreille A, Segain JP. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflamm Bowel Dis. 2010;16:684-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 57. | Scarpa M, Grillo A, Pozza A, Faggian D, Ruffolo C, Scarpa M, D’Incà R, Plebani M, Sturniolo GC, Castagliuolo I. TLR2 and TLR4 up-regulation and colonization of the ileal mucosa by Clostridiaceae spp. in chronic/relapsing pouchitis. J Surg Res. 2011;169:e145-e154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Scarpa M, Grillo A, Faggian D, Ruffolo C, Bonello E, D’Incà R, Scarpa M, Castagliuolo I, Angriman I. Relationship between mucosa-associated microbiota and inflammatory parameters in the ileal pouch after restorative proctocolectomy for ulcerative colitis. Surgery. 2011;150:56-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | Conte MP, Schippa S, Zamboni I, Penta M, Chiarini F, Seganti L, Osborn J, Falconieri P, Borrelli O, Cucchiara S. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut. 2006;55:1760-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 276] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 60. | Gophna U, Sommerfeld K, Gophna S, Doolittle WF, Veldhuyzen van Zanten SJ. Differences between tissue-associated intestinal microfloras of patients with Crohn’s disease and ulcerative colitis. J Clin Microbiol. 2006;44:4136-4141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 425] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 61. | Targan SR, Landers CJ, Yang H, Lodes MJ, Cong Y, Papadakis KA, Vasiliauskas E, Elson CO, Hershberg RM. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology. 2005;128:2020-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 351] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 62. | Fleshner P, Ippoliti A, Dubinsky M, Vasiliauskas E, Mei L, Papadakis KA, Rotter JI, Landers C, Targan S. Both preoperative perinuclear antineutrophil cytoplasmic antibody and anti-CBir1 expression in ulcerative colitis patients influence pouchitis development after ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2008;6:561-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 63. | Einerhand AW, Renes IB, Makkink MK, van der Sluis M, Büller HA, Dekker J. Role of mucins in inflammatory bowel disease: important lessons from experimental models. Eur J Gastroenterol Hepatol. 2002;14:757-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 64. | Kyo K, Parkes M, Takei Y, Nishimori H, Vyas P, Satsangi J, Simmons J, Nagawa H, Baba S, Jewell D. Association of ulcerative colitis with rare VNTR alleles of the human intestinal mucin gene, MUC3. Hum Mol Genet. 1999;8:307-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | Parker N, Tsai HH, Ryder SD, Raouf AH, Rhodes JM. Increased rate of sialylation of colonic mucin by cultured ulcerative colitis mucosal explants. Digestion. 1995;56:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Tytgat KM, van der Wal JW, Einerhand AW, Büller HA, Dekker J. Quantitative analysis of MUC2 synthesis in ulcerative colitis. Biochem Biophys Res Commun. 1996;224:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 123] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 67. | Mann SD, Pitt J, Springall RG, Thillainayagam AV. Clostridium difficile infection--an unusual cause of refractory pouchitis: report of a case. Dis Colon Rectum. 2003;46:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 68. | Shen BO, Jiang ZD, Fazio VW, Remzi FH, Rodriguez L, Bennett AE, Lopez R, Queener E, Dupont HL. Clostridium difficile infection in patients with ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2008;6:782-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 69. | Gosselink MP, Schouten WR, van Lieshout LM, Hop WC, Laman JD, Ruseler-van Embden JG. Eradication of pathogenic bacteria and restoration of normal pouch flora: comparison of metronidazole and ciprofloxacin in the treatment of pouchitis. Dis Colon Rectum. 2004;47:1519-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 70. | Ohge H, Furne JK, Springfield J, Rothenberger DA, Madoff RD, Levitt MD. Association between fecal hydrogen sulfide production and pouchitis. Dis Colon Rectum. 2005;48:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 71. | Nicholls RJ, Banerjee AK. Pouchitis: risk factors, etiology, and treatment. World J Surg. 1998;22:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 72. | Cario E. Bacterial interactions with cells of the intestinal mucosa: Toll-like receptors and NOD2. Gut. 2005;54:1182-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 234] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 73. | Haller D, Russo MP, Sartor RB, Jobin C. IKK beta and phosphatidylinositol 3-kinase/Akt participate in non-pathogenic Gram-negative enteric bacteria-induced RelA phosphorylation and NF-kappa B activation in both primary and intestinal epithelial cell lines. J Biol Chem. 2002;277:38168-38178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 142] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 74. | Ganz T, Lehrer RI. Defensins. Curr Opin Immunol. 1994;6:584-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 288] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 75. | Fellermann K, Stange EF. Defensins -- innate immunity at the epithelial frontier. Eur J Gastroenterol Hepatol. 2001;13:771-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Toiyama Y, Araki T, Yoshiyama S, Hiro J, Miki C, Kusunoki M. The expression patterns of Toll-like receptors in the ileal pouch mucosa of postoperative ulcerative colitis patients. Surg Today. 2006;36:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 77. | Lehrer RI, Barton A, Daher KA, Harwig SS, Ganz T, Selsted ME. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J Clin Invest. 1989;84:553-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 535] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 78. | Kagan BL, Selsted ME, Ganz T, Lehrer RI. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci USA. 1990;87:210-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 368] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 79. | Chertov O, Michiel DF, Xu L, Wang JM, Tani K, Murphy WJ, Longo DL, Taub DD, Oppenheim JJ. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J Biol Chem. 1996;271:2935-2940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 398] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 80. | Jones DE, Bevins CL. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem. 1992;267:23216-23225. [PubMed] |
| 81. | Cunliffe RN, Rose FR, Keyte J, Abberley L, Chan WC, Mahida YR. Human defensin 5 is stored in precursor form in normal Paneth cells and is expressed by some villous epithelial cells and by metaplastic Paneth cells in the colon in inflammatory bowel disease. Gut. 2001;48:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 163] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 82. | Wehkamp J, Schwind B, Herrlinger KR, Baxmann S, Schmidt K, Duchrow M, Wohlschläger C, Feller AC, Stange EF, Fellermann K. Innate immunity and colonic inflammation: enhanced expression of epithelial alpha-defensins. Dig Dis Sci. 2002;47:1349-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 83. | Lawrance IC, Fiocchi C, Chakravarti S. Ulcerative colitis and Crohn’s disease: distinctive gene expression profiles and novel susceptibility candidate genes. Hum Mol Genet. 2001;10:445-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 292] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 84. | O’Neil DA, Porter EM, Elewaut D, Anderson GM, Eckmann L, Ganz T, Kagnoff MF. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 1999;163:6718-6724. [PubMed] |
| 85. | Kiehne K, Brunke G, Wegner F, Banasiewicz T, Folsch UR, Herzig KH. Defensin expression in chronic pouchitis in patients with ulcerative colitis or familial adenomatous polyposis coli. World J Gastroenterol. 2006;12:1056-1062. [PubMed] |
| 86. | Harder J, Meyer-Hoffert U, Teran LM, Schwichtenberg L, Bartels J, Maune S, Schröder JM. Mucoid Pseudomonas aeruginosa, TNF-alpha, and IL-1beta, but not IL-6, induce human beta-defensin-2 in respiratory epithelia. Am J Respir Cell Mol Biol. 2000;22:714-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 317] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 87. | McLaughlin SD, Clark SK, Shafi S, Petrovska L, Tekkis PP, Ciclitira PJ, Nicholls RJ. Fecal coliform testing to identify effective antibiotic therapies for patients with antibiotic-resistant pouchitis. Clin Gastroenterol Hepatol. 2009;7:545-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 88. | Scarpa M, Grillo A, Scarpa M, Brun P, Castoro C, Pozza A, Cavallo D, Faggian D, Ruffolo C, D’Incà R. Innate immune environment in ileal pouch mucosa: α5 defensin up-regulation as predictor of chronic/relapsing pouchitis. J Gastrointest Surg. 2012;16:188-201; discussion 201-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 89. | Komanduri S, Gillevet PM, Sikaroodi M, Mutlu E, Keshavarzian A. Dysbiosis in pouchitis: evidence of unique microfloral patterns in pouch inflammation. Clin Gastroenterol Hepatol. 2007;5:352-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 90. | Tyler AD, Knox N, Kabakchiev B, Milgrom R, Kirsch R, Cohen Z, McLeod RS, Guttman DS, Krause DO, Silverberg MS. Characterization of the gut-associated microbiome in inflammatory pouch complications following ileal pouch-anal anastomosis. PLoS One. 2013;8:e66934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |