Published online Aug 7, 2014. doi: 10.3748/wjg.v20.i29.10024
Revised: January 7, 2014
Accepted: April 27, 2014
Published online: August 7, 2014
Processing time: 265 Days and 20 Hours
Video capsule endoscopy (CE) since its introduction 13 years back, has revolutionized our approach to small intestinal diseases. Obscure gastrointestinal bleed (OGIB) continues to be the most important indication for CE with a high sensitivity, specificity as well as positive and negative predictive values. It is best performed during ongoing bleed or immediately thereafter. Overt OGIB has a higher diagnostic yield than occult OGIB. However, even in iron deficiency anemia, CE is emerging as important investigation after initial negative work up. In suspected Crohn’s disease (CD), CE has been shown superior to traditional imaging and endoscopic technique and should be considered after a negative ileocolonoscopy. Although CE has also been used for evaluating established CD, a high capsule retention rate precludes its use ahead of cross-sectional imaging. Celiac disease, particularly where gastro-duodenoscopy cannot be performed or is normal, can also be investigated by CE. Small bowel tumor, hereditary polyposis syndrome, and non-steroidal anti-inflammatory drugs induced intestinal damage are other indications for CE. Capsule retention is the only significant adverse outcome of CE and occurs mostly in presence of intestinal obstruction. This can be prevented by use of Patency capsule prior to CE examination. Presence of cardiac pacemaker and intracardiac devices continue to be relative contraindications for CE, though data do not suggest interference of CE with these devices. Major limitations of CE today include failure to control its movement from outside, inability of CE to acquire tissue for diagnosis, and lack of therapeutic help. With ongoing interesting and exciting developments taking place in these areas, these issues would be solved in all probability in near future. CE has the potential to become one of the most important tools in diagnostic and possibly in the therapeutic field of gastrointestinal disorder.
Core tip: Since its discovery in the year 2000, more than 1000 articles have been published on capsule endoscopy (CE). The technology is evolving continuously with development of new concepts. This review article discusses the present status of CE and also, sheds some light on possible future solution of the current limitations of this technique. Issues related to technique, patient preparation, image interpretation, and indications have also been discussed.
- Citation: Goenka MK, Majumder S, Goenka U. Capsule endoscopy: Present status and future expectation. World J Gastroenterol 2014; 20(29): 10024-10037
- URL: https://www.wjgnet.com/1007-9327/full/v20/i29/10024.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i29.10024
Since its introduction into clinical practice in 2000[1], video capsule endoscopy (CE) has established itself as an invaluable tool for investigating a wide variety of gastrointestinal diseases. Evolving evidence has shown that CE is a reliable, non-invasive as well as a cost-effective test for examining the entire small bowel. “Esophagus” and “Colon” capsules have also been studied, and preliminary data suggest utility in certain subset of esophageal[2-5] and colonic[6,7] diseases. A clear understanding of the indications, risks, and limitations of capsule endoscopy is essential for the judicious and cost-effective use of this investigative tool. More than 1000 studies have been published in last 10 years addressing various aspects of CE, and all important gastrointestinal societies have issued guidelines from time to time[8-10]. This review will describe the technical aspects of CE along with the indications and complications associated with this test and also, outline recent advances as well as future expectations in this field.
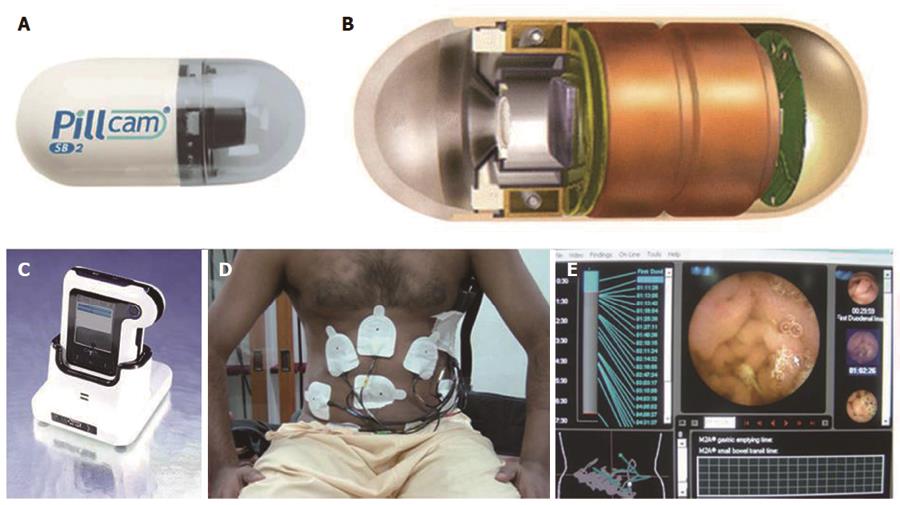
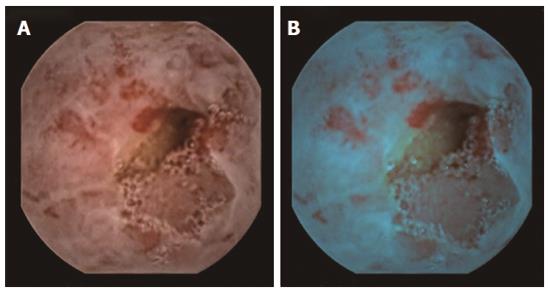
CE system was pioneered by Given Imaging, Israel and essentially, consists of three components (Figure 1): “Capsule” itself, which is actually a camera which is ingested; a data recorder, which is placed on patient’s body along with sensor pads; and a dedicated computer with software for downloading and analyzing the images from the data recorder. “Capsule” has components of camera including optical dome, light source, lens, a complementary metal oxide semiconductor imager (CMOS), battery, and a wireless transmitter-all packaged into a device. This measures approximately 26 mm × 11 mm and is easily ingested. The battery lasts for about eight h during which standard capsule takes two pictures per second totaling around 50000 images[11]. In the initial version, this camera had 140° field of view. More recent capsule introduced by Given Imaging, called PillCam SB2, now has a broader angle of view (156°vs 140°) and better optics with advanced automatic light control which allows adaptation of light in darker areas of intestine (somewhat akin to flash of a camera). Recently introduced data recorder (DR3 by Given Imaging, Israel) accompanies a screen which can show real time images during ongoing examination. This has shortened the duration of examination, as the procedure may be terminated once cecum is visualized at real time. Dedicated computer has software for viewing the images at variable speed almost like a video. In addition, the instrument also include localization system (for detecting lesion site), blood detector (for identifying site of bleed), double and quadri-viewer (can view multiple images together), quick view, and chromo-endoscopy (Fuji intelligent color enhancement i.e., FICE) (Figure 2). One can calculate inflammation score (Lewis) and compare images with incorporated atlas. It takes 45-90 min (average 60 min) to visualize all the images. With the advanced CE system, even trained nurses and resident doctors can effectively interpret the result in absence of specialist gastroenterologists[12,13]. Quick view examination reduces the time required for image reading to less than 10 min and is useful in urgent situation[14]. Evaluating FICE assisted CE (FICE-CE) to conventional white light CE, a recent study showed that FICE-CE was no better in terms of diagnosing and characterizing significant lesions in patients with obscure gastrointestinal bleeding (OGIB)[15]. However, another recent study did show some encouraging results obtained with FICE-CE in diagnosing polyp[16]. A simple three part sensory array, contained in a belt worn by the patient (without the need of wearing sensory pads), has recently being introduced. However, this does not allow localization of capsule as compared to the conventional eight-sensory pad system.
In addition to most popular Given Imaging System (PillCam) from Israel, there are other devices that are also available in the market[11]. These include MiroCam (Intromedic, South Korea), OMOM pill (Chongqing, China), and EndoCapsule (Olympus, Japan). These three systems have been launched in the market after Given Imaging Capsule; therefore having less scientific data to study. EndoCapsule has charge coupled device chip for imaging in place of CMOS used in PillCam. EndoCapsule has similar characteristics as Given Imaging PillCam Capsule and has been shown to be equally useful[17]. MiroCam measures 24 mm × 10.8 mm and has 150° field of view (170° in version 2). It can take three frames per second with a battery life of 11 h[18]. Compared to all other CE, which use radiofrequency as a mode of data transmission, MiroCam system uses electrical field propagation[18]. A recent study has compared Olympus EndoCapsule with MiroCam capsule and has found a similar overall diagnostic yield but a suboptimum concordance rate[19]. OMOM capsule is somewhat larger (27.9 mm × 13 mm) and has 140° field of view. It can take 0.5-2 frames per second and has a battery life of around 8 h[20]. A new CE system has recently been introduced in the market under the name of CapsoCam (Capso Vision Saratoga, CA, United States) having four cameras. This allows the visualization of small bowel through 360° lateral viewing[21]. It has a battery life of 15 h and takes images at a frame rate of 12-20 per second. This system, however, stores acquired image data on an internal chip. The capsule, therefore, has to be retrieved from stool. This is not only cumbersome, but the capsule with all images may be lost in a small proportion of patients. A recent study has shown comparable efficacy of Capso vision capsule compared to PillCam SB2 in terms of diagnostic yield and image quality[22].
Patients are usually placed on a clear liquid diet on the day before the test followed by an 8 to 12 h of fast prior to capsule ingestion. Drinking clear liquids is allowed after 2 h and a light meal after 4 h of capsule ingestion. The patient wears the external recorder till the exam is completed. This is usually accomplished in 8-10 h during which the patient is allowed to continue with their normal daily activities.
The presence of intraluminal contents and slow capsule transit are the two factors that negatively impact the diagnostic yield of CE. Two recent meta-analyses showed an improved diagnostic yield after a purgative preparation[23,24]. However, another meta-analysis[25] concluded that the use of bowel preparation improves mucosal visualization but did not improve diagnostic yield or completion rate. Use of bowel preparation does not significantly alter gastric or small bowel capsule transit time. Therefore, there is currently no consensus opinion on the use of purgative bowel preparation in patients undergoing small bowel CE[9,26]. The most widely practiced bowel preparation regimen involves a 2 liters polyethylene glycol-based purge administered one day before the procedure. The addition of Simethicone to the purge has been shown to reduce the formation of bubbles and improve the quality of images obtained. Use of prokinetic drugs to improve capsule propagation has not shown a consistent improvement in the diagnostic outcome[27,28]. Two recent meta-analysis studies have failed to show any benefit in diagnostic yield with CE after use of proktretics[29,30].
Indications for small bowel capsule endoscopy include obscure gastrointestinal bleeding, iron deficiency anemia, inflammatory bowel disease, celiac disease, small bowel tumors, hereditary polyposis syndrome etc.
OGIB is defined as gastrointestinal bleed either overt or occult, which remains undiagnosed in respect to underlying etiology despite doing upper and lower gastrointestinal endoscopies. OGIB is the most important and the most evaluated indication for CE[31,32]. In a meta-analysis including 227 studies and 22840 CE procedures, the diagnostic yield for OGIB was 61%[33]. In another study published subsequently including 911 patients with OGIB, 56% patients had positive finding at CE[34].
Overall diagnostic results for CE are somewhat superior for overt OGIB compared to occult OGIB. For overt OGIB, it is important to realize that CE is best performed close to index bleed. In one of our earlier studies[35], involving 385 patients with OGIB from a single center, we found that the diagnostic yield was significantly higher in subgroup where CE was performed within 48 h of index bleed (87%) compared to subgroup where it was performed later (68%). Somewhat similar results have been found in other studies as well. CE is, however, avoided when there is ongoing torrential gastrointestinal (GI) bleed because of fear of blood obscuring the vision and time lost in performing and interpreting the results.
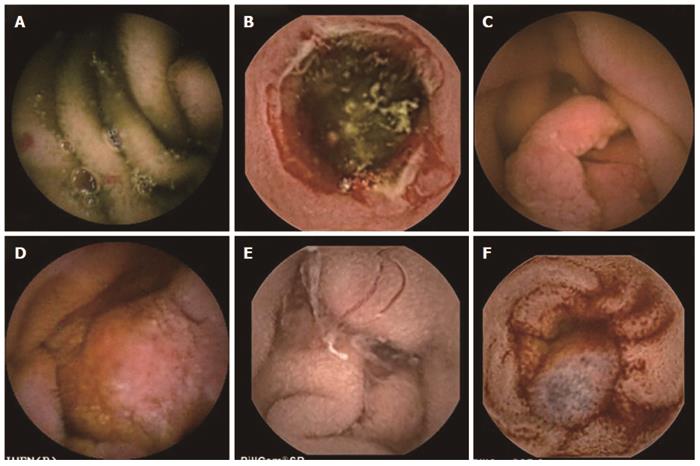
Etiology for OGIB as detected by CE has varied from study to study[36-38]. In general, arteriovenous malformations, small bowel tumors, drug-related lesions, and Crohn’s disease are the common underlying etiologies. In our published research[35], underlying causes for OGIB at CE included ulcers in 66.2%, tumors in 16.9%, arteriovenous malformation in 8.8%, and worms in 2.8% (Figure 3). In 5.3% cases, blood was noted in small intestine, but no lesion could be identified[35]. Small bowel is, as expected, the commonest site for OGIB. Interestingly, however, in 10%-15% of patients, lesions may be seen in upper GI tract or in colon, which had been missed at primary upper and lower GI endoscopy[36].
Diagnostic results of CE in OGIB have been critically analyzed in number of studies in terms of its impact on changing the management or altering the outcome. It was clearly shown that in 33%-66% of patients undergoing CE, the management strategy was changed based on CE findings[39,40]. In this respect, CE was shown to be superior to push enteroscopy[41]. A large retrospective study from Mayo Clinic showed that use of CE in OGIB resulted in reduction in number of hospitalization, additional investigations, and need for blood transfusion[42]. Recent study from South Korea, however, has shown no significant impact of CE on the long term outcome of patients with OGIB[43].
Negative CE in the setting of OGIB, has also been shown to predict the outcome. In fact, the risk of re-bleeding rate after negative CE is very low[44,45], and these patients can be justifiably managed by a conservative approach. Some retrospective studies have, however, shown a high diagnostic yield of repeat CE studies (32%) following a prior negative study[46]. The impact of this on the natural history of OGIB needs further data.
CE has been compared to other modalities for evaluating OGIB e.g., push enteroscopy, barium studies, intra-operative enteroscopy, and double balloon enteroscopy (DBE). A meta-analysis has clearly shown a significantly better yield for CE (63%) compared to push enteroscopy (23%)[47]. In another study, CE detected a source of bleeding in 72% compared to standard angiography (56%) or computed tomography (CT) angiography (24%) with positive findings at CE in more than 50% cases negative at CT or standard angiography[48]. One study compared angiography with CE in overt OGIB and found the yield to be better for CE compared to angiography (53% vs 20%); this was associated with reduced risk of re-bleeding (17% vs 33%)[49]. Compared to intra-operative enteroscopy, which is the gold standard, CE has been shown to have sensitivity, specificity, and positive and negative predictive values of 95%, 75%, 95% and 86%, respectively[50].
Studies published to date show CE in general, to have a higher diagnostic yield for OGIB compared to DBE[51,52]. One of the most recent meta-analysis involving nine studies (six prospective and three retrospective) has compared CE with DBE in OGIB and has shown similar diagnostic yield. The pooled Odd’s ratio for diagnostic yield with CE compared to DBE in this study was 1.48 (95%CI: 0.90-2.43, P = 0.16)[53].
There has been a lot of concern about cost involved in CE and other new techniques used for OGIB such as balloon-assisted enteroscopy (BAE). Contrary to the belief, recent data suggest that both CE and BAE are cost-effective when compared to conventional imaging modalities[54,55]. Overall, CE is preferred as an initial test with BAE being performed only if CE demonstrates a positive finding[54]. In this situation, CE can also guide the route, oral or anal, for BAE, thereby optimizing the time and resources[56]. In a subset of patients with high likelihood of vascular lesions with need for endotherapy, an initial BAE may, however, be more cost-effective[54]. In addition to improved diagnostic yield, CE has the added advantage over these alternative modalities of being non-invasive, patient friendly, without any major complications, and in its ability to examine almost the entire small intestine. Currently, CE is accepted as examination of choice in patients with OGIB after a negative gastroscopy and colonoscopy.
Iron deficiency anemia (IDA) is most common cause of anemia world wide. For evaluating the role of CE, patients with IDA are often clubbed with those having positive fecal occult blood test. However, a systemic review by Koulaouzidis et al[57] has recently evaluated the role of CE in patients presenting with IDA. Based on the data from 1960 patients in 24 studies included in this review, the authors found the diagnostic yield of CE to be 47%. As shown in Table 1, among 1194 patients where findings were clearly described, vascular lesions were most frequent. However, 17 of the studies included in this review were retrospective, and there was considerable heterogeneity among the studies. Despite these limitations of available data, CE is recommended as an investigation in patients with IDA with previous negative workup. A recent study also evaluated the role of CE in elderly patients presenting with IDA and demonstrated a good diagnostic yield[58]. Exact placement of CE in diagnostic algorithm of IDA, however, needs further high quality studies.
Advances in endoscopic techniques (BAE and CE) as well as cross-sectional imaging [CT and magnetic resonance (MR) enteroclysis] have revolutionized the approach to inflammatory bowel disease (IBD) and in particular, Crohn’s disease (CD). The exact position of these modalities in the algorithm of CD continues to be a matter of debate. A large meta-analysis of prospective studies comparing CE with other modalities, both in suspected and established CD, has already shown the superiority of CE. CE was found to be superior to push enteroscopy, ileo-colonoscopy, small bowel follow through, and CT enteroclysis with weighted incremental yield of 42%, 39%, 37%, and 39%, respectively[59]. MR enteroclysis, however, was found to have similar sensitivity as CE[59]. A recent study from Denmark also showed better results for CE compared to CT enteroclysis, while MR enteroclysis had similar results as CE[60]. A meta-analysis of nine studies by Pasha et al[61] compared CE with double-balloon enteroscopy and found similar diagnostic yield. Although CE being non-invasive and patient friendly has obvious advantages, it does have limitations due to the absence of well-defined criteria for abnormal findings and inability to take mucosal biopsies. A recent study has shown quick view mode of CE to be time reducing and safe for diagnosis of small bowel Crohn’s disease[62].
Findings at CE in CD include erythema, mucosal edema, ulceration, fissuring, stricture, and an occasional fistula (Figure 4). The diagnostic yield of CE in suspected CD is high (up to 55%) making it an investigation of choice[63]. However, interpreting mild abnormalities which may not always reflect CD has been a major issue. This is particularly true in the setting of non-steroidal anti-inflammatory drugs (NSAIDs) intake; hence it is recommended to avoid NSAID for a month before performing CE for suspected CD[64]. On the other hand, a normal CE finding has a negative predictive value of more than 95% highlighting the utility of CE in excluding CD[65]. Attempts have been made to increase the diagnostic yield and positive predictive value of CE in suspected CD by using high pretest probability criteria such as presence of perianal disease and negative initial work-up or high fecal calprotectin level[66,67].
A few studies have reported the use of CE for assessing disease activity in established CD, particularly to explain new symptoms and to demonstrate mucosal healing. Two inflammation scores i.e., Lewis score which is incorporated in Given Imaging Software and CE Crohn’s disease activity index, which has been recently validated, can be used for this purpose[68,69]. The correlation between Lewis score and fecal calprotectin has been demonstrated recently[70]. Use of CE standard reporting terminology is likely to enhance our ability to assess established CD at CE[71]. Capsule retention rate in established CD continues to be an area of concern. For patients needing evaluation of postoperative recurrence of CD, CE can detect proximal small bowel lesions and should be considered either after an initial ileocolonoscopy or in case of unsuccessful ileocolonoscopy[72].
CE is also an important tool to reclassify IBD of unclassified category by showing or excluding small intestinal involvement[73]. Similarly, it has been used to evaluate ulcerative colitis patients who have atypical symptoms. A prospective study, however, failed to show any utility of CE performed prior to ileal pouch-anal anastomosis in patients with IBD in predicting the outcome during 12 mo follow up[74].
Thus, CE plays an important role in diagnosis and evaluation of CD. Ileocolonoscopy would continue to be the first investigation for suspected CD, but CE is now a well-accepted second investigation of choice. For established CD, CE is generally indicated only after a stricture has been ruled out at cross-sectional imaging. However, diagnosis of CD should always be based on clinical, lab results, radiology, and endoscopy findings rather than CE alone. Differential diagnosis at CE should include non-specific jejunoileitis, lymphoma, tuberculosis, and of course NSAID induced intestinal disease.
CE is a useful non-invasive diagnostic tool in patients with suspected or established celiac disease and can demonstrate changes such as scalloping, mosaic pattern, flat mucosa, and nodularity. The ability to detect mucosal details and villous changes in the distal small bowel is particularly helpful in patients with suspected celiac disease, who have positive celiac serology and negative duodenal biopsies. Using duodenal histology as the gold standard, recent studies have reported good diagnostic sensitivity (85% to 92%) and specificity (91% to 100%) of CE, especially in previously untreated patients[75]. CE is useful in patients who are unable or unwilling to undergo conventional endoscopy as well as in those with associated alarm symptoms where a more complete small bowel evaluation is warranted. There is limited evidence to support the use of CE in the evaluation of patients with refractory celiac disease. Major limitations to the use of CE in patients with suspected celiac disease include inability to obtain mucosal biopsies and inter-observer variability in the assessment of villous atrophy.
Diagnosis of small bowel tumor is challenging in patients with negative cross-sectional abdominal imaging. There has been a rise in the diagnosis of small bowel tumors over the past decade as a result of the widespread use of CE. OGIB appears to be the most common presenting symptom in patients who have a small bowel tumor at CE. Most patients, who are eventually diagnosed with small bowel neoplasms, would have undergone three to five negative endoscopic procedures prior to CE. When compared to surgery, CE provides a satisfactory estimation of tumor location, size, and appearance. Adenocarcinomas, carcinoids, and lymphomas are the most common malignant tumors detected at CE, while majority of benign neoplasms are GI stromal tumors (GIST) and lipomas. In a recent study, CE was able to diagnose nine out of 10 patients with GIST otherwise missed by traditional endoscopic and radiological imaging[76]. Tumors are located in the jejunum (40%-60%) followed by ileum (25%-40%) and duodenum (15%-20%)[77]. Inability to obtain biopsies and the lack of definitive features to differentiate a mucosal bulge from a smooth-walled tumor are the two major limitations to the use of CE in the diagnosis and management of patients with small bowel tumors. Scoring systems such as smooth protruding index on capsule endoscopy score are recently being developed, with score greater than two suggestive of tumors with a sensitivity of 83% and specificity of 89%[78]. An automated scale with multi-scale wavelet based analysis has also been described recently[79]. These, however, need further validation before they can be incorporated into clinical decision making algorithms.
CE has also evolved into a useful tool in patients with hereditary polyposis syndromes like familial adenomatous polyposis (FAP) and Peutz-Jegher syndrome[80]. Its major clinical utility is in the detection of small polypi (< 15 mm) in the distal small bowel in patients with FAP, which are often not detected on imaging tests. These can then be removed by BAE thereby avoiding surgery. CE, however, is not the ideal method for detecting and characterizing duodenal polyps due to rapid transit of the capsule through the duodenum and the failure to adequately identify the ampulla and periampullary regions. At present, there is no clear consensus on the role of CE for small bowel surveillance in these subsets of patients[81].
CE has been used to monitor intestinal side effects of NSAIDs[82]. Recently, computer guided endoluminal image analysis has been used to extend CE utility for reliable, non-invasive, and automated test of motor disorders[83]. Other situations, where CE may have a role include gastrointestinal complications of HIV, small bowel transplant, Henoch-Schonlein purpura, and intestinal graft versus host disease[84].
There are very few contraindications to the use of CE and most of them are relative.
In view of limited experience of CE in patients with cardiac pacemakers (CP) and implantable cardioverter-defibrillators (ICD), these situations have been considered as contraindications for using CE both by manufacturers (Given Imaging) as well as by Food and Drug Administration (FDA), United States. However, Bandorski et al[85] recently reviewed the whole subject. They evaluated eight studies (including four in vitro) involving 198 patients with pacemaker and demonstrated almost no interference between pacemaker and CE. There have been at least five studies involving 81 patients with ICD, mostly in vivo, evaluating possible interference between CE and ICD. Again, there was hardly any interference noted with ICD as well. Telemetry, however, can interfere with CE videos[85]. These studies were mostly with small bowel PillCam. More data are required with other CE systems including Colon 2 capsule from Given Imaging. In particular, MiroCam System, which works with electric field propagation, needs safety data.
In view of increased risk of capsule retention in presence of obstruction, CE should not be performed in presence of bowel obstruction or if there is strong suspicion of the same. History of a major abdominal surgery in recent past is also often considered as relative contraindication for CE. This matter is further discussed later in this review.
Patients with swallowing disorders, both organic such as esophageal strictures and functional such as dysmotility, can have the capsule stuck in their esophagus itself. This can be overcome by delivery of the capsule into the stomach or duodenum with the help of a device called AdvanCE developed by US Endoscopy (United States).
Pregnancy is also considered as another relative contraindication for CE due to potential teratogenic effects of transmitted microwaves. However, there are reports of successful use of CE during pregnancy[86]. FDA, United States, has recently allowed the use of CE in children as small as two years. Main indications for CE in pediatric population are Crohn’s disease, OGIB, and small bowel polyp[87]. Diagnostic accuracy in pediatric patients has been found to be good (61.4%)[88].
CE is a safe procedure with very rare complications.
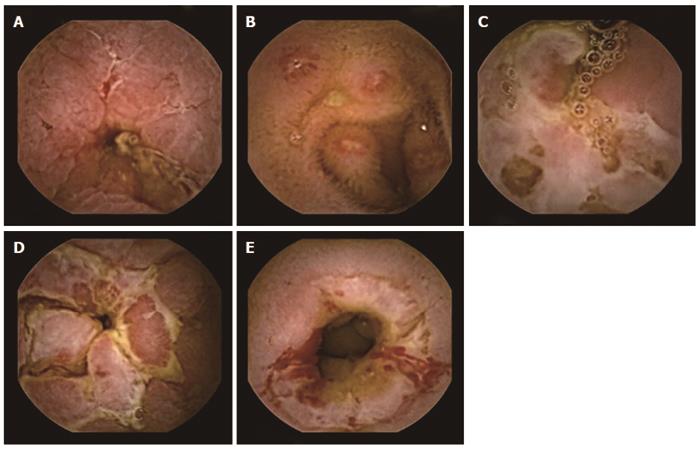
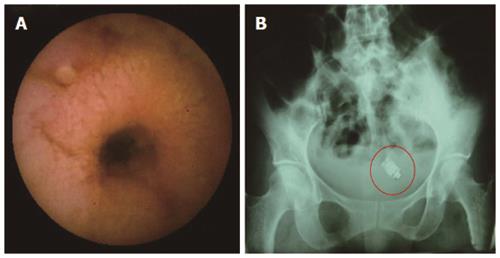
Retention of capsule is defined when it remains inside the GI tract for a minimum of two weeks (Figure 5)[89]. The proportion of patients having capsule retention is related to clinical situation, with 21% in patients with intestinal obstruction, up to 13 % in established CD, 5% in suspected CD, and 1.5% in OGIB. Radiation enteritis can also increase the risk. Mostly capsule retention has no impact on the natural history of disease. Recently, a case of asymptomatic retention of capsule for four and half years has been described[90]. Only about six cases of bowel perforation, possibly as a squeal to capsule retention, have been reported[91]. Once retention is diagnosed, one needs to reassure the patient. Retained capsule, if required, can be removed by BAE or at surgery. These interventions would also take care of the underlying cause for capsule retention i.e., stricture.
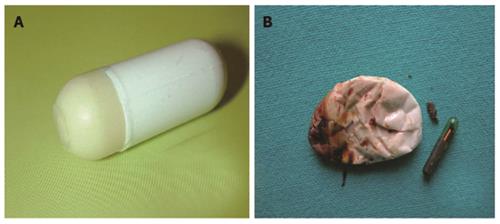
In patients with suspected small bowel strictures, the risk of capsule retention is high. Predicting this complication by performing a barium study is associated with high radiation doses as well as false negative results. Given Imaging has developed a dummy capsule system called Agile Patency capsule, which is a disintegrating, time controlled system with RF identification (RFID) tag and an RFID scanner. These capsules are of same dimension as real capsules but has a cellophane wall filled with mixture of barium and lactose and RFID at its centre. The lactose filling of capsule dissolves after 40 h leading to collapse of outer membrane, which is then excreted (Figure 6). Presence of patency capsule in body can be determined by using RF scanner. The barium containing radio-opaque capsule can be detected by plain X-ray also. Use of Agile patency capsule prior to actual CE can eliminate the risk of capsule retention to a great extent[92]. A recent retrospective study has found that Patency capsule, CT enteroclysis, and MR enteroclysis, all have a similar negative predictive value for capsule retention and can complement each other[93].
Although rare, aspiration of capsule into tracheobronchial tree is increasingly being reported. Koulaouzidis et al[11] recently compiled 25 patients with capsule aspiration. Factors associated with this complication include old age, male sex, co-morbidity, and associated swallowing disorder. While most of these patients cough out the capsule, a single case of fatal outcome has also been described[94].
Presently marketed “capsule” for CE moves in GI tract with peristalsis, which can sometimes be erratic and unpredictable. This can be responsible for “misses” and “incomplete evaluation of bowel”. This is also a hindrance to the development of capsule with capability for tissue acquisition and therapy.
Swain et al[95] first reported the possibility of controlling the movement of capsule by an external hand held magnet after incorporating neodymium-iron-boron magnet in a colonic capsule and changing capsule’s magnetic switch to a thermal switch. By this technique, the capsule could be angulated at gastro-esophageal junction and spun in the stomach. This technique has been further refined[96]. More recently, a robotic magnetic navigator system has evolved for smoother and better controlled movement[97]. This system allows integration with digital fluoroscopic scanner for exact localization of capsule and also allows a remote control, thus improving the potential for therapeutics. However, this system developed by Niobe (United States), still has issues related to cost, inability to control movement in all direction and it has also not been tested in vivo[97]. Meanwhile, an electrically propelled capsule has been introduced[98].
In order to overcome the limited battery life as well as for more liberal rate of image acquisition, attempts have been made to supply power to the capsule by an external source with a wireless transmission[99]. No clinical trials have yet been published with these modifications.
Accurate location of lesion and estimation of its size continue to be a challenge at CE. Rapid 6 system of software developed by Given Imaging has made successful attempt in these aspects. It is now possible to measure the size of the lesion just like with ultrasound images. Recently, Karargyris and Koulaouzidis[100] have described a capsule in porcine model, which is fitted with protruding wheels attached to a spring mechanism for more accurate localization.
Interpretation of images at CE may be sometimes difficult due to their large number (usually more than 50000) and their being two-dimensional (2-D). Recently, Karargyris et al[101] have utilized Shape from Shading algorithms to transform 2-D images of CE to 3-D and found this to be valuable. Hu et al[102] have introduced a feature of extraction method, using a non-linear color conversion and higher-order local auto correlation (HLAC) Features, which enables one to do a quick detection of anomaly. This method achieved 91.7% and 100% detection accuracies for mucosal swelling and bleeding, respectively[102].
Incomplete small bowel examination mainly due to slow transit of capsule has been noted in about 20% of patients. A recent study noted slow gastric transit time (> 45 min), history of previous small bowel surgery, hospitalization, and poor bowel preparation as risk factors for incomplete small bowel examination with CE[103]. In these patients device-assisted duodenal delivery of capsule may increase the yield.
Overall miss rate with CE is 11% with a range of 0.5% for ulcers to 18.9% for neoplasms. This is not surprising considering that capsule does not pursue an axial path and is known to tumble quite frequently, thereby unable to see behind the intestinal folds[17]. Routine use of double camera even for small bowel, as done for colon capsule, can potentially overcome this limitation.
One of the major limitations of CE is its inability to go beyond visualization. Optical biopsy using technology such as narrow band imaging or pathology-targeted enhance tissue markers is very soon going to be a reality.
Pilot data have shown feasibility of performing mucosal biopsies using a spring loaded Crosby capsule type device, guided by a real time imaging capability and radiofrequency controlled remote manipulation[7]. A rotational micro biopsy device with a tissue cutting razor (with a torsion controlled design) to operate sequentially is also being developed[7].
Two new capsules, Intellisite (Innovative devices; NC, United States) and Enterion (Phaeton Research; United Kingdom) have been shown to be useful for collecting absorption data in the GI tract and can be used in future for drug delivery[104].
The Nano based CE with molecular imaging and optic biopsy (NEMO) project is developing a new capsule combining optical and nanotechnologies, biosensing, and maneuvering technologies with an aim to enhance diagnostic and therapeutic potential of CE[105].
Versatile endoscopic capsule for gastrointestinal tumor recognition and therapy (VECTOR) project by European Commission, is in the process of developing a mini robot to navigate and intervene in the GI tract for early detection of cancers[106]. A prototype capsule has been tested employing an exothermic chemical reaction to generate heat by interaction of calcium oxide with water[107]. This may be potentially useful for hemostasis by thermal coagulation.
A new pill-sized endomicroscope has been developed, which enables 3D imaging of esophagus in microscopic details. The device uses optical frequency domain imaging technology. Initial results in differentiating Barrett’s esophagus from normal mucosa have been promising[108].
Celiac disease evaluation by CE is often subjective and can be labor intensive. Recent research has focused on computer analysis of CE images to detect areas of abnormality. Performing an analysis of 2-D image sequences, Tennyson et al[109] have estimated the three dimensional mucosal structure, luminal motility, and the textural properties of the images itself. It has been hypothesized that the most important finding that differentiates celiac disease patients from normal subjects is mucosal protrusion; these are blunt with lesser height and bigger diameter in patients with celiac disease. These protrusions can be measured in terms of height, width, and number in each CE image and can help us in objective assessment for both the presence of celiac disease as well as its follow up. However, more data is required in this field[110].
Given Imaging “Pillcam ESO”[2] CE system (Figure 7) uses a capsule with two cameras and collects images at a rate of 18 frames per second. In contrast to small bowel SB2 capsule, it involves only three sensory antennas. The patient swallows the capsule in right lateral position and drinks 15 mL sips of water every 30 s for 3 min. The recording continues for about 30 min.
The main indications for “ESO” have been gastro esophageal reflux disease (GERD). In a study involving 73 patients, 51 out of 55 positive findings were detected at CE (2). Sensitivity, specificity, and positive and negative predictive values for GERD at CE were 98%, 100%, 100% and 95%, respectively. These values for Barrett’s esophagus were 97%, 100%, 100% and 98%, respectively. However, the role of CE in GERD remains controversial, because histology is of great significance in Barrett’s and also, these data have not been revalidated[3,4]. Initial data does suggest that ESO-CE can be a good alternative for diagnosis of varices[5,111]. In a meta-analysis involving seven studies and 446 patients, the pooled sensitivity and specificity of CE for diagnosis of varices were 85.8% and 80.5%, respectively[5]. However, specificity was lower (54.8%) in detecting varices for screening purpose[5]. Concern also continues regarding the cost involved.
Colon capsule are the latest addition to CE system. The main indication for which the development of colonic capsule has evolved, are screening of colorectal cancers and follow-up evaluation for ulcerative colitis. Challenges for developing colon capsule include battery life to last colonic transit, cleanliness of colon and caliber of colonic lumen and no potential for air insufflation as can be done during conventional colonoscopy. Present era PillCam colon capsule called PCCE 2, from Given imaging (Figure 7) measures 31.5 mm × 11.6 mm, has two cameras with a wider angle of view about 172°, allowing a complete circumferential coverage of colonic mucosa. PCCE 2 also has an adaptive frame rate with images captured at 4 per second when capsule is stationary to 35 per second when it is moving. This feature preserves the battery life and optimizes video length. In addition, there is cross talk between data recorder and capsule allowing the change in frame rate as well as instructions to the patient being transferred via a liquid crystal display on the recorder. Rapid 6 software (given imaging) used for this system also allows polyp size estimation using a graphic interface.
Unlike small bowel capsules, colon CE requires a good bowel preparation which gives a clear ambience as well as helps to propel the capsule. After a liquid diet for a day, patient is given 2-3 liters of polyethylene glycol on previous night and 1-2 liter(s) 2 h before the procedure. Before capsule ingestion, 20 mg of domperidone is administered orally. Two h after capsule ingestion, 30-45 mL of sodium phosphate is given orally to facilitate capsule movement[6].
The results for first generation colonic capsule had been somewhat discouraging. The second generation capsule i.e., PCCE 2 was evaluated in a study involving 98 patients[6]. The sensitivity and specificity for detecting polypi bigger than 6 mm were 89% and 76%, respectively, whereas the corresponding figures for polypi bigger than 10 mm were 88% and 89%, respectively[6].
At this moment, colonic capsule cannot be advocated as standard care for colorectal screening. However, this can certainly be offered to a patient unwilling to undergo colonoscopy or where colonoscopy is contra-indicated or incomplete. Improvement in colonic preparation regimen and optimization of protocol are likely to increase the use of colon CE in near future. Interestingly, colon capsule, with its dual camera, has also been used successfully for evaluation of rest of the bowel revealing greater abnormalities compared to single camera images[112].
Capsule endoscopy has evolved very rapidly to become an important tool for mucosal visualization of the gut. While esophageal and colonic capsules are still being evaluated for their utility, small bowel capsule has now found a definite place in algorithm for investigating small intestinal diseases. In particular, OGIB and CD are two very important indications for performing CE. Celiac disease, small bowel tumors, and iron deficiency anemia are some of the other clear indications. Present limitations of CE primarily emerge from failure to be controlled externally and from its inability to acquire tissue, and offer therapeutics. Some of the developments in these areas are exciting and once in clinical practice, are likely to change our approach to luminal gastrointestinal disorders.
P- Reviewer: Arias M, Buzas GM, Koh PS, Koulaouzidis A, Kim BW S- Editor: Gou SX L- Editor: A E- Editor: Ma S
| 1. | Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417. [PubMed] |
| 2. | Eliakim R, Sharma VK, Yassin K, Adler SN, Jacob H, Cave DR, Sachdev R, Mitty RD, Hartmann D, Schilling D. A prospective study of the diagnostic accuracy of PillCam ESO esophageal capsule endoscopy versus conventional upper endoscopy in patients with chronic gastroesophageal reflux diseases. J Clin Gastroenterol. 2005;39:572-578. [PubMed] |
| 3. | Lin OS, Schembre DB, Mergener K, Spaulding W, Lomah N, Ayub K, Brandabur JJ, Bredfeldt J, Drennan F, Gluck M. Blinded comparison of esophageal capsule endoscopy versus conventional endoscopy for a diagnosis of Barrett’s esophagus in patients with chronic gastroesophageal reflux. Gastrointest Endosc. 2007;65:577-583. [PubMed] |
| 4. | Bhardwaj A, Hollenbeak CS, Pooran N, Mathew A. A meta-analysis of the diagnostic accuracy of esophageal capsule endoscopy for Barrett’s esophagus in patients with gastroesophageal reflux disease. Am J Gastroenterol. 2009;104:1533-1539. [PubMed] |
| 5. | Lu Y, Gao R, Liao Z, Hu LH, Li ZS. Meta-analysis of capsule endoscopy in patients diagnosed or suspected with esophageal varices. World J Gastroenterol. 2009;15:1254-1258. [PubMed] |
| 6. | Eliakim R, Yassin K, Niv Y, Metzger Y, Lachter J, Gal E, Sapoznikov B, Konikoff F, Leichtmann G, Fireman Z. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy. 2009;41:1026-1031. [PubMed] |
| 7. | Van Gossum A, Ibrahim M. Video capsule endoscopy: what is the future? Gastroenterol Clin North Am. 2010;39:807-826. [PubMed] |
| 8. | Sidhu R, Sanders DS, Morris AJ, McAlindon ME. Guidelines on small bowel enteroscopy and capsule endoscopy in adults. Gut. 2008;57:125-136. [PubMed] |
| 9. | Rey JF, Ladas S, Alhassani A, Kuznetsov K. European Society of Gastrointestinal Endoscopy (ESGE). Video capsule endoscopy: update to guidelines (May 2006). Endoscopy. 2006;38:1047-1053. [PubMed] |
| 10. | Mishkin DS, Chuttani R, Croffie J, Disario J, Liu J, Shah R, Somogyi L, Tierney W, Song LM, Petersen BT. ASGE Technology Status Evaluation Report: wireless capsule endoscopy. Gastrointest Endosc. 2006;63:539-545. [PubMed] |
| 11. | Koulaouzidis A, Rondonotti E, Karargyris A. Small-bowel capsule endoscopy: a ten-point contemporary review. World J Gastroenterol. 2013;19:3726-3746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 125] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 12. | Bossa F, Cocomazzi G, Valvano MR, Andriulli A, Annese V. Detection of abnormal lesions recorded by capsule endoscopy. A prospective study comparing endoscopist’s and nurse’s accuracy. Dig Liver Dis. 2006;38:599-602. [PubMed] |
| 13. | Fernández-Urién I, Espinet E, Pérez N, Betés M, Herráiz M, Carretero C, Muñoz-Navas M. [Capsule endoscopy interpretation: the role of physician extenders]. Rev Esp Enferm Dig. 2008;100:219-224. [PubMed] |
| 14. | Koulaouzidis A, Smirnidis A, Douglas S, Plevris JN. QuickView in small-bowel capsule endoscopy is useful in certain clinical settings, but QuickView with Blue Mode is of no additional benefit. Eur J Gastroenterol Hepatol. 2012;24:1099-1104. [PubMed] |
| 15. | Gupta T, Ibrahim M, Deviere J, Van Gossum A. Evaluation of Fujinon intelligent chromo endoscopy-assisted capsule endoscopy in patients with obscure gastroenterology bleeding. World J Gastroenterol. 2011;17:4590-4595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Hatogai K, Hosoe N, Imaeda H, Rey JF, Okada S, Ishibashi Y, Kimura K, Yoneno K, Usui S, Ida Y. Role of enhanced visibility in evaluating polyposis syndromes using a newly developed contrast image capsule endoscope. Gut Liver. 2012;6:218-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Cave DR, Fleischer DE, Leighton JA, Faigel DO, Heigh RI, Sharma VK, Gostout CJ, Rajan E, Mergener K, Foley A. A multicenter randomized comparison of the Endocapsule and the Pillcam SB. Gastrointest Endosc. 2008;68:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Bang S, Park JY, Jeong S, Kim YH, Shim HB, Kim TS, Lee DH, Song SY. First clinical trial of the “MiRo” capsule endoscope by using a novel transmission technology: electric-field propagation. Gastrointest Endosc. 2009;69:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Dolak W, Kulnigg-Dabsch S, Evstatiev R, Gasche C, Trauner M, Püspök A. A randomized head-to-head study of small-bowel imaging comparing MiroCam and EndoCapsule. Endoscopy. 2012;44:1012-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Liao Z, Gao R, Li F, Xu C, Zhou Y, Wang JS, Li ZS. Fields of applications, diagnostic yields and findings of OMOM capsule endoscopy in 2400 Chinese patients. World J Gastroenterol. 2010;16:2669-2676. [PubMed] |
| 21. | Friedrich K, Gehrke S, Stremmel W, Sieg A. First clinical trial of a newly developed capsule endoscope with panoramic side view for small bowel: a pilot study. J Gastroenterol Hepatol. 2013;28:1496-1501. [PubMed] |
| 22. | Pioche M, Vanbiervliet G, Jacob P, Duburque C, Gincul R, Filoche B, Daudet J, Filippi J, Saurin JC. Prospective randomized comparison between axial- and lateral-viewing capsule endoscopy systems in patients with obscure digestive bleeding. Endoscopy. 2014;46:479-484. [PubMed] |
| 23. | Rokkas T, Papaxoinis K, Triantafyllou K, Ladas SD. A meta-analysis evaluating the accuracy of colon capsule endoscopy in detecting colon polyps. Gastrointest Endosc. 2010;71:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Song HJ, Moon JS, Do JH, Cha IH, Yang CH, Choi MG, Jeen YT, Kim HJ. Guidelines for Bowel Preparation before Video Capsule Endoscopy. Clin Endosc. 2013;46:147-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Niv Y. Efficiency of bowel preparation for capsule endoscopy examination: a meta-analysis. World J Gastroenterol. 2008;14:1313-1317. [PubMed] |
| 26. | Mata A, Llach J, Bordas JM. Wireless capsule endoscopy. World J Gastroenterol. 2008;14:1969-1971. [PubMed] |
| 27. | Selby W. Complete small-bowel transit in patients undergoing capsule endoscopy: determining factors and improvement with metoclopramide. Gastrointest Endosc. 2005;61:80-85. [PubMed] |
| 28. | Almeida N, Figueiredo P, Freire P, Lopes S, Lérias C, Gouveia H, Leitão MC. The effect of metoclopramide in capsule enteroscopy. Dig Dis Sci. 2010;55:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Koulaouzidis A, Giannakou A, Yung DE, Dabos KJ, Plevris JN. Do prokinetics influence the completion rate in small-bowel capsule endoscopy? A systematic review and meta-analysis. Curr Med Res Opin. 2013;29:1171-1185. [PubMed] |
| 30. | Kotwal VS, Attar BM, Gupta S, Agarwal R. Should bowel preparation, antifoaming agents, or prokinetics be used before video capsule endoscopy? A systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2014;26:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 31. | Khan MI, Johnston M, Cunliffe R, Claydon A. The role of capsule endoscopy in small bowel pathology: a review of 122 cases. N Z Med J. 2013;126:16-26. [PubMed] |
| 32. | Freitas GP, Teixeira N, Feldman G. Capsule endoscopy in clinical practice: four years of experience from a single center. Arq Gastroenterol. 2011;48:220-222. [PubMed] |
| 33. | Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 475] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 34. | Lepileur L, Dray X, Antonietti M, Iwanicki-Caron I, Grigioni S, Chaput U, Di-Fiore A, Alhameedi R, Marteau P, Ducrotté P. Factors associated with diagnosis of obscure gastrointestinal bleeding by video capsule enteroscopy. Clin Gastroenterol Hepatol. 2012;10:1376-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Goenka MK, Majumder S, Kumar S, Sethy PK, Goenka U. Single center experience of capsule endoscopy in patients with obscure gastrointestinal bleeding. World J Gastroenterol. 2011;17:774-778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Descamps C, Schmit A, Van Gossum A. “Missed” upper gastrointestinal tract lesions may explain “occult” bleeding. Endoscopy. 1999;31:452-455. [PubMed] |
| 37. | Cellier C. Obscure gastrointestinal bleeding: role of videocapsule and double-balloon enteroscopy. Best Pract Res Clin Gastroenterol. 2008;22:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Esaki M, Matsumoto T, Yada S, Yanaru-Fujisawa R, Kudo T, Yanai S, Nakamura S, Iida M. Factors associated with the clinical impact of capsule endoscopy in patients with overt obscure gastrointestinal bleeding. Dig Dis Sci. 2010;55:2294-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Arakawa D, Ohmiya N, Nakamura M, Honda W, Shirai O, Itoh A, Hirooka Y, Niwa Y, Maeda O, Ando T. Outcome after enteroscopy for patients with obscure GI bleeding: diagnostic comparison between double-balloon endoscopy and videocapsule endoscopy. Gastrointest Endosc. 2009;69:866-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 40. | Kaffes AJ, Siah C, Koo JH. Clinical outcomes after double-balloon enteroscopy in patients with obscure GI bleeding and a positive capsule endoscopy. Gastrointest Endosc. 2007;66:304-309. [PubMed] |
| 41. | de Leusse A, Vahedi K, Edery J, Tiah D, Fery-Lemonnier E, Cellier C, Bouhnik Y, Jian R. Capsule endoscopy or push enteroscopy for first-line exploration of obscure gastrointestinal bleeding? Gastroenterology. 2007;132:855-862; quiz 1164-1165. [PubMed] |
| 42. | Carey EJ, Leighton JA, Heigh RI, Shiff AD, Sharma VK, Post JK, Fleischer DE. A single-center experience of 260 consecutive patients undergoing capsule endoscopy for obscure gastrointestinal bleeding. Am J Gastroenterol. 2007;102:89-95. [PubMed] |
| 43. | Min YW, Kim JS, Jeon SW, Jeen YT, Im JP, Cheung DY, Choi MG, Kim JO, Lee KJ, Ye BD. Long-term outcome of capsule endoscopy in obscure gastrointestinal bleeding: a nationwide analysis. Endoscopy. 2014;46:59-65. [PubMed] |
| 44. | Lai LH, Wong GL, Chow DK, Lau JY, Sung JJ, Leung WK. Long-term follow-up of patients with obscure gastrointestinal bleeding after negative capsule endoscopy. Am J Gastroenterol. 2006;101:1224-1228. [PubMed] |
| 45. | Riccioni ME, Urgesi R, Cianci R, Rizzo G, D’Angelo L, Marmo R, Costamagna G. Negative capsule endoscopy in patients with obscure gastrointestinal bleeding reliable: recurrence of bleeding on long-term follow-up. World J Gastroenterol. 2013;19:4520-4525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Viazis N, Papaxoinis K, Vlachogiannakos J, Efthymiou A, Theodoropoulos I, Karamanolis DG. Is there a role for second-look capsule endoscopy in patients with obscure GI bleeding after a nondiagnostic first test? Gastrointest Endosc. 2009;69:850-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 47. | Triester SL, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100:2407-2418. [PubMed] |
| 48. | Saperas E, Dot J, Videla S, Alvarez-Castells A, Perez-Lafuente M, Armengol JR, Malagelada JR. Capsule endoscopy versus computed tomographic or standard angiography for the diagnosis of obscure gastrointestinal bleeding. Am J Gastroenterol. 2007;102:731-737. [PubMed] |
| 49. | Leung WK, Ho SS, Suen BY, Lai LH, Yu S, Ng EK, Ng SS, Chiu PW, Sung JJ, Chan FK. Capsule endoscopy or angiography in patients with acute overt obscure gastrointestinal bleeding: a prospective randomized study with long-term follow-up. Am J Gastroenterol. 2012;107:1370-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 50. | Hartmann D, Schmidt H, Bolz G, Schilling D, Kinzel F, Eickhoff A, Huschner W, Möller K, Jakobs R, Reitzig P. A prospective two-center study comparing wireless capsule endoscopy with intraoperative enteroscopy in patients with obscure GI bleeding. Gastrointest Endosc. 2005;61:826-832. [PubMed] |
| 51. | Hadithi M, Heine GD, Jacobs MA, van Bodegraven AA, Mulder CJ. A prospective study comparing video capsule endoscopy with double-balloon enteroscopy in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2006;101:52-57. [PubMed] |
| 52. | Nakamura M, Niwa Y, Ohmiya N, Miyahara R, Ohashi A, Itoh A, Hirooka Y, Goto H. Preliminary comparison of capsule endoscopy and double-balloon enteroscopy in patients with suspected small-bowel bleeding. Endoscopy. 2006;38:59-66. [PubMed] |
| 53. | Shim KN, Moon JS, Chang DK, Do JH, Kim JH, Min BH, Jeon SR, Kim JO, Choi MG. Guideline for capsule endoscopy: obscure gastrointestinal bleeding. Clin Endosc. 2013;46:45-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 54. | Gerson LB. Small bowel endoscopy: cost-effectiveness of the different approaches. Best Pract Res Clin Gastroenterol. 2012;26:325-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Gerson L, Kamal A. Cost-effectiveness analysis of management strategies for obscure GI bleeding. Gastrointest Endosc. 2008;68:920-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 56. | Gay G, Delvaux M, Fassler I. Outcome of capsule endoscopy in determining indication and route for push-and-pull enteroscopy. Endoscopy. 2006;38:49-58. [PubMed] |
| 57. | Koulaouzidis A, Rondonotti E, Giannakou A, Plevris JN. Diagnostic yield of small-bowel capsule endoscopy in patients with iron-deficiency anemia: a systematic review. Gastrointest Endosc. 2012;76:983-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 58. | Orlando G, Luppino IM, Lerose MA, Gervasi R, Amato B, Silecchia G, Puzziello A. Feasibility of capsule endoscopy in elderly patients with obscure gastrointestinal bleeding. An up-to-date report. BMC Surg. 2012;12 Suppl 1:S30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 59. | Dionisio PM, Gurudu SR, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am J Gastroenterol. 2010;105:1240-1248; quiz 1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 276] [Article Influence: 18.4] [Reference Citation Analysis (36)] |
| 60. | Jensen MD, Nathan T, Rafaelsen SR, Kjeldsen J. Diagnostic accuracy of capsule endoscopy for small bowel Crohn’s disease is superior to that of MR enterography or CT enterography. Clin Gastroenterol Hepatol. 2011;9:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (36)] |
| 61. | Pasha SF, Leighton JA, Das A, Harrison ME, Decker GA, Fleischer DE, Sharma VK. Double-balloon enteroscopy and capsule endoscopy have comparable diagnostic yield in small-bowel disease: a meta-analysis. Clin Gastroenterol Hepatol. 2008;6:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 272] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 62. | Halling ML, Nathan T, Kjeldsen J, Jensen MD. High sensitivity of quick view capsule endoscopy for detection of small bowel Crohn’s disease. J Gastroenterol Hepatol. 2014;29:992-996. [PubMed] |
| 63. | Scapa E, Jacob H, Lewkowicz S, Migdal M, Gat D, Gluckhovski A, Gutmann N, Fireman Z. Initial experience of wireless-capsule endoscopy for evaluating occult gastrointestinal bleeding and suspected small bowel pathology. Am J Gastroenterol. 2002;97:2776-2779. [PubMed] |
| 64. | Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172-1178. [PubMed] |
| 65. | Bourreille A, Ignjatovic A, Aabakken L, Loftus EV, Eliakim R, Pennazio M, Bouhnik Y, Seidman E, Keuchel M, Albert JG. Role of small-bowel endoscopy in the management of patients with inflammatory bowel disease: an international OMED-ECCO consensus. Endoscopy. 2009;41:618-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 237] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 66. | Koulaouzidis A, Douglas S, Rogers MA, Arnott ID, Plevris JN. Fecal calprotectin: a selection tool for small bowel capsule endoscopy in suspected IBD with prior negative bi-directional endoscopy. Scand J Gastroenterol. 2011;46:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 67. | Adler SN, Yoav M, Eitan S, Yehuda C, Eliakim R. Does capsule endoscopy have an added value in patients with perianal disease and a negative work up for Crohn’s disease? World J Gastrointest Endosc. 2012;4:185-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 68. | Rosa B, Moreira MJ, Rebelo A, Cotter J. Lewis Score: a useful clinical tool for patients with suspected Crohn’s Disease submitted to capsule endoscopy. J Crohns Colitis. 2012;6:692-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 69. | Niv Y, Ilani S, Levi Z, Hershkowitz M, Niv E, Fireman Z, O’Donnel S, O’Morain C, Eliakim R, Scapa E. Validation of the Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI or Niv score): a multicenter prospective study. Endoscopy. 2012;44:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 70. | Koulaouzidis A, Douglas S, Plevris JN. Lewis score correlates more closely with fecal calprotectin than Capsule Endoscopy Crohn’s Disease Activity Index. Dig Dis Sci. 2012;57:987-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 71. | Korman LY, Delvaux M, Gay G, Hagenmuller F, Keuchel M, Friedman S, Weinstein M, Shetzline M, Cave D, de Franchis R. Capsule endoscopy structured terminology (CEST): proposal of a standardized and structured terminology for reporting capsule endoscopy procedures. Endoscopy. 2005;37:951-959. [PubMed] |
| 72. | Van Assche G, Dignass A, Bokemeyer B, Danese S, Gionchetti P, Moser G, Beaugerie L, Gomollón F, Häuser W, Herrlinger K. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 3: special situations. J Crohns Colitis. 2013;7:1-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 339] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 73. | Maunoury V, Savoye G, Bourreille A, Bouhnik Y, Jarry M, Sacher-Huvelin S, Ben Soussan E, Lerebours E, Galmiche JP, Colombel JF. Value of wireless capsule endoscopy in patients with indeterminate colitis (inflammatory bowel disease type unclassified). Inflamm Bowel Dis. 2007;13:152-155. [PubMed] |
| 74. | Murrell Z, Vasiliauskas E, Melmed G, Lo S, Targan S, Fleshner P. Preoperative wireless capsule endoscopy does not predict outcome after ileal pouch-anal anastomosis. Dis Colon Rectum. 2010;53:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Rokkas T, Niv Y. The role of video capsule endoscopy in the diagnosis of celiac disease: a meta-analysis. Eur J Gastroenterol Hepatol. 2012;24:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 76. | Urgesi R, Riccioni ME, Bizzotto A, Cianci R, Spada C, Pelecca G, Ricci R, Costamagna G. Increased diagnostic yield of small bowel tumors with PillCam: the role of capsule endoscopy in the diagnosis and treatment of gastrointestinal stromal tumors (GISTs). Italian single-center experience. Tumori. 2012;98:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 77. | Ladas SD, Triantafyllou K, Spada C, Riccioni ME, Rey JF, Niv Y, Delvaux M, de Franchis R, Costamagna G. European Society of Gastrointestinal Endoscopy (ESGE): recommendations (2009) on clinical use of video capsule endoscopy to investigate small-bowel, esophageal and colonic diseases. Endoscopy. 2010;42:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 78. | Girelli CM, Porta P, Colombo E, Lesinigo E, Bernasconi G. Development of a novel index to discriminate bulge from mass on small-bowel capsule endoscopy. Gastrointest Endosc. 2011;74:1067-1074; quiz 1115.e1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 79. | Barbosa DC, Roupar DB, Ramos JC, Tavares AC, Lima CS. Automatic small bowel tumor diagnosis by using multi-scale wavelet-based analysis in wireless capsule endoscopy images. Biomed Eng Online. 2012;11:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 80. | Brown G, Fraser C, Schofield G, Taylor S, Bartram C, Phillips R, Saunders B. Video capsule endoscopy in peutz-jeghers syndrome: a blinded comparison with barium follow-through for detection of small-bowel polyps. Endoscopy. 2006;38:385-390. [PubMed] |
| 81. | Koornstra JJ. Small bowel endoscopy in familial adenomatous polyposis and Lynch syndrome. Best Pract Res Clin Gastroenterol. 2012;26:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 82. | Fujimori S, Seo T, Gudis K, Ehara A, Kobayashi T, Mitsui K, Yonezawa M, Tanaka S, Tatsuguchi A, Sakamoto C. Prevention of nonsteroidal anti-inflammatory drug-induced small-intestinal injury by prostaglandin: a pilot randomized controlled trial evaluated by capsule endoscopy. Gastrointest Endosc. 2009;69:1339-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 83. | Malagelada C, De Iorio F, Azpiroz F, Accarino A, Segui S, Radeva P, Malagelada JR. New insight into intestinal motor function via noninvasive endoluminal image analysis. Gastroenterology. 2008;135:1155-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 84. | Yakoub-Agha I, Maunoury V, Wacrenier A, Couignoux S, Depil S, Desreumaux P, Bauters F, Colombel JF, Jouet JP. Impact of Small Bowel Exploration Using Video-Capsule Endoscopy in the Management of Acute Gastrointestinal Graft-versus-Host Disease. Transplantation. 2004;78:1697-1701. [PubMed] |
| 85. | Bandorski D, Keuchel M, Brück M, Hoeltgen R, Wieczorek M, Jakobs R. Capsule endoscopy in patients with cardiac pacemakers, implantable cardioverter defibrillators, and left heart devices: a review of the current literature. Diagn Ther Endosc. 2011;2011:376053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 86. | Hogan RB, Ahmad N, Hogan RB, Hensley SD, Phillips P, Doolittle P, Reimund E. Video capsule endoscopy detection of jejunal carcinoid in life-threatening hemorrhage, first trimester pregnancy. Gastrointest Endosc. 2007;66:205-207. [PubMed] |
| 87. | Friedt M, Welsch S. An update on pediatric endoscopy. Eur J Med Res. 2013;18:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 88. | Cohen SA. The potential applications of capsule endoscopy in pediatric patients compared with adult patients. Gastroenterol Hepatol (N Y). 2013;9:92-97. [PubMed] |
| 89. | Cave D, Legnani P, de Franchis R, Lewis BS. ICCE consensus for capsule retention. Endoscopy. 2005;37:1065-1067. [PubMed] |
| 90. | Bhattarai M, Bansal P, Khan Y. Longest duration of retention of video capsule: A case report and literature review. World J Gastrointest Endosc. 2013;5:352-355. [PubMed] |
| 91. | Palmer JS, Marenah K, El Madani F, Jain K, Gupta S. Small bowel perforation following capsule endoscopy: a case report. Ann R Coll Surg Engl. 2011;93:e69-e70. [PubMed] |
| 92. | Herrerias JM, Leighton JA, Costamagna G, Infantolino A, Eliakim R, Fischer D, Rubin DT, Manten HD, Scapa E, Morgan DR. Agile patency system eliminates risk of capsule retention in patients with known intestinal strictures who undergo capsule endoscopy. Gastrointest Endosc. 2008;67:902-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 93. | Yadav A, Heigh RI, Hara AK, Decker GA, Crowell MD, Gurudu SR, Pasha SF, Fleischer DE, Harris LA, Post J. Performance of the patency capsule compared with nonenteroclysis radiologic examinations in patients with known or suspected intestinal strictures. Gastrointest Endosc. 2011;74:834-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 94. | Parker C, Davison C, Panter S. Tracheal aspiration of a capsule endoscope: not always a benign event. Dig Dis Sci. 2012;57:1727-1728. [PubMed] |
| 95. | Swain P, Toor A, Volke F, Keller J, Gerber J, Rabinovitz E, Rothstein RI. Remote magnetic manipulation of a wireless capsule endoscope in the esophagus and stomach of humans (with videos). Gastrointest Endosc. 2010;71:1290-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 96. | Rey JF. The future of capsule endoscopy. Keio J Med. 2013;62:41-46. [PubMed] |
| 97. | Carpi F, Kastelein N, Talcott M, Pappone C. Magnetically controllable gastrointestinal steering of video capsules. IEEE Trans Biomed Eng. 2011;58:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 98. | Woo SH, Kim TW, Mohy-Ud-Din Z, Park IY, Cho JH. Small intestinal model for electrically propelled capsule endoscopy. Biomed Eng Online. 2011;10:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 99. | Kusuda Y. A further step beyond wireless capsule endoscopy. Sensor Rev. 2005;25:259-260. [DOI] [Full Text] |
| 100. | Karargyris A, Koulaouzidis A. Capsule-odometer: a concept to improve accurate lesion localisation. World J Gastroenterol. 2013;19:5943-5946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 101. | Karargyris A, Rondonotti E, Mandelli G, Koulaouzidis A. Evaluation of 4 three-dimensional representation algorithms in capsule endoscopy images. World J Gastroenterol. 2013;19:8028-8033. [PubMed] |
| 102. | Hu E, Nosato H, Sakanashi H, Murakawa M. A modified anomaly detection method for capsule endoscopy images using non-linear color conversion and Higher-order Local Auto-Correlation (HLAC). Conf Proc IEEE Eng Med Biol Soc. 2013;2013:5477-5480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 103. | Westerhof J, Weersma RK, Koornstra JJ. Risk factors for incomplete small-bowel capsule endoscopy. Gastrointest Endosc. 2009;69:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 104. | Wilding I, Hirst P, Connor A. Development of a new engineering-based capsule for human drug absorption studies. Pharm Sci Technolo Today. 2000;3:385-392. [PubMed] |
| 105. | Fisher LR, Hasler WL. New vision in video capsule endoscopy: current status and future directions. Nat Rev Gastroenterol Hepatol. 2012;9:392-405. [PubMed] |
| 106. | Schostek S, Schurr MO. European research on wireless endoscopy--the VECTOR project. Stud Health Technol Inform. 2013;189:193-199. [PubMed] |
| 107. | Swain P. The future of wireless capsule endoscopy. World J Gastroenterol. 2008;14:4142-4145. [PubMed] |
| 108. | Ray K. Endoscopy: Tethered capsule endomicroscopy of the oesophagus--an easy pill to swallow. Nat Rev Gastroenterol Hepatol. 2013;10:129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 109. | Tennyson CA, Ciaccio EJ, Lewis SK. Video capsule endoscopy in celiac disease. Gastrointest Endosc Clin N Am. 2012;22:747-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 110. | Ciaccio EJ, Tennyson CA, Lewis SK, Krishnareddy S, Bhagat G, Green PH. Distinguishing patients with celiac disease by quantitative analysis of videocapsule endoscopy images. Comput Methods Programs Biomed. 2010;100:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 111. | Ishiguro H, Saito S, Imazu H, Aihara H, Kato T, Tajiri H. Esophageal Capsule Endoscopy for Screening Esophageal Varices among Japanese Patients with Liver Cirrhosis. Gastroenterol Res Pract. 2012;2012:946169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 112. | Remes-Troche JM, Jiménez-García VA, García-Montes JM, Hergueta-Delgado P, Roesch-Dietlen F, Herrerías-Gutiérrez JM. Application of colon capsule endoscopy (CCE) to evaluate the whole gastrointestinal tract: a comparative study of single-camera and dual-camera analysis. Clin Exp Gastroenterol. 2013;6:185-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |