Published online Jul 28, 2014. doi: 10.3748/wjg.v20.i28.9564
Revised: March 11, 2014
Accepted: April 21, 2014
Published online: July 28, 2014
Processing time: 199 Days and 17.7 Hours
AIM: To assess the expression of nuclear hepatoma-derived growth factor (HDGF) in benign and malignant gallbladder lesions and to determine its clinicopathological significance.
METHODS: We studied 40 patients with gallbladder cancer (GBC) and a control group of 40 patients with cholelithiasis. All diagnoses of GBC and cholelithiasis were confirmed by histopathological examination after surgery. None of the patients received chemotherapy or radiotherapy before surgery. All tissue samples were fixed in 4% formalin immediately after removal and embedded in paraffin for immunohistochemical staining. The HDGF expression in the GBC and cholelithiasis specimens was examined by immunohistochemical staining. The relationship between the HDGF expression and the clinicopathological parameters of GBC was analyzed.
RESULTS: Nuclear HDGF expression was significantly higher (77.5%) in GBC than in chronic cholelithiasis (21.5%, P < 0.001). High nuclear HDGF levels were associated with histopathological subtype (P < 0.05), clinical stage (P < 0.01), and perineural invasion (P < 0.01) but not with sex, age, history of gallstones, or lymph node metastasis. A univariate Kaplan-Meier analysis showed that positive nuclear HDGF expression was associated with decreased overall survival (P < 0.01). Multivariate Cox regression analysis showed that nuclear HDGF expression and lymph node metastasis were independent risk factors for disease-free survival.
CONCLUSION: The expression of nuclear HDGF might be closely related to the carcinogenesis, clinical biological behaviors, and prognosis of gallbladder adenocarcinoma.
Core tip: Gallbladder cancer (GBC) is a highly invasive, rapidly proliferating tumor. Unfortunately, most conventional treatments for this tumor remain associated with poor survival. Therefore, it is necessary to identify prognostic biomarkers that are independently correlated with tumor aggressiveness. This study documents hepatoma-derived growth factor (HDGF) immunostaining in gallbladder carcinomas for the first time and shows that the expression of nuclear HDGF is closely related to the carcinogenesis, clinical biological behaviors, and prognosis of GBC.
- Citation: Tao F, Ye MF, Sun AJ, Lv JQ, Xu GG, Jing YM, Wang W. Prognostic significance of nuclear hepatoma-derived growth factor expression in gallbladder cancer. World J Gastroenterol 2014; 20(28): 9564-9569
- URL: https://www.wjgnet.com/1007-9327/full/v20/i28/9564.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i28.9564
Gallbladder cancer (GBC) is the most common biliary tract malignancy and the seventh most common gastrointestinal cancer[1-3]. According to the data from the SEER Program, the incidence of GBC is estimated at 2.5 per 100000 persons. Despite its comparatively lower incidence rate, GBC-associated mortality is higher than the mortality of other cancers[4]. The median survival of GBC patients is less than 1 year due to the early spread of tumors via lymphatic, perineural, and hematogenous routes as well as direct invasion of the liver[5,6]. Therefore, the development of novel and effective therapeutic strategies for GBC, as well as an increased understanding of biomarkers and their effects on therapeutic responses, may improve patient prognosis.
Hepatoma-derived growth factor (HDGF), a heparin-binding growth factor, has a wide range of biological functions, including roles in mitogenic activity and vascular development[7]. Consistent with these critical roles, HDGF has been characterized as an oncogene. Thus, it plays important roles in promoting cancer cell proliferation, angiogenesis, invasion, and migration in several malignancies, including oral squamous cell carcinoma as well as gastric, colonic, lung, and esophageal cancers[8-12]. Furthermore, clinicopathological analysis has shown that a high level of HDGF expression is associated with poor prognosis in several types of cancers, such as breast cancer and pancreatic ductal carcinoma[13,14], and that HDGF overexpression is correlated with an increasing rate of lymph node metastasis in gastric cancer[15]. However, the significance of HDGF expression in the prognosis of GBC has not been fully evaluated.
In this study, using immunohistochemistry (IHC), we investigated the HDGF expression levels in benign and malignant lesions of the gallbladder and studied the clinicopathological significance of its expression in the prognosis of GBC.
Samples from 40 patients with GBC pathologically confirmed after surgery were collected at the Shaoxing Hospital of Zhejiang University, China. None of the patients received chemotherapy or radiotherapy before surgery. The patients included 11 males and 29 females with an average age of 70.6 years. According to the Union for International Cancer Control staging system, 2 (5.0%), 3 (7.5%), 12 (30.0%), 12 (30.0%), and 11 (27.5%) patients were diagnosed with stage 0, I, II, III, and IV disease, respectively. Additionally, 21 cases were diagnosed with lymph node metastases. A total of 40 patients with cholelithiasis who had undergone simple cholecystectomy were included as controls. All diagnoses of GBC, cholelithiasis, and lymph node metastasis were confirmed by histopathological examination, and all tissue samples were fixed in 4% formalin immediately after removal and embedded in paraffin for immunohistochemical staining. The survival information for the 40 GBC patients was obtained through phone calls. This study was approved by the Ethics Committee of Shaoxing Hospital of Zhejiang University. Written informed consent was obtained from each participant.
Immunohistochemical staining was performed using the standard immunoperoxidase staining procedure, and HDGF expression in the specimens was evaluated according to the methods described by Pinheiro et al[16]. The sections were semi-quantitatively scored according to the extent of immunoreactivity as follows: 0, 0% immunoreactive cells; 1, < 5% immunoreactive cells; 2, 5%-50% immunoreactive cells; and 3, > 50% immunoreactive cells. The staining intensity was scored semi-quantitatively as follows: 0, negative; 1, weak; 2, intermediate; and 3, strong. The final immunoreaction score was defined as the sum of the two parameters (extent and intensity), and the samples were classified as negative (0), weakly stained (1-2), moderately stained (3), or strongly stained (4-6). For statistical purposes, only moderate and strong final immunoreaction scores were considered positive; negative and weakly stained final scores were considered negative.
The data were analyzed using SPSS 19.0 (Statistical Package for the Social Sciences version 19.0). The relationship between HDGF expression and histology or clinical factors was analyzed using the χ2 independence test or Fisher’s exact test. The Kaplan-Meier test was used for univariate survival analysis. The Cox proportional hazard model was used for multivariate analysis and to determine the 95%CI. P < 0.05 was considered statistically significant.
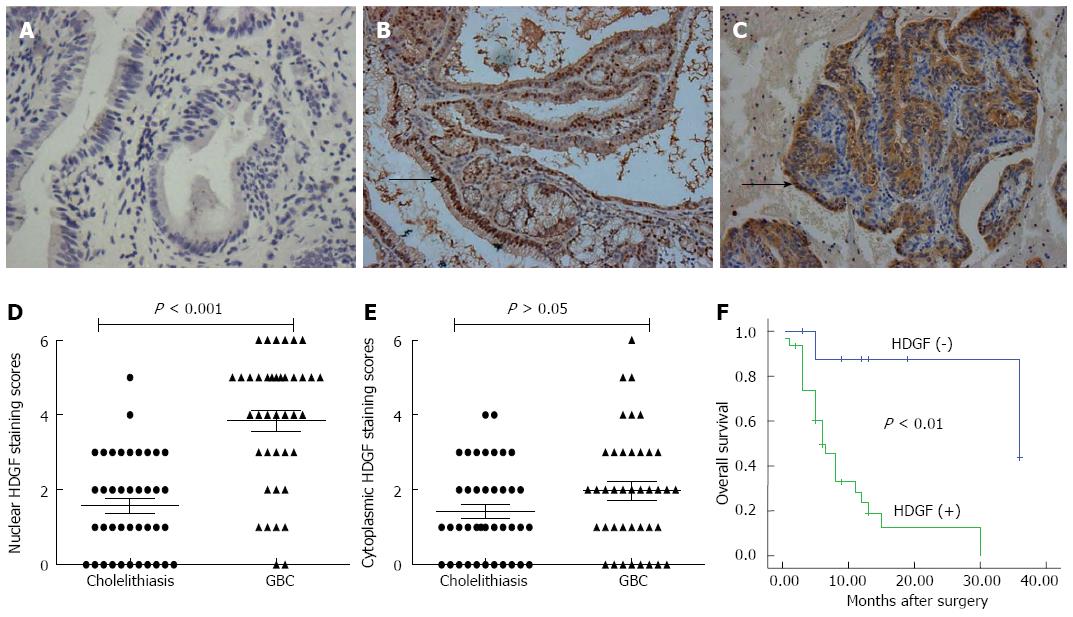
To determine the potential role of HDGF in GBC progression, we evaluated HDGF expression in GBC and cholelithiasis tissues by IHC (Figure 1A-E). HDGF was mainly located in the nuclei, which was consistent with the known cellular function of HDGF protein as a nuclear-targeted mitogen that exhibits mitogenic activity in various cells after translocation to the nucleus[17]. Approximately 77.5% (31/40) of the GBC cases had positive nuclear HDGF staining in the tumor cells. In contrast, only 21.5% (11/40) of the cases had positive staining in the cholelithiasis tissues (Table 1, Figure 1D, P < 0.001). However, the incidence of cytoplasmic HDGF was comparable between the tumor and cholelithiasis tissues: positive staining was observed in tumor cells in 32.5% (13/40) of cases and in cholelithiasis tissues in 22.5% (9/40) of cases (Table 1, Figure 1E, P > 0.05). Thus, the differences in HDGF protein levels between the tumor and cholelithiasis tissues were much more significant in the nuclei than in the cytoplasm.
| Tissue types | Cases (n) | Nuclear HDGF | Cytoplasmic HDGF | ||||
| Positive cases | χ2 | P value | Positive cases | χ2 | P value | ||
| GBC | 40 | 31 (77.5) | 20.05 | < 0.001 | 13 (32.5) | 1.003 | 0.317 |
| Cholelithiasis | 40 | 11 (21.5) | 9 (22.5) | ||||
As shown in Table 2, HDGF overexpression was found to be significantly correlated with histopathological subtypes (P < 0.05), TNM stage (P < 0.01), and perineural invasion (P < 0.01), indicating a potential role for HDGF in promoting aggressive phenotypes in GBC. However, HDGF expression was not significantly associated with the presence of lymph node metastasis or other clinicopathological characteristics, such as gender, age, or history of gallstones (P > 0.05).
| Parameter | Category | Cases (n) | HDGF | ||
| Positive cases | χ2 | P value | |||
| Age | < 60 | 9 | 8 (88.9) | 0.227 | 0.634 |
| ≥ 60 | 31 | 23 (74.2) | |||
| Sex | Male | 11 | 7 (63.6) | 0.755 | 0.385 |
| Female | 29 | 24 (82.8) | |||
| Histopathological subtypes | High | 8 | 3 (37.5) | 8.027 | 0.018 |
| Middle | 14 | 12 (85.7) | |||
| Low | 18 | 16 (88.9) | |||
| TNM stage | 0-I | 5 | 1 (20.0) | 7.349 | 0.007 |
| II-IV | 35 | 30 (85.7) | |||
| Lymph node metastasis | Negative | 19 | 14 (73.7) | 0.029 | 0.865 |
| Positive | 21 | 17 (83.0) | |||
| Perineural invasion | Absent | 12 | 5 (41.7) | 9.858 | 0.002 |
| Present | 28 | 26 (92.9) | |||
| Gallbladder stones | Negative | 17 | 14 (82.4) | 0.152 | 0.697 |
| Positive | 23 | 17 (77.3) | |||
Correlation between HDGF expression and survival in patients with gallbladder cancer.
A total of 12 patients survived at least 1 year, and 28 patients survived less than 1 year, with an average survival time of 9.7 mo. The patients who survived less than 1 year were found to have significantly higher HDGF expression than the patients who survived over 1 year (Table 3). The Kaplan-Meier survival analysis revealed that the histopathological subtype (P < 0.01), the TNM stage (P < 0.05), the presence of lymph node metastasis (P < 0.01), and the presence of perineural invasion (P < 0.05) were significantly associated with the average survival time. The average survival time for HDGF-negative patients was significantly higher than for patients with positive HDGF expression (P < 0.01) (Table 4, Figure 1F). To obtain a more precise estimate of prognosis, the Cox proportional hazard regression model was applied. The results confirmed that HDGF expression and the presence of lymph node metastasis were negatively correlated with post-operative survival, suggesting that high HDGF expression is a risk factor for disease-free survival (Table 5).
| Survival time | Cases (n) | HDGF | ||
| Positive cases | χ2 | P value | ||
| < 1 yr | 28 | 25 (89.3) | 5.352 | 0.021 |
| ≥ 1 yr | 12 | 6 (50.0) | ||
| Parameter | Category | Cases (n) | Mean survival time (mo) 95%CI | P value |
| Age | < 60 | 8 | 14.8 (4.3-25.2) | 0.930 |
| ≥ 60 | 32 | 14.3 (9.2-19.4) | ||
| Sex | Male | 11 | 20.2 (10.4-30.1) | 0.142 |
| Female | 29 | 12.7 (7.9-17.5) | ||
| Histopathological subtypes | High | 8 | 30.4 (21.5-39.2) | 0.003 |
| Middle | 14 | 12.1 (5.6-18.5) | ||
| Low | 18 | 7.2 (4.8-9.7) | ||
| TNM stage | 0-I | 5 | 36.0 (36.0-36.0) | 0.015 |
| II-IV | 35 | 11.4 (7.4-15.4) | ||
| Lymph node metastasis | Negative | 19 | 19.4 (11.4-27.4) | 0.005 |
| Positive | 21 | 9.1 (4.4-13.8) | ||
| Perineural invasion | Absent | 12 | 27.6 (18.3-37.0) | 0.013 |
| Present | 28 | 10.3 (6.3-14.2) | ||
| HDGF | Negative | 9 | 32.1 (22.1-42.2) | 0.001 |
| Positive | 31 | 9.6 (6.1-13.1) |
| Parameter | Category | HR | 95%CI | P value |
| HDGF expression | Negative | 4.298 | 1.134-88.887 | 0.038 |
| Positive | ||||
| TNM stage | 0-I | 0.008 | 0.084-15.014 | 0.930 |
| II-IV | ||||
| Lymph node metastasis | Negative | 4.368 | 1.060-6.134 | 0.037 |
| Positive | ||||
| Histopathological subtypes | High | 2.061 | 0.828-3.402 | 0.151 |
| Middle | ||||
| Low | ||||
| Perineural invasion | Absent | 0.073 | 0.218-3.170 | 0.787 |
| Present |
GBC is associated with highly invasive, rapidly proliferating tumors. Unfortunately, the most aggressive conventional treatments for GBC still remain associated with poor survival[18,19]. Therefore, it is necessary to identify prognostic biomarkers that are independently correlated with tumor aggressiveness. This study documents HDGF immunostaining in GBC for the first time, showing that a high level of nuclear HDGF expression is found in approximately 77.5% of cases.
HDGF, located on chromosome 1q21-23, is a heparin-binding growth factor that was originally purified from media conditioned with the human hepatoma cell line HuH7[20]. HDGF comprises 240 amino acids and contains multiple functional domains, including a conserved N-terminal domain 100 amino acids in length that is responsible for DNA binding and 2 bipartite nuclear localization sequences for nuclear localization[21]. HDGF is a nuclear-targeted mitogen that exhibits mitogenic activity and stimulates the proliferation of various cells, including fibroblasts, vascular smooth muscle cells, endothelial cells, and a variety of cancer cell lines, after translocation to the nucleus[17,22]. Consistent with these findings, Chen et al[13] determined that the nuclear HDGF staining level, but not the cytoplasmic staining level, was correlated with the proliferation index in breast cancer. In this study, we also detected significantly increased nuclear HDGF expression in the vast majority of GBC tissues compared with cholecystitis tissues, while cytoplasmic HDGF localization had no prognostic significance. Although the role of cytoplasmic HDGF expression has not yet been clarified, it is thought to be associated with HDGF receptors located at the plasma membrane[23]. Additional studies are necessary to identify the precise biological role of cytoplasmic HDGF in different cancers.
Invasion and metastasis are the predominant characteristics of malignant diseases[24]. In multiple studies, it has been shown that elevated levels of HDGF in different types of tumors are correlated with metastatic potential, increased malignancy, and an unfavorable prognosis for the patients[8-12,25]. However, the molecular mechanism underlying HDGF-induced metastasis remains unclear. One possibility is that HDGF may promote tumor progression through the modulation of angiogenesis or facilitate epithelial-mesenchymal transition to create a niche environment for metastasis[23,26]. In this study, we also found that upregulated nuclear HDGF expression was strongly correlated with the degree of tumor differentiation, perineural invasion, and TNM stage in GBC patients. Further survival analysis showed that after surgery, the survival time of cases with positive nuclear HDGF expression was significantly lower than the survival time of cases with negative expression. Cox multivariate analysis showed that HDGF expression was negatively correlated with the postoperative survival ratio and was an independent indicator of poor prognosis. These findings indicate the potential role of HDGF in promoting aggressive phenotypes of GBC and the potential utility of HDGF expression levels as a prognostic biomarker of GBC.
This study was a retrospective analysis and had some limitations. The sample size of the study was relatively small, with only 40 cases. To further understand the specific role of HDGF in the prognosis of GBC, additional comprehensive prospective studies must be performed.
In conclusion, nuclear HDGF overexpression is present in a substantial proportion of GBC cases and is significantly correlated with the clinical biological behaviors of GBC. More importantly, HDGF overexpression, along with advanced stages and poor differentiation, represents a significant prognostic factor for reduced disease-specific survival. Our data suggest that nuclear HDGF overexpression could predict a poor prognosis in GBC patients, suggesting HDGF as a potential molecular target for GBC therapy.
Despite its comparatively lower incidence rate, gallbladder cancer (GBC) has a higher mortality rate than other cancers. Unfortunately, most conventional treatments for GBC remain associated with poor survival. Therefore, the development of novel and effective therapeutic strategies for GBC as well as an increased understanding of biomarkers and their effects on therapeutic responses may improve patient prognosis.
Hepatoma-derived growth factor (HDGF), a heparin-binding growth factor, plays important roles in the promotion of cancer cell proliferation, angiogenesis, invasion, and migration in several cancers. However, the significance of HDGF expression in the prognosis of GBC has not been fully evaluated. In this study, the authors demonstrate that the overexpression of nuclear HDGF is closely related to the clinical biological behaviors and prognosis of GBC.
Recent research has highlighted the significant increase in nuclear HDGF staining in GBC. In this study, the authors found that HDGF overexpression, along with advanced stages and poor differentiation, represents a significant prognostic factor for reduced disease-specific survival.
The study results suggest that nuclear HDGF overexpression could predict a poor prognosis in GBC patients and suggest HDGF as a potential molecular target for GBC therapy.
HDGF, located on chromosome 1q21-23, is a heparin-binding growth factor that was originally purified from media conditioned with the human hepatoma cell line HuH7.
This work is a good descriptive study in which the authors examined the expression of HDGF in gallbladder cancer. The results are interesting and suggest that nuclear HDGF overexpression is present in a substantial proportion of GBC cases and is significantly correlated with clinical biological behaviors. More importantly, nuclear HDGF overexpression was predictive of a poor prognosis in GBC patients. The value of the manuscript is very clear.
P- Reviewer: Nagem RG, Yang H S- Editor: Gou SX L- Editor: Ma JY E- Editor: Zhang DN
| 1. | Zhang Z, Wang X, Wu W, Wang J, Wang Y, Wu X, Fei X, Li S, Zhang J, Dong P. Effects of matrine on proliferation and apoptosis in gallbladder carcinoma cells (GBC-SD). Phytother Res. 2012;26:932-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Dong P, Zhang Y, Gu J, Wu W, Li M, Yang J, Zhang L, Lu J, Mu J, Chen L. Wogonin, an active ingredient of Chinese herb medicine Scutellaria baicalensis, inhibits the mobility and invasion of human gallbladder carcinoma GBC-SD cells by inducing the expression of maspin. J Ethnopharmacol. 2011;137:1373-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Boutros C, Gary M, Baldwin K, Somasundar P. Gallbladder cancer: past, present and an uncertain future. Surg Oncol. 2012;21:e183-e191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Quan Z, Gu J, Dong P, Lu J, Wu X, Wu W, Fei X, Li S, Wang Y, Wang J. Reactive oxygen species-mediated endoplasmic reticulum stress and mitochondrial dysfunction contribute to cirsimaritin-induced apoptosis in human gallbladder carcinoma GBC-SD cells. Cancer Lett. 2010;295:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Wang JW, Peng SY, Li JT, Wang Y, Zhang ZP, Cheng Y, Cheng DQ, Weng WH, Wu XS, Fei XZ. Identification of metastasis-associated proteins involved in gallbladder carcinoma metastasis by proteomic analysis and functional exploration of chloride intracellular channel 1. Cancer Lett. 2009;281:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Dong P, He XW, Gu J, Wu WG, Li ML, Yang JH, Zhang L, Ding QC, Lu JH, Mu JS. Vimentin significantly promoted gallbladder carcinoma metastasis. Chin Med J (Engl). 2011;124:4236-4244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Shih TC, Tien YJ, Wen CJ, Yeh TS, Yu MC, Huang CH, Lee YS, Yen TC, Hsieh SY. MicroRNA-214 downregulation contributes to tumor angiogenesis by inducing secretion of the hepatoma-derived growth factor in human hepatoma. J Hepatol. 2012;57:584-591. [PubMed] |
| 8. | Lin YW, Li CF, Chen HY, Yen CY, Lin LC, Huang CC, Huang HY, Wu PC, Chen CH, Chen SC. The expression and prognostic significance of hepatoma-derived growth factor in oral cancer. Oral Oncol. 2012;48:629-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Mao J, Xu Z, Fang Y, Wang H, Xu J, Ye J, Zheng S, Zhu Y. Hepatoma-derived growth factor involved in the carcinogenesis of gastric epithelial cells through promotion of cell proliferation by Erk1/2 activation. Cancer Sci. 2008;99:2120-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Liao F, Dong W, Fan L. Apoptosis of human colorectal carcinoma cells is induced by blocking hepatoma-derived growth factor. Med Oncol. 2010;27:1219-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Meng J, Xie W, Cao L, Hu C, Zhe Z. shRNA targeting HDGF suppressed cell growth and invasion of squamous cell lung cancer. Acta Biochim Biophys Sin (Shanghai). 2010;42:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Yamamoto S, Tomita Y, Hoshida Y, Morii E, Yasuda T, Doki Y, Aozasa K, Uyama H, Nakamura H, Monden M. Expression level of hepatoma-derived growth factor correlates with tumor recurrence of esophageal carcinoma. Ann Surg Oncol. 2007;14:2141-2149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Chen X, Yun J, Fei F, Yi J, Tian R, Li S, Gan X. Prognostic value of nuclear hepatoma-derived growth factor (HDGF) localization in patients with breast cancer. Pathol Res Pract. 2012;208:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Uyama H, Tomita Y, Nakamura H, Nakamori S, Zhang B, Hoshida Y, Enomoto H, Okuda Y, Sakon M, Aozasa K. Hepatoma-derived growth factor is a novel prognostic factor for patients with pancreatic cancer. Clin Cancer Res. 2006;12:6043-6048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Yamamoto S, Tomita Y, Hoshida Y, Takiguchi S, Fujiwara Y, Yasuda T, Doki Y, Yoshida K, Aozasa K, Nakamura H. Expression of hepatoma-derived growth factor is correlated with lymph node metastasis and prognosis of gastric carcinoma. Clin Cancer Res. 2006;12:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Pinheiro C, Longatto-Filho A, Scapulatempo C, Ferreira L, Martins S, Pellerin L, Rodrigues M, Alves VA, Schmitt F, Baltazar F. Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows Arch. 2008;452:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 17. | Everett AD, Bushweller J. Hepatoma derived growth factor is a nuclear targeted mitogen. Curr Drug Targets. 2003;4:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Li ML, Wang XF, Tan ZJ, Dong P, Gu J, Lu JH, Wu XS, Zhang L, Ding QC, Wu WG. Ethyl pyruvate administration suppresses growth and invasion of gallbladder cancer cells via downregulation of HMGB1-RAGE axis. Int J Immunopathol Pharmacol. 2012;25:955-965. [PubMed] |
| 19. | Srivastava K, Srivastava A, Sharma KL, Mittal B. Candidate gene studies in gallbladder cancer: a systematic review and meta-analysis. Mutat Res. 2011;728:67-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Huang JS, Chao CC, Su TL, Yeh SH, Chen DS, Chen CT, Chen PJ, Jou YS. Diverse cellular transformation capability of overexpressed genes in human hepatocellular carcinoma. Biochem Biophys Res Commun. 2004;315:950-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Kishima Y, Yamamoto H, Izumoto Y, Yoshida K, Enomoto H, Yamamoto M, Kuroda T, Ito H, Yoshizaki K, Nakamura H. Hepatoma-derived growth factor stimulates cell growth after translocation to the nucleus by nuclear localization signals. J Biol Chem. 2002;277:10315-10322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Bremer S, Klein K, Sedlmaier A, Abouzied M, Gieselmann V, Franken S. Hepatoma-derived growth factor and nucleolin exist in the same ribonucleoprotein complex. BMC Biochem. 2013;14:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Tsai HE, Wu JC, Kung ML, Liu LF, Kuo LH, Kuo HM, Chen SC, Chan EC, Wu CS, Tai MH. Up-regulation of hepatoma-derived growth factor facilitates tumor progression in malignant melanoma [corrected]. PLoS One. 2013;8:e59345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Wistuba II, Gazdar AF. Gallbladder cancer: lessons from a rare tumour. Nat Rev Cancer. 2004;4:695-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 323] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 25. | Zhang A, Long W, Guo Z, Guo Z, Cao BB. Downregulation of hepatoma-derived growth factor suppresses the malignant phenotype of U87 human glioma cells. Oncol Rep. 2012;28:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Tsai HE, Liu GS, Kung ML, Liu LF, Wu JC, Tang CH, Huang CH, Chen SC, Lam HC, Wu CS. Downregulation of hepatoma-derived growth factor contributes to retarded lung metastasis via inhibition of epithelial-mesenchymal transition by systemic POMC gene delivery in melanoma. Mol Cancer Ther. 2013;12:1016-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |