Published online Jul 28, 2014. doi: 10.3748/wjg.v20.i28.9528
Revised: April 9, 2014
Accepted: April 30, 2014
Published online: July 28, 2014
Processing time: 177 Days and 22.2 Hours
AIM: To explore the value of liver fibrosis assessment by acoustic radiation force impulse (ARFI) and the AST/PLT ratio index (APRI) in chronic hepatitis C patients.
METHODS: One hundred and twenty eight patients with chronic hepatitis C were examined using ARFI elastometry and APRI, calculated according to known formulae. The gold standard of liver biopsy was referred; ROC curve analysis was used to assess all ARFI and APRI values. The corresponding cut-off values, sensitivities, and specificities were calculated and compared. In addition, the correlation of liver fibrosis stages in chronic hepatitis C patients with ARFI measurements and APRI were also tested to evaluate significant data.
RESULTS: The values of ARFI in S1-S4 were 1.23 ± 0.34 m/s, 1.48 ± 0.43 m/s, 2.06 ± 0.45 m/s, and 2.30 ± 0.87 m/s. The values of APRI in S1-S4 were 0.31 ± 0.45 m/s, 0.28 ± 0.38 m/s, 0.58 ± 0.59 m/s and 0.65 ± 0.34 m/s. ARFI (r = 0.649, P < 0.05) showed a better correlation with liver fibrosis stages in chronic hepatitis C than APRI (r = 0.478, P < 0.05). The areas under the ROC curves for ARFI and APRI were 0.775 and 0.721 for stages ≥ S2, 0.901 and 0.787 for stages ≥ S3, and 0.792 and 0.780 for S = 4, respectively.
CONCLUSION: Both ARFI and APRI could evaluate liver fibrosis stages in chronic hepatitis C. ARFI is more accurate than the APRI index.
Core tip: This study has utilized acoustic radiation force impulse (ARFI) technology and the AST/PLT ratio index (APRI) model to detect and analyze the degree of liver fibrosis in 128 patients with hepatitis C. The clinical value and accuracy of these two methods were explored in the evaluation of liver fibrosis stages of patients with chronic hepatitis C. ARFI could more accurately evaluate liver fibrosis in patients with chronic hepatitis C than the APRI.
- Citation: Li SM, Li GX, Fu DM, Wang Y, Dang LQ. Liver fibrosis evaluation by ARFI and APRI in chronic hepatitis C. World J Gastroenterol 2014; 20(28): 9528-9533
- URL: https://www.wjgnet.com/1007-9327/full/v20/i28/9528.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i28.9528
In recent years, the incidence of chronic hepatitis C has shown an upward trend. The body’s stress repair response keeps on working in response to continuous inflammatory stimuli, thus accumulating a large number of extracellular matrixes in the liver tissue[1]. If effective degradation is not performed, cirrhosis or even cancer will occur. Hence, early diagnosis is important for timely prevention and treatment of liver fibrosis. The degree of liver fibrosis, caused by chronic hepatitis C, is an important factor that affects the treatment and prognosis of patients with liver diseases[2]. Therefore, the method of quantitatively assessing the stage and grading of liver fibrosis is very important. The traditional method to evaluate the degree of liver fibrosis and cirrhosis is an invasive technique - liver biopsy[3]. However, this method is not suitable for continuous observation of liver fibrosis and complications limit its usage[4]. At present, there are two kinds of widely used noninvasive measurements for detecting liver fibrosis: acoustic radiation force impulse (ARFI) and the AST/PLT (aspartate aminotransferase/platelet) ratio index (APRI). ARFI evaluates liver stiffness non-invasively and was invented recently. This technique can easily and accurately assess the degree of liver fibrosis in clinical practice[4-6]. Fierbinteanu-Braticevici et al[7] reported that ARFI elastography showed very good accuracy in assessing all stages of liver fibrosis. In addition, a meta-analysis by Nierhoff et al[8] also demonstrated good diagnostic accuracy. The APRI is a mathematical model that can be used as a noninvasive diagnostic method for evaluating liver fibrosis[9]. Abd El Rihim et al[10] reported that APRI appeared to be clinically useful to detect cirrhosis. Lin et al[11] observed that APRI showed an acceptable accuracy for the assessment of liver fibrosis in patients with chronic hepatitis C. Only a few studies of liver fibrosis have reported the accuracy and reliability of both ARFI and APRI. Hence, the present study used ARFI and the APRI model to detect and analyze the degree of liver fibrosis in 128 patients with hepatitis C. We also explored the clinical value and accuracy of these two methods in the evaluation of liver fibrosis stages of patients with chronic hepatitis C. This study aimed to provide a reference to develop practical guidance for diagnosing liver fibrosis in patients with chronic hepatitis C.
One hundred and twenty-eight patients with chronic hepatitis C, who were treated in our hospital from February 2011 to June 2013, were enrolled as the subjects. Patients with other types of hepatitis and liver cancer were excluded. All the subjects gave informed consent, and the ethics committee of our hospital approved the study. There were 86 males and 42 females, with an average age of 69.1 ± 4.7 years. The diagnostic standard of hepatitis C was based on the Viral Hepatitis Prevention and Treatment Programs, which were revised at the Xi’an Conference in 2000[12].
ARFI detection: The ARFI elastometry used in this study was a color Doppler ultrasound technique (Philips IU22) with a convex array probe. To avoid the influence of respiration and cardiac impulse, the right anterior lobe of the liver was detected while patients were in the lateral decubitus position, with the right upper limb elevated. During measurements with the probe in the vertical position, the patients were asked to hold their breath; pipeline structuring was avoided. The sampling depth was 3-4 cm from the body surface. The region of interest was set far away from the pipe and was continuously measured 8-9 times. The shear wave velocity and depth of sampling were recorded. The recorded liver stiffness index was the average value of the measurements.
Liver biopsy method: Liver biopsy was taken within 3 d after ARFI. Beforehand, hepatic function and routine blood tests were done. Liver samples were taken from the region of interest by a Bard Magnum biopsy gun, which was then sliced, stained and observed under a microscope. The Scheuer scheme was adopted as the pathological diagnosis standard. Generally, liver fibrosis is divided into five stages; S0, S1, S2, S3 and S4. S0: no fibrosis; S1: fibrotic portal regions, expanded local perisinusoidal and intralobular fibrosis; S2: fibrosis around portal regions, fibrous septum formation, and lobular structure preservation; S3: a lot of fibrous septum formation and lobular structure disorder without cirrhosis; and S4: early period of cirrhosis[13].
APRI index determination: An automatic biochemistry analyzer and an automated hematology analyzer detected the AST and PLT levels just before, or one day before, the liver biopsy. APRI = AST (ULN)/PLT (109/L)[14].
SPSS 19.0 statistical software was used to measure (mean ± SD) and the t test or variance analysis were used for comparisons. Spearman’s analysis was used to determine any correlations. P < 0.05 indicated that the differences were statistically significant. ROC curve analysis was used to analyze the accuracy of diagnosis of the degrees of hepatic fibrosis. The area under the ROC curve value was close to one, indicating high diagnostic accuracy.
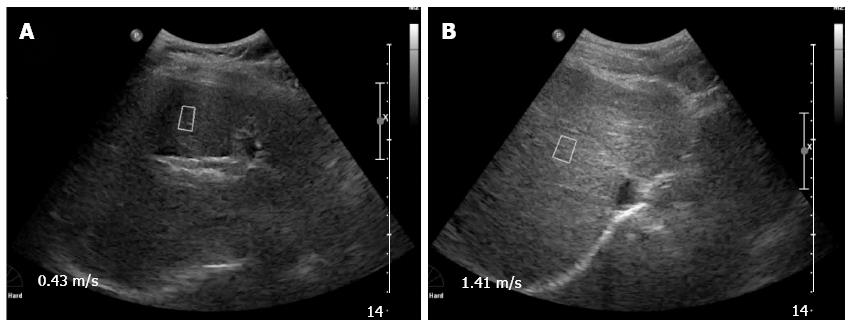
The flexibility or elasticity grade of liver tissue can be obtained from real time ultrasound, after excluding influencing factors, such as intrahepatic vasculature and fat. Representative ARFI detection results are shown in Figure 1. Figure 1A shows ARFI detection of chronic hepatitis C patients without liver fibrosis, while Figure 1B shows ARFI detection of chronic hepatitis C patients with liver fibrosis. The value in Figure 1B is significantly higher than in Figure 1A.
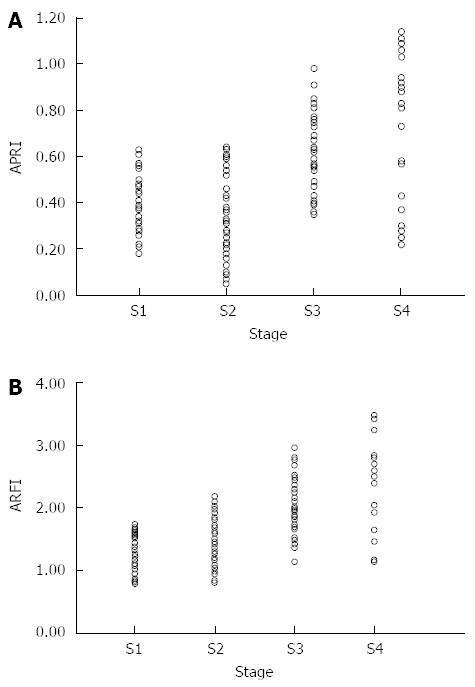
Table 1 shows the comparison of ARFI measurements and APRI in different stages of liver fibrosis of patients with chronic hepatitis C. The results showed that the ARFI and APRI values significantly increased with increasing liver fibrosis; the differences were statistically significant (P < 0.05).
In patients with chronic hepatitis C, ARFI and APRI values were positively correlated with liver fibrosis stage. Both showed increasing trends with increased degree of fibrosis. The Spearman correlation coefficients were 0.649 (P = 0.001) for ARFI and 0.478 (P = 0.001) for APRI (Figure 2).
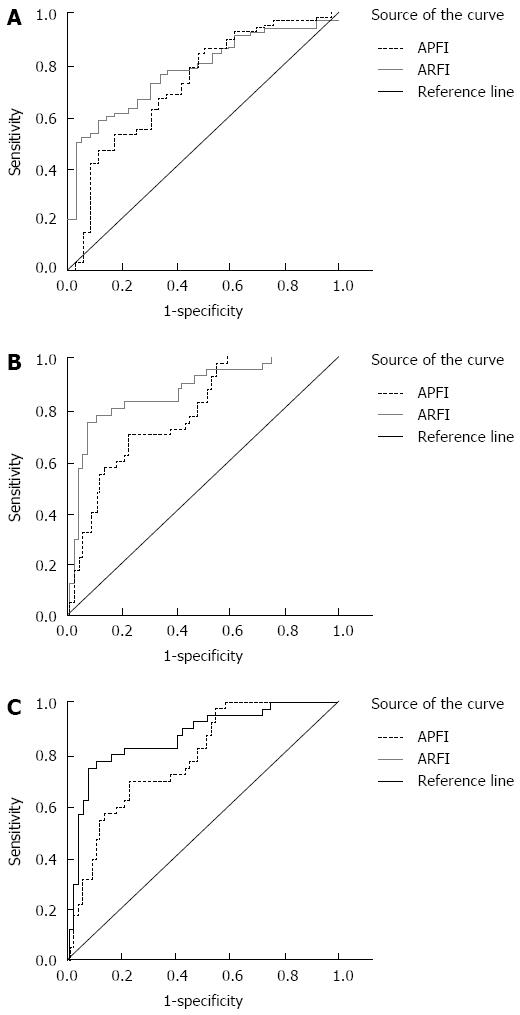
The comparison of diagnostic parameters, such as diagnostic threshold, sensitivity and specificity of ARFI and APRI at different stages of liver fibrosis is shown in Table 2. The ROC curves of different stages are shown in Figure 3. The area under the ROC curve was used to evaluate different degrees of liver fibrosis in patients with hepatitis C. The results showed that in stages ≥ S2, ≥ S3, and = S4; all ARFI values were greater than the corresponding APRI value, indicating that ARFI had a higher credibility rating in detecting liver fibrosis than APRI. The highest value of the ROC curve (0.901) was in the ARFI detection at stage ≥ S3.
| Diagnostic parameters | ≥S2 | ≥S3 | S4 | |||
| ARFI | APRI | ARFI | APRI | ARFI | APRI | |
| Diagnostic threshold | 1.530 | 0.171 | 1.790 | 0.278 | 1.789 | 0.443 |
| Area under curve | 0.775 | 0.721 | 0.901 | 0.787 | 0.792 | 0.780 |
| 95%CI | 0.692-0.867 | 0.620-0.837 | 0.786-0.954 | 0.721-0.886 | 0.664-0.933 | 0.678-0.876 |
| Sensitivity | 0.576 | 0.876 | 0.764 | 0.703 | 0.789 | 0.789 |
| Specificity | 0.895 | 0.564 | 0.965 | 0.765 | 0.754 | 0.812 |
This study used ARFI and APRI models to detect and analyze the degree of liver fibrosis in 128 patients with hepatitis C. The test results of these two methods were assessed and compared to determine their accuracy. Their clinical significance in evaluating liver fibrosis stages of patients with chronic hepatitis C was also determined. The findings of the present study should initiate and promote the use of these noninvasive methods as new tools for quick detection and treatment of patients with chronic hepatitis C.
The incidence of chronic hepatitis C has been increasing gradually, and is a potentially deadly threat to people’s health. Sustained and repeated inflammatory stimuli can cause a stress repair response in the body, leading to the accumulation of a large number of extracellular matrixes in the liver tissue, with accompanying fibrosis hyperplasia[15-19]. Untimely and ineffective degradation of fibrosis can cause cirrhosis or even liver cancer. Therefore, early diagnosis is important for the timely prevention and treatment of liver fibrosis and cirrhosis. Currently, liver biopsy is the main diagnostic method for liver fibrosis. However, it is an invasive method and associated complications limit its safe use[20].
Liver fibrosis assessment was evaluated based on two facts; (1) liver stiffness is caused by fibrosis; and (2) Elastrography - an objective and reliable technique to measure hepatic tissue elasticity[21-24]. Castera et al[25] used transient elastography technology and reported that chronic hepatitis C significantly correlated with the stages of liver fibrosis. However, the disadvantages of early transient elastography findings were their instability and inaccuracy. These disadvantages were influenced by certain factors during assessment, e.g., obesity, width of the intercostal space and ascites. It may also limit the use of elastography in evaluating the degrees of liver fibrosis of patients with hepatitis C[26,27]. ARFI is a new noninvasive real-time dynamic ultrasound imaging technology for evaluating liver tissue elasticity. It can measure the degree of liver fibrosis by detecting and evaluating stages of liver stiffness[22,23,28]. As a complement to ARFI technology, various mathematical modalities for assessing liver fibrosis objectively have ben developed in recent years, such as the AAR index, the APRI and the Shata index. The APRI is a mathematical technique to assess the degree of liver fibrosis. Its advantage is that only two simple biochemical markers (AST and PLT) are used. Therefore, the APRI is receiving more attention as a non-invasive diagnostic tool[15,29,30].
In this study, the comparison of ARFI and APRI in different stages of liver fibrosis showed that, with increasing severity of liver fibrosis, ARFI APRI values increased significantly. ARFI and APRI values at stages S3 and S4 were higher than at S1 and S2 stages. However, the ARFI and APRI values were significantly different from each other. This result was similar to the report of Fierbinteanu-Braticevici et al[7], who also reported that, with increasing degree of liver fibrosis, ARFI and APRI values increased significantly. This implies that ARFI and APRI can effectively evaluate the different stages of liver fibrosis of patients with chronic hepatitis C.
In this study, the degrees of liver fibrosis of patients with chronic hepatitis C were positively correlated with ARFI and APRI values (Spearman correlation coefficients were 0.649 and 0.478, all P < 0.05). Furthermore, a correlation coefficient analysis showed that the correlation between ARFI measurements and the stages of liver fibrosis was better than that of the APRI. The difference may reflect the basic principle of these methods. The theory of ARFI is to emit low-frequency pulse shear waves via the convex array probe. The elastic modulus of the underlying tissue is obtained by longitudinal focal compression and lateral displacement, which are produced by the high frame rate of ultrasonic imaging. The state of elasticity of the liver tissue can be measured without any external pressure[31]. APRI index is calculated using determined AST and PLT levels. AST is easily influenced by liver protection and immuno-enhancement activities, which cannot reflect the real content. Therefore, correlations between the APRI and the stages of liver fibrosis are less accurate than the ARFI.
The area under the ROC curve was used in this study to evaluate the different degrees of liver fibrosis in hepatitis C patients. The results showed that in the stages ≥ S2, ≥ S3, and = S4; all the ARFI values were greater than the corresponding APRI values. Generally, the area under the ROC curve, if greater than 0.8, provides highly credible evidence of the clinical significance of a particular diagnostic method. In this study, only the area under the curve in stages ≥ S3 was greater than 0.8. This suggests that ARFI measurements were more credible than the APRI mathematical model. Friedrich-Rust et al[17] reported that the area under the curve in stages ≥ S2 was greater than 0.8. The results are somewhat different to ours. This might reflect a different sample size or pathological examination standard. Generally, the findings on ARFI technology were similar; i.e., ARFI has a higher diagnostic efficiency for liver fibrosis staging in chronic hepatitis C.
The present study suggested that 1.779 m/s can be used as an ARFI diagnostic threshold to diagnose severe liver fibrosis (S3-S4). Patients with < 1.779 m/s real-time hepatic tissue elasticity had lower grades of fibrosis; therefore, liver biopsy was temporarily postponed for close monitoring, which could help the patients avoid early biopsy and associated complications. In performing an ARFI elastometry, the patient’s respiration and shear-wave measurements, near large vessels, might be unsuccessful because of subcutaneous fat thickness, which can be avoided by careful and professional handling.
In conclusion, both ARFI and APRI could evaluate the degree of liver fibrosis objectively. ARFI measurements were more accurate and provided quantitative values to assess the real time hepatic tissue elasticity states (liver fibrosis stages) for patients with chronic hepatitis C, compared with the APRI. These preliminary findings could be used as an important reference for clinical investigations on noninvasive modalities for diagnosing liver fibrosis stages in patients with chronic hepatitis C.
The degree of liver fibrosis caused by chronic hepatitis C is an important factor affecting the treatment and prognosis of patients with liver diseases. Therefore, quantitative assessment of the stage and grading of liver fibrosis is very important.
Liver biopsy is not suitable for continuously evaluating the degree of liver fibrosis and cirrhosis. At present, acoustic radiation force impulse (ARFI) and the APRI - AST/PLT (aspartate aminotransferase/platelet) - are widely used noninvasive measurements for detecting liver fibrosis.
This study used ARFI and APRI models to detect and analyze the degree of liver fibrosis in 128 patients with hepatitis C; to explore the clinical value of these two methods, and to compare their accuracy in the evaluation of liver fibrosis stages for patients with chronic hepatitis C.
This study attempted to develop practical guidance for diagnostic purposes.
This manuscript is very well written. In this manuscript, the authors explored the diagnostic value of the acoustic radiation force impulse technology and the AST/PLT ratio index for the assessment of liver fibrosis in chronic hepatitis C patients. This manuscript provided a reference for developing practical guidance for diagnosing liver fibrosis in patients with chronic hepatitis C.
P- Reviewer: Okamoto T S- Editor: Ma YJ L- Editor: Stewart G E- Editor: Wang CH
| 1. | Ichino N, Osakabe K, Nishikawa T, Sugiyama H, Kato M, Kitahara S, Hashimoto S, Kawabe N, Harata M, Nitta Y. A new index for non-invasive assessment of liver fibrosis. World J Gastroenterol. 2010;16:4809-4816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Fierbinteanu-Braticevici C, Dina I, Petrisor A, Tribus L, Negreanu L, Carstoiu C. Noninvasive investigations for non alcoholic fatty liver disease and liver fibrosis. World J Gastroenterol. 2010;16:4784-4791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 1541] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 4. | Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, Takahashi H, Yoneda M, Suda T, Zeuzem S. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212-e219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 363] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 5. | Kircheis G, Sagir A, Vogt C, Vom Dahl S, Kubitz R, Häussinger D. Evaluation of acoustic radiation force impulse imaging for determination of liver stiffness using transient elastography as a reference. World J Gastroenterol. 2012;18:1077-1084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Nightingale K. Acoustic Radiation Force Impulse (ARFI) Imaging: a Review. Curr Med Imaging Rev. 2011;7:328-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 7. | Fierbinteanu-Braticevici C, Andronescu D, Usvat R, Cretoiu D, Baicus C, Marinoschi G. Acoustic radiation force imaging sonoelastography for noninvasive staging of liver fibrosis. World J Gastroenterol. 2009;15:5525-5532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 133] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | Nierhoff J, Chávez Ortiz AA, Herrmann E, Zeuzem S, Friedrich-Rust M. The efficiency of acoustic radiation force impulse imaging for the staging of liver fibrosis: a meta-analysis. Eur Radiol. 2013;23:3040-3053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 9. | Shin WG, Park SH, Jang MK, Hahn TH, Kim JB, Lee MS, Kim DJ, Jun SY, Park CK. Aspartate aminotransferase to platelet ratio index (APRI) can predict liver fibrosis in chronic hepatitis B. Dig Liver Dis. 2008;40:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Abd El Rihim AY, Omar RF, Fathalah W, El Attar I, Hafez HA, Ibrahim W. Role of fibroscan and APRI in detection of liver fibrosis: a systematic review and meta-analysis. Arab J Gastroenterol. 2013;14:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 786] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 12. | Chinese Society of Infectious and Parasitic Diseases, Chinese Society of Hepatology Diseases. Prevention and treatment program of viral hepatitis. Zhonghua Ganzangbing Zazhi. 2000;8:324. |
| 13. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2159] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 14. | Snyder N, Gajula L, Xiao SY, Grady J, Luxon B, Lau DT, Soloway R, Petersen J. APRI: an easy and validated predictor of hepatic fibrosis in chronic hepatitis C. J Clin Gastroenterol. 2006;40:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3234] [Article Influence: 147.0] [Reference Citation Analysis (0)] |
| 16. | Hourigan LF, Macdonald GA, Purdie D, Whitehall VH, Shorthouse C, Clouston A, Powell EE. Fibrosis in chronic hepatitis C correlates significantly with body mass index and steatosis. Hepatology. 1999;29:1215-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 476] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 17. | Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, Herrmann E, Poynard T, Dietrich CF, Vermehren J. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 468] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 18. | Rifai K, Cornberg J, Mederacke I, Bahr MJ, Wedemeyer H, Malinski P, Bantel H, Boozari B, Potthoff A, Manns MP. Clinical feasibility of liver elastography by acoustic radiation force impulse imaging (ARFI). Dig Liver Dis. 2011;43:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 19. | Goertz RS, Amann K, Heide R, Bernatik T, Neurath MF, Strobel D. An abdominal and thyroid status with Acoustic Radiation Force Impulse Elastometry--a feasibility study: Acoustic Radiation Force Impulse Elastometry of human organs. Eur J Radiol. 2011;80:e226-e230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1796] [Cited by in RCA: 1847] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 21. | Zhai L, Madden J, Foo WC, Palmeri ML, Mouraviev V, Polascik TJ, Nightingale KR. Acoustic radiation force impulse imaging of human prostates ex vivo. Ultrasound Med Biol. 2010;36:576-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Fahey BJ, Nightingale KR, Nelson RC, Palmeri ML, Trahey GE. Acoustic radiation force impulse imaging of the abdomen: demonstration of feasibility and utility. Ultrasound Med Biol. 2005;31:1185-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Takahashi H, Ono N, Eguchi Y, Eguchi T, Kitajima Y, Kawaguchi Y, Nakashita S, Ozaki I, Mizuta T, Toda S. Evaluation of acoustic radiation force impulse elastography for fibrosis staging of chronic liver disease: a pilot study. Liver Int. 2010;30:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 24. | Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1090] [Cited by in RCA: 1095] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 25. | Castera L, Pinzani M, Bosch J. Non invasive evaluation of portal hypertension using transient elastography. J Hepatol. 2012;56:696-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 26. | Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1077] [Article Influence: 63.4] [Reference Citation Analysis (1)] |
| 27. | Ophir J, Alam SK, Garra B, Kallel F, Konofagou E, Krouskop T, Varghese T. Elastography: ultrasonic estimation and imaging of the elastic properties of tissues. Proc Inst Mech Eng H. 1999;213:203-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 425] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 28. | Nightingale K, Soo MS, Nightingale R, Trahey G. Acoustic radiation force impulse imaging: in vivo demonstration of clinical feasibility. Ultrasound Med Biol. 2002;28:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 646] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 29. | Zhu X, Wang LC, Chen EQ, Chen XB, Chen LY, Liu L, Lei XZ, Liu C, Tang H. Prospective evaluation of FibroScan for the diagnosis of hepatic fibrosis compared with liver biopsy/AST platelet ratio index and FIB-4 in patients with chronic HBV infection. Dig Dis Sci. 2011;56:2742-2749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Sotgia F, Martinez-Outschoorn UE, Howell A, Pestell RG, Pavlides S, Lisanti MP. Caveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanisms. Annu Rev Pathol. 2012;7:423-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 234] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 31. | D’Onofrio M, Gallotti A, Mucelli RP. Tissue quantification with acoustic radiation force impulse imaging: Measurement repeatability and normal values in the healthy liver. AJR Am J Roentgenol. 2010;195:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |