Published online Jul 28, 2014. doi: 10.3748/wjg.v20.i28.9486
Revised: February 8, 2014
Accepted: April 1, 2014
Published online: July 28, 2014
Processing time: 234 Days and 2.9 Hours
AIM: To determine the relationship between host immunity and the characteristics of viral infection or nucleoside analogues (NAs) themselves in patients with chronic hepatitis B (CHB) receiving NA therapy.
METHODS: Fifty-two hepatitis B envelope antigen (HBeAg) positive CHB patients were enrolled and divided equally into two groups. One group received telbivudine (LDT, 600 mg/d), and the other group received lamivudine (LAM, 100 mg/d). Clinical, virological and immunological parameters were assessed at the baseline and at 4, 12, 24, 36 and 48 wk.
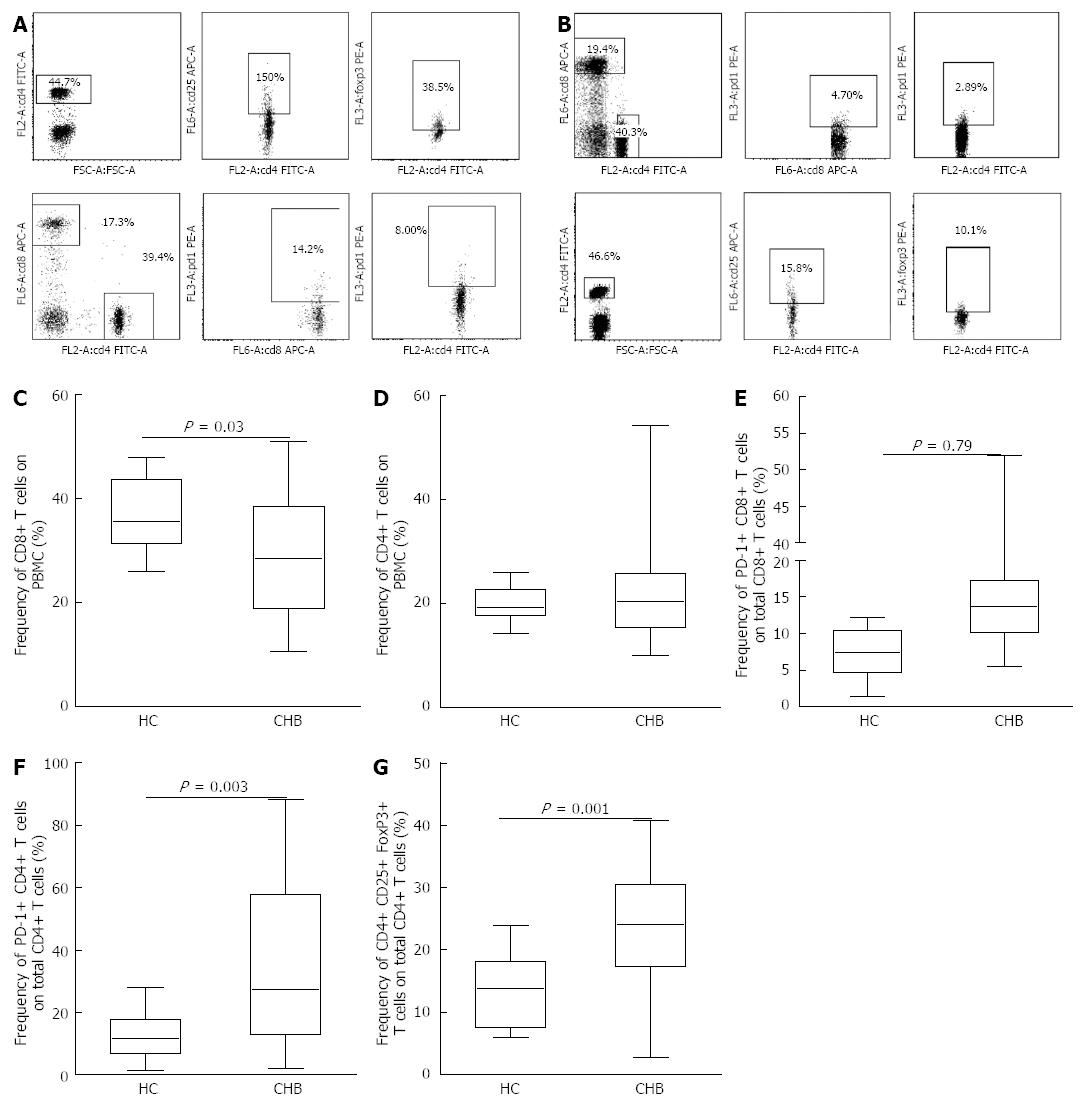
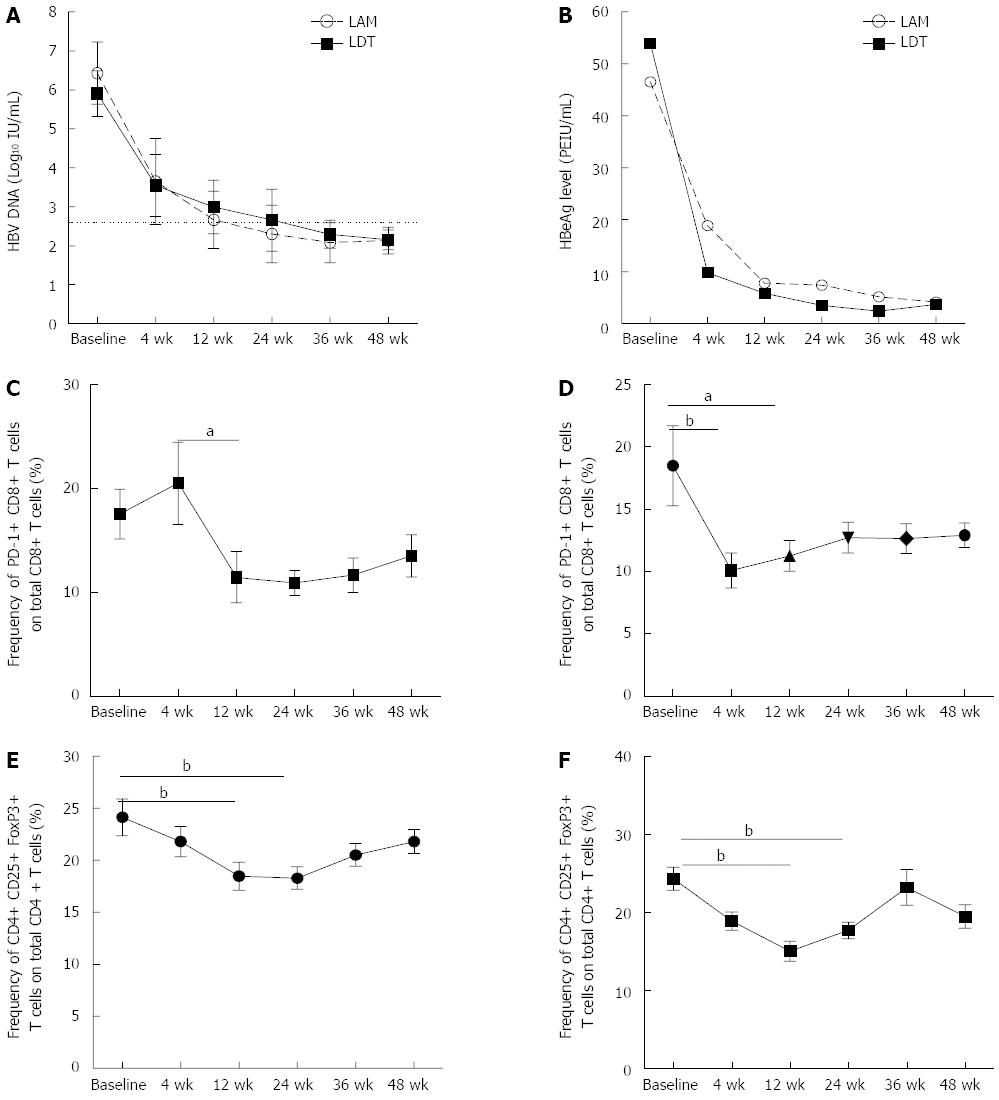
RESULTS: Both groups achieved significant hepatitis B virus (HBV) replication inhibition and alanine aminotransferase normalization at 48 wk. At the baseline, compared to healthy controls, CHB patients had a lower circulating CD8 T cell frequency (29.44% ± 11.55% vs 37.17% ± 7.30%, P = 0.03) and higher frequencies of programmed death 1 positive CD8 T cells (PD-1+ CD8 T) (16.48% ± 10.82% vs 7.02% ± 3.62%, P = 0.0001) and CD4+ CD25+ FoxP3+ T regulatory cells (Tregs) (23.64% ± 9.38% vs 13.60% ± 6.06%, P = 0.001). On therapy, at the beginning 24 wk with the levels of hepatitis B virus deoxyribonucleic acid (HBV DNA) and HBeAg declining, the frequencies of PD-1+ CD8 T cells and Treg cells gradually and significantly declined at 12 and 24 wk in both therapy groups. At treatment week 4, patients treated with LDT had a lower frequency of PD-1+ CD8 T cells compared to patients treated with LAM (10.08% ± 6.83% vs 20.51% ± 20.96%, P = 0.02). The frequency of PD-1+ CD8 T cells in all of the CHB patients was significantly correlated with both the HBV DNA level (r = 0.45, P = 0.01) and HBeAg level (r = 0.47, P = 0.01) at treatment week 24, but the frequency of Treg cells was only significantly correlated with the HBeAg level (r = 0.44,P = 0.02). Furthermore, the ability of CD8 T cells to secrete pro-inflammatory cytokines was partially restored after 24 wk of therapy.
CONCLUSION: NA-mediated HBV suppression could down-regulate the production of negative regulators of host immunity during the first 24 wk of therapy and could partially restore the ability of CD8 T cells to secrete pro-inflammatory cytokines. This immune modulating response may be correlated with the levels of both HBV DNA and HBeAg.
Core tip: We compared the host immunity of hepatitis B envelope antigen (HBeAg)-positive patients treated with telbivudine (LDT) to those treated with lamivudine (LAM) and longitudinally investigated the relationship between two important negative immune modulating factors and viral infection parameters in HBeAg-positive chronic hepatitis B (CHB) patients that were treated with the nucleoside analogues (NAs) LDT and LAM. NA-mediated inhibition of hepatitis B virus replication could cause the downregulation of PD-1+ CD8 T cells and Treg cells during the first 24 wk of therapy and could also partially restore the ability of CD8 T cells to secrete pro-inflammatory cytokines. The immune modulating effect associated with NA treatment in CHB patients was correlated with the levels of both HBV DNA and HBeAg.
- Citation: Li CZ, Hu JJ, Xue JY, Yin W, Liu YY, Fan WH, Xu H, Liang XS. Viral infection parameters not nucleoside analogue itself correlates with host immunity in nucleoside analogue therapy for chronic hepatitis B. World J Gastroenterol 2014; 20(28): 9486-9496
- URL: https://www.wjgnet.com/1007-9327/full/v20/i28/9486.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i28.9486
Persistent infection with hepatitis B virus (HBV) remains a challenging global health problem. Currently more than 370 million people are chronically infected, and this rate is estimated to increase by 4 million per year. Hepatitis B is a leading cause of chronic hepatitis, cirrhosis and hepatocellular carcinoma and accounts for approximately 1 million deaths annually[1]
Multiple types of interferon (IFN), lamivudine (LAM), adefovir dipivoxil (ADV), entecavir (ETV), telbivudine (LDT) and tenofovir disoproxil fumarate have been approved for the treatment of chronic hepatitis B (CHB). These agents vary with respect to antiviral and clinical efficacy, resistance profiles, tolerability and safety[2]. LDT (β-L-2′-deoxythymidine) is an orally bioavailable L-nucleoside with a potent and specific anti-HBV activity[3]. In the absence of head-to-head studies, current cross-trial data suggest that LDT therapy tends to yield a higher rate of HBeAg seroconversion over time, particularly in patients who demonstrate an early virologic response (i.e., undetectable viremia at treatment week 24) compared with other nucleoside agents (NAs)[4-8].
Several studies have shown that NAs have immunomodulating capabilities in patients with CHB. Boni et al[9,10] showed that LAM treatment restores T-cell responsiveness and overcomes cytotoxic T-cell hyporesponsiveness in CHB patients. Recently, Cooksley et al[11] showed that ADV treatment caused increased CD4+ T-cell responses in patients with CHB compared to the placebo. Restoration of the T-lymphocyte subpopulation has been detected in CHB patients treated with ETV[12]. Additionally, recent evidence suggests that interactions between LDT and the host immune system may be involved in its putative benefits[13-16]. Although these data suggest that NAs might restore the host immune system by reducing HBV replication, there remains a lack of evidence of whether there are differences between different NAs. Furthermore, little is known about the relationship between the change in host immunity and hepatitis B envelope antigen (HBeAg) levels during NA therapy.
To gain further understanding of the effects of direct antiviral therapy on host immunity in HBeAg-positive CHB patients, we investigated the longitudinal relationship between two important negative immune modulating factors in chronic HBV infection, PD-1+ CD8 T and FoxP3+ Treg cell frequencies, and measurements of viral infection in HBeAg-positive CHB patients undergoing treatment with LDT and LAM, the most widely prescribed anti-hepatitis B agents worldwide. We also examined pro-inflammatory cytokine secretion by T cells in some patients on antiviral therapy.
Fifty-two treatment-naïve patients with CHB who attended Changhai Hospital (Shanghai, China) were enrolled in the study. All patients were seropositive for hepatitis B surface antigen (HBsAg) and HBeAg for at least 6 mo, with HBV deoxyribonucleic acid (HBV-DNA) levels ≥ 105 IU/mL. All patients were negative for anti-hepatitis D virus (anti-HDV), anti-hepatitis C virus (anti-HCV), anti-human immunodeficiency virus 1/2 (anti-HIV1/2), and autoantibodies. Forty-four of the 52 patients were male, and the mean age of all patients was 39.0 ± 10.0 years.
The recruited healthy controls (HCs, n = 14) had no previous history or current evidence of any liver disease, with normal serum ALT values. They were also negative for HBsAg, anti-hepatitis A virus, anti-HCV, and anti-HIV IgM antibodies.
Patients were randomly assigned into two therapy groups in a 1:1 ratio; one group received LAM (100 mg/d), whereas the other group received LDT (600 mg/d), and both groups were followed serially with protocol visits for a period of 12 mo. The nature and possible consequences of this study were explained to all patients, and all of them gave written informed consent. The study protocol was approved by the Ethics Committee of Changhai Hospital. The study was registered on ClinicalTrials.gov with the identifier NCT01480492.
Clinical, virological, and immunological parameters were assessed in the studied patients at the baseline and at 4, 12, 24, 36 and 48 wk during antiviral therapy.
At each assessment, the patients were evaluated for HBV-DNA, HBeAg, hepatitis B envelope antibody, HBsAg, and hepatitis B surface antibody. An adverse event inquiry was completed, and blood samples were drawn for immunoassays, blood chemistry and hematology.
HBsAg, anti-HBs, total and IgM hepatitis B core antibody and HBeAg were determined using a chemiluminescent microparticle immunoassay (Abbott Laboratories, North Chicago, IL). The S/CO values of HBeAg were converted to PEI U/mL with a HBeAg quantitation conversion formula[17]. Anti-HCV, anti-HDV, anti-HGV, anti-HIV-1, and anti-HIV-2 antibodies were measured using commercially available kits (Abbott Laboratories, North Chicago, IL) in our clinical lab. Serum HBV-DNA levels were measured by fluorescent quantitative PCR with commercially available kits (PE/B/MJ/L, Shenzhen, China) according to the manufacturer’s instructions. The threshold of the HBV DNA detection limit was 500 IU/mL. All specimens were examined in the Clinical Laboratory Center of Changhai Hospital, Shanghai.
EDTA- and heparin-anticoagulated blood (5-7 mL) was collected from each patient and used directly for fluorescence-activated cell sorting (FACS) or for peripheral blood mononuclear cell (PBMC) isolation. PBMCs (2 × 106-6 × 106) were isolated by Ficoll-Hypaque density gradient centrifugation, washed twice in phosphate-buffered saline and analyzed immediately.
For PD-1 expression on CD4 and CD8 T cells, peripheral blood (100 μL) was stained with fluorescein isothiocyanate (FITC)-conjugated anti-human CD4 (San Diego, CA), allophycocyanin (APC)-conjugated anti-human CD8 (San Diego, CA) and phycoerythrin (PE)-conjugated anti-PD-1 monoclonal antibodies (BD Biosciences, San Jose, CA) according to the manufacturers’ instructions. Flow cytometry was performed using a FACSCalibur (Becton Dickinson, San Jose, CA). FACS data were analyzed using the CellQuest software (Becton Dickinson Rutherford, NJ).
For FoxP3+ Treg cell examination, peripheral blood (100 μL) was first surface-stained with FITC-conjugated anti-human CD4 antibody and APC-conjugated anti-human CD25 antibody for 30 min, then lysed with FACSTM lysis solution (BD Pharmingen) and treated with fix/perm mixture (eBiosciences) according to the manufacturer’s instructions. Finally, the cells were incubated with PE-conjugated anti-human FoxP3 antibody overnight. Isotope controls were used to ensure antibody specificity. The stained cells were analyzed by flow cytometry.
Ex vivo-purified PBMCs were stimulated at 2 × 106-3 × 106 cells/mL in RPMI-1640 medium containing phorbol myristate acetate (0.1 μg per 100 μL), ionomycin (0.75 μg per 100 μL) and 1 µL Brefeldin A (Sigma Aldrich, Poole, Dorset, United Kingdom), then incubated for 6 h at 37 °C with 5% CO2. The cells were washed, stained with APC-conjugated anti-CD8 antibody, permeabilized and fixed using Cytofix/Cytoperm (Pharmingen, San Diego, CA, United States) according to the manufacturer’s instructions. FITC-conjugated anti-interleukin-2 (IL-2) and APC-conjugated anti-IFN-γ antibodies were added (30 min, 22 °C), washed twice and analyzed by flow cytometry.
ANOVA with Tukey’s multiple comparison test was used to compare the means of the independent groups. Mean log10 values of HBV DNA at the baseline and at follow-up were analyzed using Student’s t-test. The R value was calculated for the HBV DNA or HBeAg levels and for the frequencies of Treg or PD-1+ CD8 T and PD-1+ CD4 T cells at the baseline and during the follow-up visits. The frequencies of IFN-γ and IL-2 producing T cells derived from patients before and during antiviral treatment were compared using Student’s t-test, and paired data were compared using the Mann-Whitney U test. Frequencies of significant proliferative responses were compared using χ2 analysis. P < 0.05 was considered statistically significant.
The clinical characteristics of the 52 CHB patients and age- and sex-matched HCs enrolled in this study are shown in Table 1. The patient population was predominantly male, with raised serum ALT and elevated HBV DNA levels compared to the HCs. Of the 52 patients, 28 were in the LAM therapy group and 24 received LDT. Both patient groups had similar baseline characteristics for AST, ALT, serum albumin, bilirubin levels and HBV DNA or HBeAg levels.
| HCs (n = 14) | LAM (n = 28) | LDT (n = 24) | P (LAM vs LDT) | |
| Sex ratio (male/female) | 11:3 | 26:2 | 18:6 | NS |
| Age (mean ± SD) | 40 ± 11.4 | 39.86 ± 10.9 | 34.71 ± 8.40 | NS |
| ALT median (U/L, range) | 30 (30-34) | 145 (60-453) | 227 (60-957) | NS |
| AST median (U/L, range) | 23 (17-30) | 87.5 (20-274) | 129.5 (29-585) | NS |
| TBil median (μmol/L, range) | 4.0 (3.0-7.0) | 19.25 (7.7-35.6) | 37.5 (35-47) | NS |
| Median albumin (g/dL, range) | 3.6 (2.4-4.4) | 3.7 (3.4-4.7) | NS | |
| HBV DNA (Log10 IU/mL) (mean ± SD) | 6.28 ± 0.78 | 5.85 ± 0.64 | NS | |
| HBeAg (PEIU/mL) (mean ± SD) | 46.53 ± 48.63 | 53.91 ± 83.59 | NS |
After antiviral treatment with LAM or LDT for 48 wk, the LDT group achieved a higher HBeAg/HBeAb seroconversion rate than the LAM group (18.18% vs 7.69%, P = 0.57). No patient achieved HBsAg/HBsAb seroconversion in either group. Serum HBV loads were significantly reduced in both groups as early as at treatment week 12 (HBV DNA PCR negative rate; LAM: 53.86% vs LDT: 55.56%). By treatment week 48, all patients had undetectable serum HBV loads, and serum ALT returned to normal. No patients withdrew from the treatment due to intolerable side effects.
At baseline, the patients with CHB had significantly lower levels of CD8 T cells (29.44% ± 11.55%) than the HCs (37.17% ± 7.30%, P = 0.03) (Figure 1C). The frequency of CD4 T cells did not change significantly in the CHB patients compared to the HCs. Next, we determined the expression of PD-1 in both CD4 and CD8 T cells and found that the CHB patients had higher frequencies of PD-1 expression in both CD4 T cells (34.10% ± 24.26%) and CD8 T cells (16.48% ± 10.82%) compared to the HCs (12.75% ± 8.03% for CD4, P = 0.003; vs 7.02% ± 3.62% for CD8, P = 0.0001) (Figure 1E and F). Patients with CHB also had a significantly higher frequency of Treg cells (23.64% ± 9.38%) than the HCs (13.60% ± 6.06%, P = 0.001) (Figure 1G).
The HBV DNA level decreased by more than 2 logs from the pretreatment level at week 12 in both treatment groups (Figure 2A), while the HBeAg level decreased to more than 5 times lower than the pretreatment level at week 12 in both groups (Figure 2B). In addition to these changes, the negative regulators of host immunity, including PD-1 expression on CD8 T cells and the frequency of Treg cells, also decreased (Figure 2C-F). In patients receiving LAM, there was no obvious change in the frequencies of PD-1+ CD8 T cells and Treg cells at week 4 (baseline vs 4 wk; 17.53% ± 12.61% vs 20.96% ± 20.53%, P = NS for PD-1 and 24.14% ± 9.40% vs 21.81% ± 7.70%, P = NS for Treg), even though both the HBV DNA and HBeAg levels declined significantly (Figure 2C). However, in patients receiving LDT, there was a significant decrease in the frequency of PD-1+ CD8 T cells at treatment week 4 compared to the baseline (Figure 2D).
At treatment week 12, there was a significant reduction in the frequencies of both Treg cells and PD-1+ CD8 T cells in patients receiving either LAM or LDT treatment (Figure 2C-F). At week 24, the only significant difference was observed in the frequency of Treg cells compared to the baseline in both groups (Figure 2E and F). At treatment week 36, the frequencies of both PD-1+ CD8 T cells and Treg cells increased again in both treatment groups, though there was continued reduction in the HBV DNA and HBeAg levels on therapy compared to the baseline (Figure 2A and B).
Finally, we compared the frequencies of PD-1+ CD8 T cells and Treg cells in the two groups at different time points during therapy and found that there were no differences between the two groups at any time point, except at week 4, when the LDT therapy group had a lower frequency of PD-1+ CD8 T cells than the LAM group (10.08% ± 6.83% vs 20.51% ± 20.96%, P = 0.02).
Based on the similar pattern of changes in host immunity in the two groups, we can infer that the reduction in virus levels caused by the antiviral therapy induced the change in the host immune system and that this change was not due to the NAs themselves. Thus, we combined the two groups to analyze the correlation between viral load and changes in the host immune system.
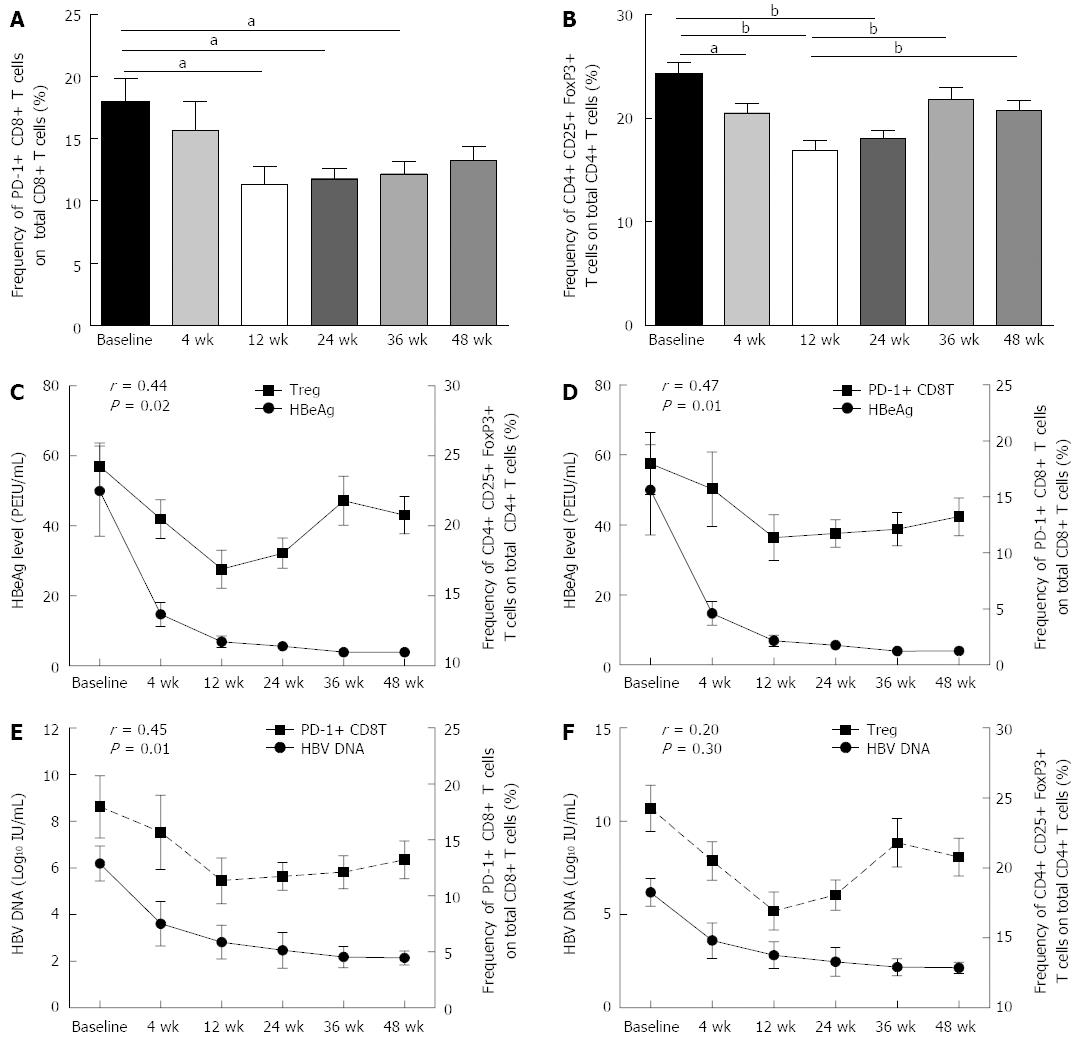
During therapy, the frequencies of PD-1+ CD8 T cells and Treg cells decreased gradually, and at treatment weeks 12 and 24, the frequencies of both PD-1+ CD8 T cells and Treg cells significantly decreased from the baseline levels (Figure 3A and B).
In the first 24 wk of treatment, the frequencies of PD-1+ CD8 T cells and Treg cells declined concurrently with the decline of the HBV DNA and HBeAg levels. At week 24, the viral load and HBeAg level were positively correlated with the frequencies of PD-1+ CD8 T cells and Treg cells (Figure 3C-F). However, the trend towards decline only continued to week 36, and then the frequencies of PD-1+ CD8 T cells and Treg cells both increased gradually from week 36, even though the viral load and HBeAg level declined continually (Figure 3C-F).
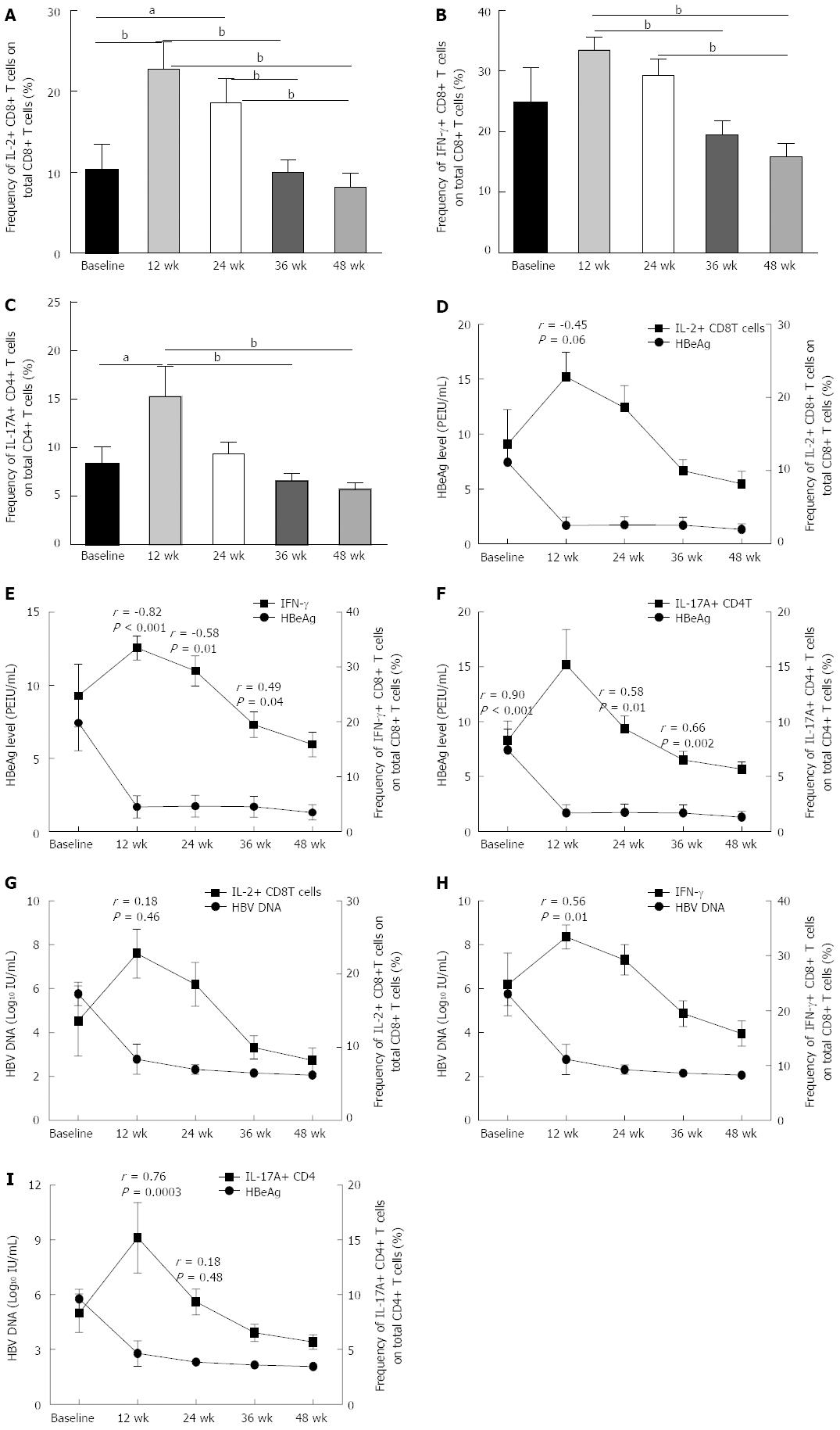
To determine whether the decrease in viral load or HBeAg level caused by antiviral therapy could affect the quantity and quality of CD8 and CD4 T cells, we determined the frequencies of pro-inflammatory cytokine-secreting CD8 T cells and IL-17A+ CD4 T cells during the treatment and analyzed the correlations between viral load or HBeAg level and the frequency of pro-inflammatory cytokine-secreting CD8 T and CD4 T cells at different time points. We found that along with the decline in viral load, the frequencies of pro-inflammatory cytokine-secreting CD8 T cells, IL-2 and IFN-γ positive CD8 T cells increased significantly (Figure 4A-C). At treatment week 12, the frequencies of pro-inflammatory cytokine-secreting CD8 T cells, IL-2 and IFN-γ positive CD8 T cells increased to the highest level compared to all other time points during therapy (Figure 4A-C). From treatment week 24 on, though the viral load and HBeAg level continued to decline, the pro-inflammatory cytokine secreting ability of CD8 and CD4 T cells began to decline, and at treatment weeks 36 and 48, the frequencies of pro-inflammatory cytokine-secreting CD8 and CD4 T cells declined significantly compared to those at treatment week 12 (Figure 4A-C).
We further analyzed the correlation between the frequency of pro-inflammatory cytokine-secreting CD8 or CD4 T cells with the viral load or HBeAg level at time points when viral replication was significantly inhibited by NAs. We found that at treatment week 12, the HBeAg level was negatively correlated with the frequency of IL-2+ and IFN-γ+ CD8 T cells (Figure 4D and E). At treatment weeks 24 and 36, though the quantities of pro-inflammatory cytokine-secreting CD8 and CD4 T cells decreased slightly compared to those at treatment week 12, the HBeAg level was still significantly correlated with the frequency of IFN-γ+ CD8 T cells (Figure 4E). Notably, the viral load was only negatively correlated with the frequency of IFN-γ+ CD8 T cells at treatment week 12, while the viral load was positively correlated with the frequency of IL-17A+ CD4 T cells at baseline and at treatment weeks 12, 24 and 36 (Figure 4G-I).
The results of this novel study demonstrate that both LAM and LDT can significantly suppress HBV replication as early as at week 4 of treatment. Along with the suppression of HBV replication by LAM or LDT, both groups displayed the downregulation of negative immune regulators, including PD-1 expression on CD8 T cells and the frequency of Treg cells. A lower Treg cell frequency was observed at weeks 12 and 24 in both treatment groups, but downregulation of PD-1 expression on CD8 T cells only occurred in patients receiving LDT at weeks 4 and 12. Unfortunately, the decrease in negative immune regulators was only maintained through treatment week 24, although the HBV DNA and HBeAg levels continued to decline. These results suggest that the downregulation of negative immune regulators during NA therapy was primarily related to the decline in viral load and HBeAg level and was not directly related to the NAs themselves. Furthermore, the rebound of negative immune regulators in the later periods of antiviral therapy may contribute to the low HBeAg conversion rate and HBV persistence in patients treated with NAs.
Cellular immunity plays a critical role in clearing the virus from HBV-infected individuals[9,10,18,19]. Differences in the host immune response may significantly affect the outcome of HBV infection. Previous studies have suggested that Treg cells and PD-1 expression on T cells play a major role in the fine-tuning of T cell functions during chronic HBV infection[20-24]. We also observed that the immune profile of HBeAg+ CHB patients consisted of a significantly lower CD8+ T cell frequency and higher frequencies of Treg cells and PD-1+ CD8 and CD4 T cells (Figure 1). It is plausible that Treg activity and PD-1 expression on T cells may be induced by HBV replication. Recent studies have found that reducing the HBV viral load is followed by a decrease in the Treg cell frequency and lower PD-1 expression on host T cells[25-27]. We found that in the first 24 wk of treatment, in addition to a reduction in viral load, there was also a reduction in the Treg cell frequency, and the frequency of PD-1 expressing CD8 T cells decreased gradually. Particularly at week 24, these negative immune regulatory factors decreased significantly, and their reduction was positively correlated with the change in HBV DNA level (Figure 3E and F). These results suggested that the HBV load plays an important role in host immune tolerance.
The function of the HBV precore or HBeAg is largely unknown because it is not required for viral assembly, infection, or replication. However, HBeAg does appear to play a role in viral persistence. It has been suggested that HBeAg may promote chronic HBV infection by functioning as an immunoregulatory protein. Peng et al[23] found that HBeAg persistence in CHB patients likely increased the expression of PD-1 and CTLA-4 on HBV-specific CD8 T cells, which may be associated with the low intensity of T cell responses and the high HBV DNA load. Evans et al[13] longitudinally studied 18 HBeAg-positive patients undergoing treatment with direct antivirals to determine the relationship between treatment-induced viremia reduction and HBeAg seroconversion with respect to PD-1 levels and T-cell reactivity and found that HBeAg seroconversion (in 6/18 patients) resulted in a further PD-1 decrease, with a 50% reduction in the frequency of PD-1+ CD8 T cells, which was not observed in patients that remained HBeAg-positive. We found that in the first 24 wk of therapy, along with a decline in the HBeAg level, the frequencies of both PD-1+ CD8 T cells and Treg cells decreased gradually, and at week 24, the HBeAg level was positively correlated with both the PD-1+ CD8 T cell and the Treg cell frequencies (Figure 3D and E). Furthermore, we determined the correlation between the HBeAg level and the ability of CD8 T cells to secrete pro-inflammatory cytokines and found that in the first 24 wk of therapy, concurrent with the decline in HBeAg level, the percentage of IL-12 and IFN-γ+ CD8 T cells gradually increased, and at some time points, the HBeAg level was negatively correlated with the ability of CD8 T cells to secrete pro-inflammatory cytokines. All these data suggested that HBeAg drives the upregulation of negative immune regulatory factors in chronic HBV infection, resulting in T cell impairment.
It is well recognized that oral NAs demonstrate superior antiviral potency, but with relatively lower HBeAg seroconversion rates compared to PegIFN[28]. Notably, compared with the other oral NAs, relatively higher HBeAg responses have been reported with LDT treatment[5,6]. In the GLOBE study participants (baseline ALT ≥ 2 × ULN), HBeAg loss was achieved for 41% of the patients treated with LDT for 2 years vs 32% in the LAM group (P = 0.021); 36% of the patients in the LDT group achieved HBeAg seroconversion vs 27% in the LAM group (P = 0.022)[8]. Currently, we know little about why patients treated with LDT experienced a higher HBeAg response. Recently, some researchers have described the immune modulatory effects associated with LDT and other NA treatments in CHB patients. The two main categories of the immune response are adaptive immunity (e.g., CD4+/CD8+ T cells, Th1/Th2, Treg, PD-1/PD-L1, Th17, IL-21, TFH) and innate immunity (e.g., NK, NKT, TLR, dendritic cells and macrophages)[9-16,18-21]. We first directly compared the immune systems of HBeAg-positive patients treated with LDT vs LAM and found that the main negative immune regulators involved in chronic HBV infection in both groups declined along with virus replication, but there were no significant differences between the two groups. We only found that the mean frequency of PD-1+ CD8 T cells in patients treated with LDT was lower than that in patients treated with LAM during treatment week 4 (LAM: 20.51% ± 20.96% vs LDT: 10.08% ± 6.83%, P = 0.02). Further analysis revealed that the difference may be caused by the significant decline in the HBeAg level in patients receiving LDT treatment from the baseline to week 4. Our results not only further provide evidence of the immune modulatory effect associated with NA treatment in CHB patients but also suggested that the immune modulatory effect was associated with the decrease in the levels of HBV DNA or HBeAg caused by NA therapy.
In summary, we have demonstrated a role for distinct negative immune modulators in chronic HBV infection. Direct inhibition of HBV replication by NA treatment could cause the down-regulation of the host negative immune modulators PD-1 on CD8 T cells and a decrease in the frequency of FoxP3+ Treg cells in the first 24 wk of therapy and could also restore the ability of CD8 T cells to secrete pro-inflammatory cytokines. The immune modulatory effect associated with NA treatment in CHB patients was correlated with the HBV DNA and HBeAg levels. Unfortunately, we did not carry out accurate quantitative examination of HBsAg during the study, and thus we cannot determine whether host immune changes during therapy were also correlated with the HBsAg level.
We would like to thank all of the patients enrolled in this study for their kind understanding and support.
Different nucleoside agents (NAs) lead to different hepatitis B envelope antigen (HBeAg) conversation rates. NA therapy accompanied short-term host immune restoration. However, until now, there has been a lack of evidence on whether there are differences between different NAs, and little is known about the relationship between the changes in host immunity and the HBeAg level during NA therapy.
Several studies have shown that NA therapy could cause short-term restoration of the host immune response.
In this study, the authors compared the immune systems of HBeAg-positive patients treated with telbivudine (LDT) or lamivudine (LAM) head-to-head and longitudinally investigated the relationship between PD-1 expression on CD8 T cells and the frequency of Treg cells with the virological parameters of HBeAg-positive chronic hepatitis B (CHB) patients receiving treatment with LDT or LAM. Direct inhibition of hepatitis B virus (HBV) replication by NA treatment could decrease the frequencies of PD-1+ CD8 T cells and FTreg cells during the first 24 wk of therapy and could also restore the ability of CD8 T cells to secrete pro-inflammatory cytokines. The immune modulatory effect associated with NA treatment in CHB patients was correlated with the HBV DNA and HBeAg levels.
By discussing the alterations in the host immune systems of HBeAg-positive CHB patients during treatment with different NAs, this study may present a future strategy for combined therapy with immune modulators and NAs for these patients.
This is an interesting paper with a reasonable hypothesis, logical workflow and nice illustration. The data suggested that NAs might restore the host immune response by reducing HBV replication, but there is a lack of evidence on whether there are differences between different NAs; additionally, little is known about the relationship between the alterations in the host immune response and the HBeAg levels during nucleoside analogue therapy. The aim of this paper was to determine the relationship between host immunity and virological parameters or the NAs themselves in patients with chronic hepatitis B receiving treatment with different NAs.
P- Reviewer: Yang YF, Wong GLH S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373:582-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 993] [Article Influence: 62.1] [Reference Citation Analysis (1)] |
| 2. | Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1794] [Cited by in RCA: 1778] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 3. | Standring DN, Bridges EG, Placidi L, Faraj A, Loi AG, Pierra C, Dukhan D, Gosselin G, Imbach JL, Hernandez B. Antiviral beta-L-nucleosides specific for hepatitis B virus infection. Antivir Chem Chemother. 2001;12 Suppl 1:119-129. [PubMed] |
| 4. | Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, Chen Y, Heathcote EJ, Rasenack J, Bzowej N. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357:2576-2588. [PubMed] |
| 5. | Hou J, Yin YK, Xu D, Tan D, Niu J, Zhou X, Wang Y, Zhu L, He Y, Ren H. Telbivudine versus lamivudine in Chinese patients with chronic hepatitis B: Results at 1 year of a randomized, double-blind trial. Hepatology. 2008;47:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Liaw YF, Gane E, Leung N, Zeuzem S, Wang Y, Lai CL, Heathcote EJ, Manns M, Bzowej N, Niu J. 2-Year GLOBE trial results: telbivudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136:486-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 443] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 7. | Liaw YF, Lau GK, Kao JH, Gane E. Hepatitis B e antigen seroconversion: a critical event in chronic hepatitis B virus infection. Dig Dis Sci. 2010;55:2727-2734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Gane EJ, Wang Y, Liaw YF, Hou J, Thongsawat S, Wan M, Moon YM, Jia J, Chao YC, Niu J. Efficacy and safety of prolonged 3-year telbivudine treatment in patients with chronic hepatitis B. Liver Int. 2011;31:676-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Boni C, Bertoletti A, Penna A, Cavalli A, Pilli M, Urbani S, Scognamiglio P, Boehme R, Panebianco R, Fiaccadori F. Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B. J Clin Invest. 1998;102:968-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 369] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 10. | Boni C, Penna A, Ogg GS, Bertoletti A, Pilli M, Cavallo C, Cavalli A, Urbani S, Boehme R, Panebianco R. Lamivudine treatment can overcome cytotoxic T-cell hyporesponsiveness in chronic hepatitis B: new perspectives for immune therapy. Hepatology. 2001;33:963-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 275] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 11. | Cooksley H, Chokshi S, Maayan Y, Wedemeyer H, Andreone P, Gilson R, Warnes T, Paganin S, Zoulim F, Frederick D. Hepatitis B virus e antigen loss during adefovir dipivoxil therapy is associated with enhanced virus-specific CD4+ T-cell reactivity. Antimicrob Agents Chemother. 2008;52:312-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | You J, Sriplung H, Geater A, Chongsuvivatwong V, Zhuang L, Li YL, Lei H, Liu J, Chen HY, Tang BZ. Impact of viral replication inhibition by entecavir on peripheral T lymphocyte subpopulations in chronic hepatitis B patients. BMC Infect Dis. 2008;8:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Evans A, Riva A, Cooksley H, Phillips S, Puranik S, Nathwani A, Brett S, Chokshi S, Naoumov NV. Programmed death 1 expression during antiviral treatment of chronic hepatitis B: Impact of hepatitis B e-antigen seroconversion. Hepatology. 2008;48:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Wu ZG, Yan WM, Guo W, Chen T, Zou Y, Wang HW, Wang XJ, Yang XJ, Lu YL, Luo XP. Telbivudine preserves T-helper 1 cytokine production and downregulates programmed death ligand 1 in a mouse model of viral hepatitis. J Viral Hepat. 2010;17 Suppl 1:24-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Shi TD, Zhang JM, Wang XF, Chen M, Sun H, Chen CB, Ren H. Effects of antiviral therapy with Telbivudine on peripheral iNKT cells in HBeAg(+) chronic hepatitis B patients. Clin Exp Med. 2012;12:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Jiang X, Zhang M, Lai Q, Huang X, Li Y, Sun J, Abbott WG, Ma S, Hou J. Restored circulating invariant NKT cells are associated with viral control in patients with chronic hepatitis B. PLoS One. 2011;6:e28871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Xu J. Quantitative detection of serum HBeAg levels using Paul-Ehrlich international (PEI) reference standard on the Architect platform. Hepatol Int. 2012;6:7-10. |
| 18. | Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 764] [Article Influence: 34.7] [Reference Citation Analysis (1)] |
| 19. | Webster GJ, Reignat S, Brown D, Ogg GS, Jones L, Seneviratne SL, Williams R, Dusheiko G, Bertoletti A. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J Virol. 2004;78:5707-5719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 330] [Article Influence: 15.7] [Reference Citation Analysis (1)] |
| 20. | Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, Kusters JG, Janssen HL. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology. 2005;41:771-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 407] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 21. | Xu D, Fu J, Jin L, Zhang H, Zhou C, Zou Z, Zhao JM, Zhang B, Shi M, Ding X. Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol. 2006;177:739-747. [PubMed] |
| 22. | Liang XS, Zhou Y, Li CZ, Wan MB. Natural course of chronic hepatitis B is characterized by changing patterns of programmed death type-1 of CD8-positive T cells. World J Gastroenterol. 2010;16:618-624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Peng G, Luo B, Li J, Zhao D, Wu W, Chen F, Chen Z. Hepatitis B e-antigen persistency is associated with the properties of HBV-specific CD8 T cells in CHB patients. J Clin Immunol. 2011;31:195-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Ye P, Weng ZH, Zhang SL, Zhang JA, Zhao L, Dong JH, Jie SH, Pang R, Wei RH. Programmed death-1 expression is associated with the disease status in hepatitis B virus infection. World J Gastroenterol. 2008;14:4551-4557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Nan XP, Zhang Y, Yu HT, Sun RL, Peng MJ, Li Y, Su WJ, Lian JQ, Wang JP, Bai XF. Inhibition of viral replication downregulates CD4(+)CD25(high) regulatory T cells and programmed death-ligand 1 in chronic hepatitis B. Viral Immunol. 2012;25:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Zheng Y, Huang Z, Chen X, Tian Y, Tang J, Zhang Y, Zhang X, Zhou J, Mao Q, Ni B. Effects of telbivudine treatment on the circulating CD4⁺ T-cell subpopulations in chronic hepatitis B patients. Mediators Inflamm. 2012;2012:789859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | TrehanPati N, Kotillil S, Hissar SS, Shrivastava S, Khanam A, Sukriti S, Mishra SK, Sarin SK. Circulating Tregs correlate with viral load reduction in chronic HBV-treated patients with tenofovir disoproxil fumarate. J Clin Immunol. 2011;31:509-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, Jeffers L, Goodman Z, Wulfsohn MS, Xiong S. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1060] [Cited by in RCA: 1019] [Article Influence: 46.3] [Reference Citation Analysis (0)] |