INTRODUCTION
Malignant bile duct obstruction can be a devastating consequence of pancreatic cancer. Its development may contribute to poor outcomes including cholangitis, delay in treatment (including chemotherapy or surgery), decreased quality of life and increased mortality. Pancreatic ductal adenocarcinoma has a dismal five-year survival rate of only 6%, and biliary obstruction correlates with decreased survival times[1]. As many as 70% of patients have some degree of biliary obstruction at the time of their initial diagnosis with pancreatic cancer[2]. In most of these situations, the adverse nature of biliary obstruction can be improved with decompression. For purposes of palliation, decompression can improve patient comfort by relieving jaundice and pruritus[3]. It can also facilitate treatment by allowing total bilirubin levels to drop to less than 1.5 times the upper limit of normal, which is necessary to prevent toxicity in some chemotherapy regimens[4].
The role of biliary stents in achieving biliary decompression has been well established for the past 20 years, with more recent studies showing an increased role for self-expandable metal stents (SEMSs) compared to plastic stents[5-10]. Endoscopic biliary stenting is technically successful in over 90% of attempted cases[9]. Thus, endoscopic retrograde cholangiopancreaticogram (ERCP) with biliary stent placement has become the standard of care in situations where biliary decompression is desired.
The purpose of this article is to review the efficacy and outcomes of different strategies including stenting in relieving malignant bile duct obstruction. The strategy and choice of stent may differ based on clinical scenario and disease stage, including resectable disease, locally advanced disease treated with neoadjuvant therapy, and metastatic disease in which only palliative therapy is available. Second-line methods including percutaneous drainage, endoscopic ultrasound (EUS) guided biliary drainage and surgical bypass will be discussed and compared to current methods utilized as the standard of care. In cases of concurrent gastric outlet obstruction and malignant biliary obstruction, strategies such as double stenting, double surgical bypass, and EUS guided biliary drainage with duodenal stenting will also be discussed.
BACKGROUND ON STENTS
Polyethylene or plastic stents are used for relief of biliary obstruction in numerous settings, and offer excellent patency for short-term use. These stents are available in multiple diameters ranging from 7 French to 11.5 French, though 10 French stents are the most commonly used with distal common bile duct obstruction. The benefits of polyethylene stents include low cost for the prosthesis itself, as well as removability at the time of surgical procedures.
One of the factors that initially led to plastic stents being used preferentially in pancreatic cancer was the notion that uncovered SEMS could complicate pancreaticoduodenectomy by interfering with transection of the bile duct proximal to the resection specimen[6]. Experience has shown that as long as > 2 cm of common hepatic duct is exposed proximal to the SEMS, then surgery is no more complex than in the presence of a plastic stent[7]. Thus, the choice of stents in treatment of malignant biliary obstruction relies on other factors such as cost-effectiveness, expected length of survival, and certainty of the diagnosis of malignancy.
Any stent is subject to occlusion in the setting of distal common bile duct obstruction, though the mechanisms can differ by stent design. For plastic stents, the development of biofilm and bacterial colonization is the most important factor[8]. For uncovered SEMS, tissue ingrowth through the mesh interstices at the level of the tumor remains the most likely source of occlusion. For partially or fully covered SEMS, occlusion may occur due to stent migration, overgrowth of tissue at the ends of the stent, or food debris. Duodenal contents can also flow back up the biliary system in a retrograde fashion, as demonstrated by contrast studies[11]. This duodenal biliary reflux may cause stent occlusion in any type of stent.
RESECTABLE DISEASE
The choice of modality for decompression varies by the clinical setting and expected treatment for each patient. For pancreatic cancer without local advancement or metastases, prompt surgical resection is the definitive method of treatment and the only hope of cure[12]. There is debate over whether jaundice should be relieved prior to surgery. In theory, relieving jaundice would improve surgical outcomes by overcoming the impaired immune response and coagulopathy associated with cholestasis. Over the past 10 years, multiple studies have examined the role of biliary decompression with stenting in localized disease prior to pancreaticoduodenectomy. In many cases, preoperative biliary drainage was found to be associated with increased complications including infections, abscesses, pancreatic fistulas and wound infection[13,14]. Authors have hypothesized that these complications are due to infection of bile from instrumentation with foreign objects into the biliary system[13,15].
In 2010, a Dutch multicenter randomized trial demonstrated that preoperative biliary drainage and stenting was associated with increased complications compared to surgery alone in resectable disease[16]. In this trial, 202 patients were randomized to undergo either preoperative biliary drainage followed by surgery within 4-6 wk, or surgery alone within 1 wk of diagnosis. Rates of serious complications were 39% in the early - surgery group and 74% in the group with biliary drainage (RR = 0.54, P < 0.001). There was also no mortality benefit or shortened length of stay with preoperative drainage.
The trial garnered much attention due to its large size, as well as its randomized prospective multicenter design. However, some methodological issues may limit the generalizability of the above conclusions. As the preoperative biliary drainage group waited 4-6 wk for surgery, one could theorize that a shorter interval between stent placement and surgery may have allowed fewer stent-related complications. (The authors chose this length of time prior to surgery to allow normal synthetic and clearance functions of the liver). The use of plastic stents rather than larger diameter SEMS was also cited as a factor in the poor performance of the biliary decompression group. These issues may warrant further studies to address them and may guide clinical practice.
Based on the data from this study, routine preoperative biliary decompression is not currently recommended. However, for patients who present with cholangitis or intractable pruritus stent placement is an appropriate intervention prior to pancreaticoduodenectomy.
LOCALLY ADVANCED DISEASE AND NEOADJUVANT THERAPY
Plastic stents vs self-expanding metal stents
The poor long-term survival for pancreatic cancer even in surgically resected disease has prompted interest in the use of neoadjuvant therapy in operable pancreatic cancer to improve patient survival[17]. This strategy has also been used in locally advanced cases that may require down- staging to permit eventual surgical resection. Patients who receive neoadjuvant therapy such as gemcitabine-based regimens require biliary decompression to allow the safe use of such chemotherapeutic agents[4]. Biliary stenting during the neoadjuvant period has been the common method for achieving this decompression, with the goal of stent patency up until surgery. Unfortunately, the performance of plastic stents during the preoperative period has been lackluster. A retrospective study showed that the median time from stent placement to surgery was 150 d, while the median duration of stent patency was 134.5 d[5]. In order to achieve a longer duration of stent patency during this time, SEMSs have been studies for use rather than plastic stents.
Multiple studies have now demonstrated that the use of SEMSs in such patients lead to improved outcomes during neoadjuvant therapy[5,6,10,11,18]. A retrospective review of plastic stent performance during neoadjuvant therapy revealed that over half the patients with plastic stents required repeat stent exchange due to stent occlusion or cholangitis[5]. Adams et al[10] demonstrated in 2012 that in a retrospective cohort of 52 patients, the complication rate was almost 7 times higher with plastic stents. It was also estimated the rate of hospitalization for these cases was 3 times higher than patients with metal stents. Although SEMSs are more expensive than plastic stents, data thus far indicates that their superiority in patency and improved patient outcomes make them the safer and ultimately the more cost effective choice for patients in whom attempted surgical resection is planned[19].
TYPES OF SEMSs: NO PERFECT CHOICE
More recent literature has focused on comparing the different types of SEMS for use in patients undergoing neoadjuvant therapy or in palliative cases. Major categories of SEMSs include uncovered (USEMSs) and covered (CSEMSs) groups. USEMSs have a mesh design that allows them to embed in the biliary duct wall but it also makes them susceptible to tissue ingrowth, which can lead to occlusion in as many as 20% of patients. CSEMSs were designed to prevent tissue ingrowth, but because of this they are known to have increased rates of migration[20].
Several studies have demonstrated the trade-off between tissue ingrowth in USEMS and migration in CSEMS. A meta-analysis by Saleem et al[21] concluded that CSEMSs had a significantly longer duration of patency than USEMSs (average of 61 more days) in palliative cases, but also noted their increased incidence in migration (RR = 8.11). The study also noted that CSEMSs and USEMSs had similar rates of cholecystitis (approximately 2% in each group). A more recent retrospective cohort study by Lee et al[22] had different conclusions, with no difference in overall recurrent obstruction (CSEMSs 35% vs USEMSs 38%) among 749 patients. While tumor ingrowth associated with obstruction was higher in the USEMS group (76% vs 9%, P < 0.001), other mechanisms of obstruction still occurred with CSEMSs, including tumor overgrowth, sludge formation, food debris, and migration. CSEMSs were found to have higher rates of migration (36% vs 2%, P < 0.001) and acute pancreatitis (6% vs 1%, P < 0.001). Despite its large size, limitations to the study include its nonrandomized and retrospective design, with lack of uniform follow-up data for patients.
In an effort to decrease rates of migration while maintaining the patency achieved with CSEMSs, partially covered SEMSs have also been used in practice. Through subgroup analysis, Saleem et al[21] did not find any difference in rate of migration or stent patency in partially covered SEMSs compared to fully covered SEMSs. A multicenter randomized trial by Telford et al[23] compared partially covered SEMSs to USEMSs, and found no significant difference in rates of obstruction and patient survival. It did note a statistically significant increase in adverse events (62% vs 44%, P = 0.046) and stent migration (12% vs 0%, P = 0.0061) with partially covered SEMSs. Limitations to this study included some imbalance in the distribution of patients to the treatment group, and difficulties in recruiting an adequate number of patients.
A more recent study by Kitano et al[24] demonstrated the use of a modified CSEMS aimed at reducing stent migration. The anti-migration system in this model of CSEMS used low axial force and uncovered flare ends, and was compared to USEMSs of a similar design. A total of 120 patients were included in the prospective randomized multicenter study. Patients were randomized to receive the modified CSEMS or USEMS. The CSEMS cohort had significantly longer durations of stent patency (mean of 219.3 d vs 166.9 d, P = 0.047) and less need for reintervention (23% vs 37%, P = 0.08) compared to USEMSs. The rate of tumor ingrowth was also significantly less in the CSEMSs group (0% vs 25%, P < 0.01). Neither group demonstrated stent migration; survival time (median 285 d in CSEMSs vs 223 d in USEMSs, P = 0.68) and serious adverse events also did not differ significantly. It would be useful for a study to compare these CSEMSs with partially covered SEMSs and USEMSs concurrently to show which type has overall superiority.
The limitations found in each category of SEMSs make it difficult to use one type as the ideal stent for biliary decompression in obstruction caused by pancreatic cancer. In addition, some of the studies do have conflicting data, which indicates the need for further investigation to determine which type of stent should be used in the future. While SEMS show improved patency rates compared to plastic stents, occlusion still occurs with disturbing frequency. A recent Korean review of SEMS patency among 107 patients with unresectable pancreatic cancer showed stent occlusion in 36% of patients during a median survival period of 33 d[25]. This appears consistent with the experience of other large studies as detailed above. There is still a need for other types of SEMSs that would minimize the flaws of existing designs.
ALTERNATIVE STENT DESIGNS AND STRATEGIES
Drug eluting stents have been designed in an attempt to improve SEMS patency by eluting a chemotherapeutic agent such as paclitaxel to prevent tumor ingrowth and stent occlusion[26]. Prior studies have shown that they effectively inhibit cells responsible for stent occlusion[27] and can be safely used in animal models[28] and humans[29]. Recently Jang et al[30] conducted a multicenter prospective comparative study to compare the efficacy of this type of stent to covered SEMS in patients with unresectable distal malignant biliary obstruction. In a non-randomized fashion, 60 patients were enrolled into a paclitaxel coated SEMS group while 46 were enrolled to the covered SEMS group. There was no significant difference in rates of stent patency between both groups. There are ongoing efforts to design new drug eluting stents with different chemotherapeutic agents such as gemcitabine. Further trials are needed to determine whether these stents can improve upon the performance of the current generation of SEMSs.
Other stents have been designed to prevent reflux of duodenal contents into the biliary system, which is another known cause of stent occlusion[11]. Dua et al[31] demonstrated through a randomized prospective trial that an anti-reflux plastic biliary stent (AR-PBS) could stay patent for a longer duration compared to traditional plastic stents (median patency 145 d for AR-PBS compared to 101 d for PBS, P = 0.002). Subsequent studies focused on the use of anti-reflux metal stents (ARMSs). In a retrospective single center case series, Hu et al[32] described the use and outcomes of ARMSs in 23 patients. Median patency of the stents was 14 mo and overall patency was reported at 3, 6 and 12 mo (95%, 74%, 56% respectively). A separate study group designed their own anti-reflux SEMS (AR-SEMS), and in their own prospective case series described the placement of AR-SEMSs in five patients with unresectable hilar malignant biliary obstruction[33]. Stent occlusion occurred early in four patients with patency durations ranging 4-26 d, and the fifth patient’s stent remained patent for 235 d. The authors noted that the outcomes may have been different from Hu et al[32] due to differences in stent design, as their stent was an end-flared type. Hu et al[32], in comparison, used stents that were hemispheric type and were covered in a hemispheric silicon membrane. Further studies need to be conducted to confirm the efficacy of AR-SEMSs and show superiority to current stents before they can be applied to more widespread use.
RECOMMENDED STRATEGY FOR STENTING IN LOCALLY ADVANCED DISEASE
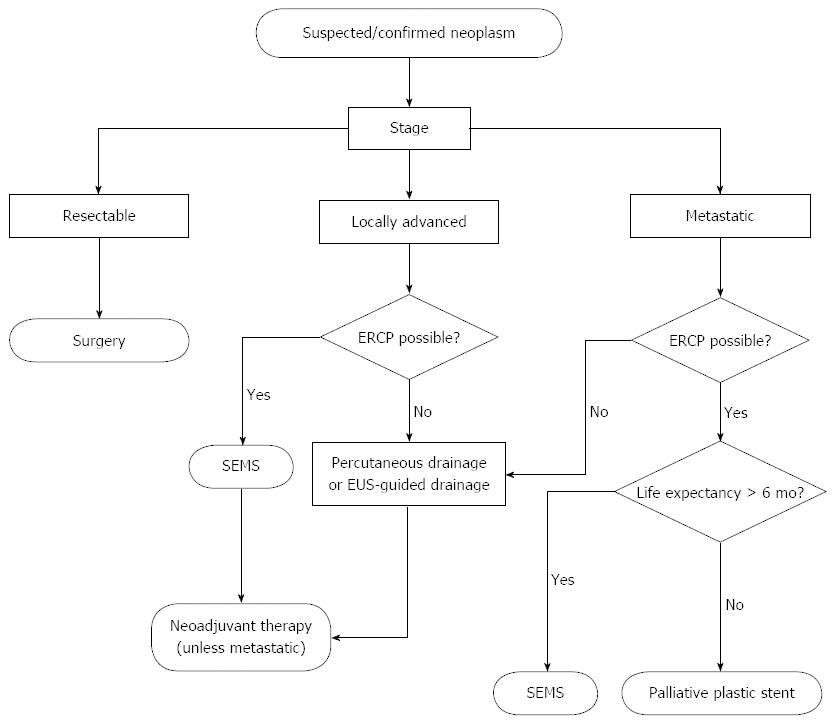
The use of a plastic stent for biliary decompression in locally advanced disease appears largely unwarranted based on the studies reviewed above. However, practitioners may be hesitant to place a SEMS if the diagnosis of malignancy is uncertain at that time of ERCP. Routine use of EUS-guided fine needle aspiration with on-site cytologic review is limited to certain centers, and the differential diagnosis for distal common bile duct strictures may include chronic pancreatitis or autoimmune cholangiopathy, in which case a removable stent is the best option. With the recent availability of fully covered SEMS and data to show their comparable efficacy with uncovered SEMS, the use of a fully covered SEMS appears to be a sound strategy when suspicion of malignancy is high and life expectancy is greater than 4 mo (Figure 1).
Figure 1 Algorithm for relief of malignant biliary obstruction from pancreas cancer.
SEMS: Self-expanding metallic stents; ERCP: Endoscopic retrograde cholangiopancreaticogram; EUS: Endoscopic ultrasound.
Palliative stenting
Another area of focus in malignant biliary obstruction is the placement of stents for palliative purposes in incurable pancreatic cancer. The rationale for their placement is similar in many respects to that of patients undergoing neoadjuvant therapy: relief of jaundice and pruritus, normalization of bilirubin levels to allow palliative chemotherapy, and prevention of other adverse outcomes such as cholangitis and frequent hospitalizations. However in patients with advanced disease and shorter life expectancies, it may be difficult in justifying the use of SEMSs as long- term patency is less of a goal in these cases. A meta-analysis by Moss et al[19] showed that SEMSs cost 15-40 times more than most plastic stents, and are only cost effective if the patient survives > 4 mo. One of the included studies demonstrated that the presence of liver metastases was independently related to survival (P < 0.0005 with multivariate analysis), with a median survival of 2.7 mo compared to 5.3 mo without liver metastases[34]. Cost analysis demonstrated that plastic stents were more cost effective in the patients with liver metastases compared to SEMSs. Soderlund et al[35] reached similar conclusions, showing that the survival period for patients with distant metastases was similar to patency time in plastic stents. They concluded that SEMSs should only be reserved for patients who did not have distant metastases. While some other prognostic factors have been identified to predict mortality in pancreatic cancer, the data is limited and generally used to determine surgical risk[36-38].
Recently there has been growing use of double layer stents (DLSs) as a cost-effective alternative to SEMSs in palliative cases. Such stents are designed with a stiff outer layer to allow stricture cannulation and a smooth inner layer that is less likely to occlude. A recent retrospective review demonstrated DLSs had a longer duration of stent patency than plastic stents (95 d vs 59 d, P = 0.014). There was also no significant difference in patency between the DLS and SEMS group[39]. These results were consistent to past studies[40-42], and may pave the way in routine use of DLSs in palliative relief of obstruction due to advanced pancreatic cancer.
OTHER METHODS FOR BILIARY DRAINAGE
Percutaneous transhepatic biliary drainage
In cases where patients are not candidates for ERCP or have failed attempted transpapillary stent placement, percutaneous transhepatic cholangiography (PTC) has traditionally been used as a method for biliary drainage. This method can offer the same benefits of biliary decompression in improving patient comfort and preventing adverse outcomes. In most cases an internal-external biliary drain is passed through the site of malignant biliary obstruction to the duodenum, where it can reestablish internal bile drainage and normal enterohepatic circulation. Efforts are made to discontinue the external drainage component unless it continues to have high output or the patient is in a state of sepsis, in which case internalization is delayed. In some of these situations, or cases when exclusively external percutaneous biliary drains are placed due to inability to transverse the site of obstruction, having continued external drainage can be cumbersome and uncomfortable for patients. External drains can require significant maintenance, including emptying and flushing of the drain as well as routine drain exchange to prevent occlusion[43]. It should also be noted that PTC can cause bacteremia, cholangitis and hemobilia. Internal-external and external biliary drains can also be prone to leakage, dislodgement and obstruction. However, in cases when ERCP fails these drains are an appropriate means of biliary decompression.
Percutaneous stent placement
Percutaneous stent placement has been another option to relieve malignant biliary obstruction, but historically have been avoided for similar reasons as above. In a study conducted 25 years ago, Speer et al[44] randomized patients into groups receiving biliary stents by percutaneous or endoscopic routes. It concluded that endoscopically placed stents had improved efficacy and lower mortality, due to complications including hemorrhage and bile leaks in the percutaneous group. These results have contributed to the use of endoscopic stent placement as first-line treatment. However, recent studies using improved technology including the percutaneous placement of SEMSs have shown to be safe and effective[45-47]. This may lead to more routine us of percutaneous stent placement, especially in cases when ERCP is not possible.
EUS guided biliary drainage
Other endoscopic alternatives are being used in relieving malignant biliary obstruction not amenable to stent placement via ERCP. Endoscopic ultrasound-guided biliary drainage (EUS-BD) has been demonstrated to be a safe and effective means of biliary drainage. EUS-BD can be achieved by multiple techniques including EUS-guided rendezvous, EUS-guided choledochoduodenostomy (EUS-CDS), and EUS- guided hepatic gastrostomy (EUS-HGS)[48]. In situations when the endoscope can reach the ampulla, rendezvous can be achieved by inserting an FNA needle into the common bile duct or left intrahepatic duct under EUS guidance, followed by navigating a guidewire through the bile duct past the stricture into the duodenum. A duodenoscope can then be used to allow over-the-wire cannulation with ERCP and retrograde stent placement. When the papilla cannot be reached due to malignant obstruction of the duodenum, EUS-CDS and EUS- HGS offer ways to create tracts to the bile duct with extrahepatic and intrahepatic approaches respectively. Both these methods also utilize guidewires that are advanced past the ampulla into the duodenum, which is followed by anterograde dilation of the tract and stent placement. At this time, these procedures remain technically complex and limited to high-volume centers with expertise in therapeutic endoscopy[49]. Complications may include bile leak, bleeding, or pneumoperitoneum. Future trials will further assess the efficacy of these methods and seek to improve their feasibility and safety.
Adjunctive techniques may hold some promise for improving the long-term performance of stents in malignant obstruction. An emerging method known as endobiliary bipolar radiofrequency ablation (RFA) has been able to achieve safe and effective biliary decompression and patency. The procedure involves placement of a bipolar RFA catheter across the biliary stricture under fluoroscopic guidance, with subsequent energy delivery to cause local tumor necrosis. In an open-label pilot study, Steel et al[50] recruited 22 patients with unresectable obstruction with the goal of using bipolar RFA prior to placing uncovered SEMSs in each patient. In all, 21 of the patients had successful RFA catheter deployment and SEMS placement. At 90 d, 76% (16/21) of those patients still had stent patency. Further studies are investigating this novel method, and will need to determine the long-term efficacy and safety of RFA, along with direct comparison to existing SEMS performance.
Surgical biliary drainage
Finally, surgical biliary bypass for drainage remains an option. Glazer et al[51] performed a meta-analysis of trials comparing surgical bypass and endoscopic stent placement in patients with unresectable pancreatic cancer. Recurrent biliary obstruction was less likely in surgically treated patients (3.1% vs 28.7% of stent-treated patients) over a mean survival time of 4 mo. The length of hospital stay for either treatment type was fairly long (21.8 d for surgical bypass, 14.6 d for stent treatment) and the majority of the studies used older data which preceded the use of SEMS. Another meta-analysis using the same studies demonstrated high rate of complications in surgical bypass, but similar overall mortality to patients treated with endoscopically placed stents[52]. Both of these meta-analyses included studies in which plastic biliary stents were the stent of choice; newer data comparing surgical bypass to SEMS is lacking but might be expected to yield a different result favoring endoscopic treatment. Nonetheless, surgical biliary bypass remains an appropriate treatment for patients with a life expectancy exceeding six months.
MANAGEMENT OF CONCURRENT GASTRIC OUTLET AND BILIARY OBSTRUCTION
Surgical bypass can also be used to relieve concomitant malignant biliary and gastric outlet obstruction caused by pancreatic cancer. However many patients with this presentation are either poor surgical candidates, or may decline a comparatively invasive surgery that would include both gastrojejunostomy and biliary bypass. With the advent of enteral and biliary SEMSs in the past decade, biliary and gastroduodenal obstruction can be relieved using endoscopic double stenting in a safe and less invasive manner. In 2002, Kaw et al[53] conducted a retrospective review of 18 patients who underwent simultaneous biliary and duodenal SEMS placement. Median survival time for the cohort was 78 d and only 4 out of 18 patients experienced recurrent obstruction (2 with biliary obstruction and 2 with duodenal obstruction; all were successfully stented). Similar results have been obtained in subsequent studies[54-56], with SEMS placement at the duodenal stricture followed by an endoscopic approach to the papilla for ERCP guided biliary stent placement. If the stricture involved the papilla, biliary stenting was performed through the mesh of the duodenal SEMS with high rate of success. In patients where the transpapillary approach fails, EUS-BD can be performed after the initial endoscopic duodenal SEMS placement to allow biliary stenting[55,56]. Given the limited use of EUS-BD and its recent development, this approach may be limited to high volume centers.
CONCLUSION
Malignant biliary obstruction caused by pancreatic cancer is associated with poor outcomes and decreased survival in patients. Biliary decompression through the interventions discussed in this review can be performed to improve patient quality of life and mortality. Although the available data seems to indicate that resectable disease should proceed straight to surgery, there may still be benefit in preoperative stenting if surgery will be delayed or if jaundice is associated with complications. The role for stenting in biliary decompression is clearer in locally advanced disease and with incurable patients for palliative purposes.
Numerous studies have shown the overall superiority of SEMS compared to plastic stents in terms of long-term stent patency and improved patient outcomes. At this time, there is no ideal type of SEMS as both uncovered and covered SEMSs are associated with their own benefits and limitations. A recent study has demonstrated a modified covered SEMS with less migration and similar patency to traditional CSEMS, but further studies using this stent will be needed to demonstrate clear superiority. Drug eluting stents may offer a more effective option in the future, although current designs have not shown superiority to the current generation of SEMSs. In palliative stenting, it is potentially not cost effective to use SEMSs in patients with shorter life expectancy given their expense relative to plastic stents. The presence or absence of distant metastases can help guide what type of stent should be used, but predictive mortality models may offer a way to further stratify patients in an accurate and cost effective fashion. Double layer stents may provide a less expensive option for some patients, while still maintaining superiority to regular plastic stents.
Although percutaneous transhepatic cholangiography with internal-external and external drainage catheters have been used when ERCP and stent placement is not possible, it has some disadvantages such as patient discomfort, need for maintenance and routine exchanges, and can be associated with hemobilia, cholangitis and sepsis. Percutaneous SEMS placement may be an underutilized strategy given successful trials utilizing it, but it can still have some of the complications mentioned above. EUS-guided biliary drainage and RFA ablation may provide a better alternative, but require more research into their safety and efficacy. Surgical bypass may be appropriate in cases when life expectancy exceeds 6 months, or in patients with concomitant duodenal obstruction. Endoscopic double SEMS placement or EUS-BD with endoscopic duodenal stent placement may be safe and less invasive methods for palliation of malignant and duodenal obstruction due to pancreatic cancer.
P- Reviewer: Ho CS, Lim JB, Morris-Stiff G, Nakai Y S- Editor: Zhai HH L- Editor: A E- Editor: Ma S