Published online Jul 21, 2014. doi: 10.3748/wjg.v20.i27.9146
Revised: March 16, 2014
Accepted: April 15, 2014
Published online: July 21, 2014
Processing time: 181 Days and 0.6 Hours
AIM: To investigate perioperative outcomes in patients undergoing modified laparoscopic splenectomy or open splenectomy and azygoportal disconnection for portal hypertension.
METHODS: This study included 44 patients who underwent modified laparoscopic splenectomy and azygoportal disconnection (MLSD) and 71 who underwent open procedures for portal hypertension. Blood samples were collected before surgery and on days 1, 3, and 7 after surgery. Markers of liver and renal function, C-reactive protein (CRP), interleukin-6 (IL-6), and procalcitonin (PCT) were measured, and perioperative variables were compared between the two groups.
RESULTS: The modified laparoscopic group showed significantly better and faster recovery, better liver and renal function, and fewer complications than the open group. CRP, IL-6, and PCT concentrations on postoperative days 1, 3, and 7 were significantly lower in the modified laparoscopic group than in the open group.
CONCLUSION: MLSD was associated with lower inflammatory immune responses, less impairment of liver and renal function, and faster and better recovery.
Core tip: Minimal surgical trauma is an important goal to both surgeons and patients. A novel technique, in which massively enlarged spleens are removed from the abdominal cavity with an electromechanical morcellator through an existing 12-mm port, was first developed by our surgical team for laparoscopic splenectomy and azygoportal disconnection, and greatly reduces surgical trauma for cirrhotic patients with bleeding portal hypertension and secondary hypersplenism. This technique resulted in minimal postoperative pain and scarring, faster and better postoperative recovery, and lower inflammatory immune responses.
-
Citation: Jiang GQ, Chen P, Qian JJ, Yao J, Wang XD, Jin SJ, Bai DS. Perioperative advantages of modified laparoscopic
vs open splenectomy and azygoportal disconnection. World J Gastroenterol 2014; 20(27): 9146-9153 - URL: https://www.wjgnet.com/1007-9327/full/v20/i27/9146.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i27.9146
Surgical trauma results in the activation of systemic immunologic and inflammatory responses; a process called surgical stress. Acute inflammatory responses are initiated by direct tissue trauma caused by incisions, dissections, organ manipulation, and vascular compromise[1-4]. The minimally invasive nature of laparoscopic surgery is thought to generate weaker systemic immune and inflammatory responses than traditional open surgery. These weaker responses are likely caused by the minimal manipulation of organs, as well as smaller surgical incisions.
Modified laparoscopic splenectomy and azygoportal disconnection (MLSD), which was first developed by our surgical team, is a novel technique in which massively enlarged spleens are removed from the abdominal cavity with an electromechanical morcellator through an existing 12-mm port[5]. Although resulting in less surgical trauma than open procedures, little is known about the exact pathophysiologic mechanisms that occur during MLSD. Other types of laparoscopic surgical techniques have shown immunologic advantages over traditional open surgery[1,3,6], with lower concentrations of interleukin-6 (IL-6) and C-reactive protein (CRP) induced by laparoscopic than by open surgical procedures[7-10]. Laparoscopic splenectomy and azygoportal disconnection (LSD) has become increasingly popular for the treatment of cirrhotic patients with bleeding portal hypertension and secondary hypersplenism. However, the immunologic effects of LSD, and especially MLSD, have not yet been well clarified. To our knowledge, this report is the first to compare the immunologic effects of MLSD vs open splenectomy and azygoportal disconnection (OSD). We therefore compared systemic inflammatory indices and perioperative outcomes in patients undergoing MLSD or OSD for portal hypertension, focusing specifically on the immunologic markers IL-6, the main indicator of surgical trauma[11]; CRP, as an important acute phase reactant produced by the liver; and procalcitonin (PCT), a pro-peptide of calcitonin produced by the thyroid gland and an early and specific biologic marker of infection.
All cirrhotic patients who underwent LSD or MLSD for bleeding portal hypertension and secondary hypersplenism at the Clinical Medical College of Yangzhou University in China from January 2010 and May 2013 were eligible for inclusion. Data on these patients were retrospectively entered into a database. Of the 115 cirrhotic patients with bleeding portal hypertension and secondary hypersplenism, 44 elected to undergo MLSD and 71 chose OSD.
This study was not a randomized trial. During the pre-operation discussion, all patients were informed that MLSD is a minimally invasive procedure, but still in the experimental stage compared with the more typical OSD. Procedures were selected by individual patients, who provided written informed consent. This study was approved by the Ethics Committee of the Clinical Medical College of Yangzhou University.
Data collected included patient gender, age, etiology of cirrhosis, Acute Physiology and Chronic Health Evaluation (APACHE) II score, Child-Pugh class, longitudinal diameter of the spleen, operation time, estimated intraoperative blood loss, and volume of intraoperative blood transfusion. Other factors analyzed included Visual Analogue Scale (VAS) pain score on the first day after surgery, time to first oral intake, first passage of flatus, and off-bed activity, postoperative hospital stay, perioperative complications, number of days of postoperative body temperature > 38.0 °C, and incidence of non-fever and normal white blood cell (WBC) counts on postoperative days 1, 3, and 7. Blood analysis included WBC count, hemoglobin (Hb) concentration, platelet (PLT) count; and concentrations of aspartate transaminase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine (CER), CRP, IL-6, and PCT determined preoperatively and 1, 3, and 7 d after surgery.
VAS pain score was evaluated by direct interview using a questionnaire that rated pain intensity on a scale of 0-10[12-14], with 0 representing no pain and 10 representing very severe pain.
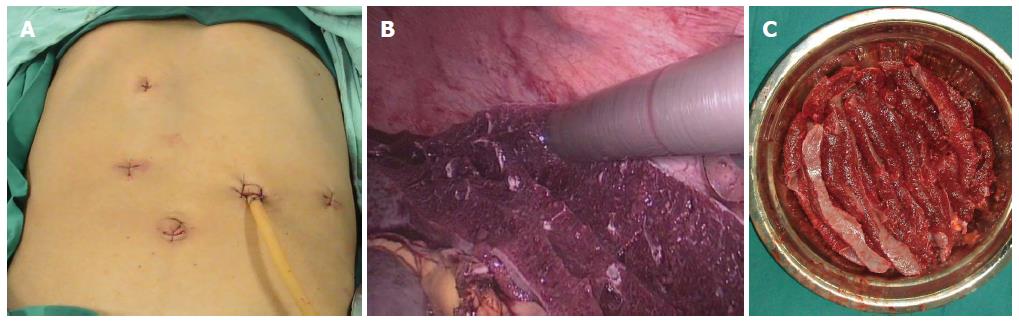
MLSD: After induction and intubation, patients received general anesthesia and were placed in the supine and parted-legs position. A pneumoperitoneum of 13 mm Hg was obtained with a Veress needle. A five-port method (Figure 1A) was used, including one 5-mm, three 10-mm, and one 12-mm port. The splenic artery was dissociated and clipped with a hem-o-lok, and the ligaments surrounding the spleen were divided with a LigaSure vessel-sealing device (Covidien, Boulder, CO, United States). The splenic artery and vein were transected en bloc through the 12-mm port using a linear laparoscopic vascular stapler (EndoGIA). During laparoscopic azygoportal disconnection, all paraesophageal venous collaterals were divided by the LigaSure vessel-sealing device, from back to front, from below to above, and from left to right.
The spleen was removed from the abdominal cavity through the 12-mm port using an electromechanical morcellator (TSCS, Hangzhou, China), consisting of a motor-driven cutting tube and large claw forceps. The spleen was grasped by these forceps through the cutting tube and extracted by rolling the tube (Figure 1B), allowing a cylindrical spleen sample (Figure 1C) to be cut and removed from the tube using a mild pulling force. These steps were repeated until the entire spleen was removed. The entire upper quadrant was irrigated and carefully inspected for residual tissue and bleeding.
OSD: OSD was performed through either a midline laparotomy or a left subcostal incision using traditional methods. Splenectomy was performed before azygoportal disconnection.
Data are presented as the mean ± SD, median (range), or number (%). Group means were compared using Student’s t-test or the Mann-Whitney U test, as appropriate, and χ2 tests were used to compare percentages. A P value < 0.05 was considered significant. SPSS 13.0 software (SPSS, Chicago, IL, United States) was used for statistical analysis.
Of 115 cirrhotic patients with bleeding portal hypertension and secondary hypersplenism, 44 underwent MLSD and 71 underwent OSD. There were no significant differences between groups in terms of patient gender, age, etiology of cirrhosis, APACHE II score, Child-Pugh class, longitudinal spleen diameter, preoperative WBC counts, or preoperative Hb, PLT, TBIL, AST, ALT, BUN, and CER concentrations (Table 1).
| Variable | MLSD (n = 44) | OSD (n = 71) | P value |
| Gender, M/F, n | 27/17 | 41/30 | 0.701 |
| Age, mean ± SD, yr | 54.98 ± 10.41 | 52.46 ± 10.33 | 0.209 |
| Etiology, n | |||
| HBV cirrhosis | 23 | 46 | 0.183 |
| HCV cirrhosis | 3 | 5 | 0.963 |
| Schistosome cirrhosis | 6 | 9 | 0.882 |
| Alcoholic cirrhosis | 3 | 4 | 0.796 |
| Autoimmunity liver cirrhosis | 9 | 7 | 0.111 |
| APACHE II score, mean ± SD | 3.32 ± 2.27 | 3.49 ± 2.57 | 0.712 |
| Child-Pugh classification, A/B, n | 29/15 | 39/32 | 0.244 |
| Longitudinal diameter of spleen, mean ± SD, mm | 179.45 ± 27.88 | 185.44 ± 33.24 | 0.321 |
| WBC, mean ± SD, 109/L | 2.89 ± 1.65 | 3.07 ± 1.85 | 0.596 |
| Hb , mean ± SD, g/dL | 104.16 ± 26.95 | 95.34 ± 28.14 | 1.000 |
| PLT, mean ± SD, 109/L | 40.32 ± 7.74 | 38.24 ± 9.18 | 0.214 |
| TBIL, mean ± SD, μmol/L | 19.21 ± 9.76 | 20.88 ± 12.66 | 0.454 |
| AST, mean ± SD, U/L | 34.93 ± 21.26 | 35.89 ± 15.08 | 0.779 |
| ALT, mean ± SD, U/L | 31.14 ± 23.91 | 29.41 ± 15.28 | 0.637 |
| BUN, mean ± SD, mmol/L | 5.93 ± 2.25 | 5.38 ± 1.95 | 0.168 |
| CER, mean ± SD, μmol/L | 72.32 ± 17.63 | 71.15 ± 19.81 | 0.748 |
The median operation time was significantly longer for the MLSD than for the OSD group (P < 0.0001; Table 2). Median intraoperative estimated blood loss was significantly lower for MLSD than for OSD (P < 0.0001), as was median intraoperative volume of blood transfused (P < 0.05) (Table 2).
| Variable | MLSD (n = 44) | OSD (n = 71) | P value |
| Operation time, median (range), min | 210 (140-390) | 180 (110-300) | < 0.0001 |
| Estimated blood loss, median (range), mL | 150 (50-800) | 300 (50-1200) | < 0.0001 |
| Blood transfused, median (range), mL | 0 (0-400) | 0 (0-700) | 0.024 |
| VAS pain score on the first day, mean ± SD | 2.50 ± 0.85 | 5.06 ± 1.08 | < 0.0001 |
| Time to first oral intake, mean ± SD, d | 1.52 ± 0.63 | 2.76 ± 0.62 | < 0.0001 |
| Time to first flatus, mean ± SD, d | 2.36 ± 0.97 | 3.18 ± 1.00 | 0.0004 |
| Time to off-bed activity, mean ± SD, d | 2.59 ± 0.69 | 5.96 ± 0.93 | < 0.0001 |
| Postoperative hospital stay, median (range), d | 10 (7-18) | 15 (7-28) | < 0.0001 |
| Perioperative complications, n | 7 | 26 | 0.017 |
| Incision complications | 0 | 9 | 0.035 |
| Incision hernia | 0 | 1 | |
| Superficial SSI | 0 | 5 | |
| Deep SSI | 0 | 3 | |
| Pneumonia | 0 | 3 | 0.436 |
| Organ space SSI | 0 | 2 | 0.697 |
| Emergency operation for bleeding | 0 | 3 | 0.436 |
| Pancreatic fistula | 2 | 1 | 0.672 |
| Asymptomatic portal vein thrombosis | 5 | 8 | 1.000 |
The mean ± SD VAS pain score on the first day after surgery was significantly lower in the MLSD than the OSD group (P < 0.0001; Table 2). Mean times to first oral intake, first flatus, and off-bed activity were significantly shorter in the MLSD than the OSD group (all P < 0.001), as was median hospital stay (P < 0.0001) (Table 2).
Seven of the 44 patients (15.91%) in the MLSD group and 26 of 71 (36.62%) in the OSD group experienced postoperative complications (P < 0.05). The seven complications in the MSLD group included two patients with pancreatic fistulae and five with asymptomatic portal vein thrombosis. In the OSD group, one patient had a pancreatic fistula, eight had asymptomatic portal vein thrombosis, nine had incision complications [including five with superficial surgical site infections (SSI), three with deep SSI, and one with an incision hernia], three had pneumonia, two had organ space SSI, and three required emergency laparotomy for bleeding. All complications were successfully managed. The incision complication rate was significantly lower in the MLSD than in the OSD group (0% vs 12.68%, P < 0.05). Emergency laparotomy operation for bleeding was not necessary following MLSD (Table 2).
Before surgery, none of the patients in either group had fever. The mean ± SD number of days of postoperative body temperature > 38.0 °C was significantly lower after MLSD than after OSD (P < 0.0001; Table 3). Seven patients in the MLSD and two in the OSD group did not have fever postoperatively, making the rate significantly higher in the MLSD than in the OSD group (15.91% vs 2.82%, P < 0.05; Table 3). Although WBC count at admission was similar in both the MLSD and OSD groups (P > 0.05 each), median WBC counts on postoperative days 1 (P < 0.0001), 3 (P < 0.05), and 7 (P < 0.05) were significantly lower after MLSD than after OSD (Table 3). The percentage of patients with normal WBC counts on postoperative days 1 (34.09% vs 4.22%, P < 0.0001), 3 (31.82% vs 14.08%, P < 0.05), and 7 (81.82% vs 54.93%, P < 0.01) were all significantly higher after MLSD than after OSD (Table 3).
| Variable | MLSD (n = 44) | OSD (n = 71) | P value |
| Postoperative fever, mean ± SD, d | 4.09 ± 3.16 | 6.89 ± 3.55 | < 0.0001 |
| No fever, n | 7 | 2 | 0.029 |
| WBC 0 d, mean ± SD, 109/L | 2.89 ± 1.65 | 3.07 ± 1.85 | 0.596 |
| WBC 1 d, median (range), 109/L | 11.45 (5.2-17.4) | 16.9 (6.1-33.90) | < 0.0001 |
| WBC 3 d, mean ± SD, 109/L | 11.63 ± 3.29 | 13.72 ± 4.67 | 0.011 |
| WBC 7 d, median (range), 109/L | 8.4 (5-23.1) | 9.5 (4.9-38) | 0.035 |
| Normal WBC, 1 d, n | 15 | 3 | < 0.0001 |
| Normal WBC, 3 d, n | 14 | 10 | 0.023 |
| Normal WBC, 7 d, n | 36 | 39 | 0.003 |
Preoperative AST and ALT concentrations were similar in the two groups (Table 1). Although AST concentrations were similar on postoperative day 1 (P > 0.05), mean AST on postoperative days 3 (P < 0.05) and 7 (P < 0.001) were significantly lower after MLSD than after OSD (Table 4). Moreover, although ALT concentrations on postoperative days 1 and 3 were similar in the two groups, ALT was significantly lower after MLSD than after OSD on day 7 (P < 0.01) (Table 4).
| Variable | MLSD (n = 44) | OSD (n = 71) | P value |
| AST 1 d, mean ± SD, U/L | 57.32 ± 27.89 | 87.14 ± 135.13 | 0.152 |
| AST 3 d, mean ± SD, U/L | 33.84 ± 22.16 | 50.86 ± 43.83 | 0.018 |
| AST 7 d, mean ± SD, U/L | 25.66 ± 11.28 | 33.63 ± 16.32 | 0.005 |
| ALT 1 d, mean ± SD, U/L | 40.14 ± 18.81 | 61.66 ± 105.43 | 0.183 |
| ALT 3 d, mean ± SD, U/L | 31.93 ± 25.21 | 46.93 ± 56.60 | 0.100 |
| ALT 7 d, mean ± SD, U/L | 21.14 ± 12.63 | 29.68 ± 17.36 | 0.003 |
| BUN 1 d, mean ± SD, mmol/L | 5.52 ± 1.76 | 6.54 ± 2.33 | 0.014 |
| BUN 3 d, mean ± SD, mmol/L | 6.63 ± 2.20 | 7.33 ± 2.85 | 0.171 |
| BUN 7 d, mean ± SD, mmol/L | 4.76 ± 2.02 | 5.52 ± 2.21 | 0.069 |
| CER 1 d, mean ± SD, μmol/L | 75.27 ± 16.22 | 84.99 ± 20.92 | 0.010 |
| CER 3 d, mean ± SD, μmol/L | 59.75 ± 16.64 | 66.04 ± 19.08 | 0.074 |
| CER 7 d, mean ± SD, μmol/L | 60.88 ± 13.92 | 60.83 ± 16.17 | 0.986 |
Preoperative BUN and CER concentrations were similar in the two groups (Table 1). Although mean BUN was significantly lower after MLSD than after OSD on postoperative day 1 (P < 0.05), BUN concentrations were similar in the two groups on postoperative days 3 and 7 (Table 4). Similarly, mean CER was significantly lower after MLSD than after OSD on day 1 (P < 0.05), but was similar in the two groups on postoperative days 3 and 7 (Table 4).
Median CRP concentrations were similar preoperatively in the MLSD and OSD groups, but were significantly lower in the MLSD group on postoperative days 1 (P < 0.001), 3 (P < 0.05), and 7 (P < 0.05) (Table 5). Similarly, IL-6 concentrations did not differ preoperatively, but were significantly lower in the MLSD group on postoperative days 1, 3, and 7 (P < 0.05 each) (Table 5). Additionally, PCT concentrations were similar in the two groups preoperatively, but were significantly lower in the MLSD than in the OSD group on postoperative days 1, 3, and 7 (P < 0.0001 each) (Table 5).
| Variable | MLSD (n = 44) | OSD (n = 71) | P value |
| CRP day 0, median (range), mg/L | 1.03 (0.02-49.21) | 0.96 (0.03-13.32) | 0.715 |
| CRP day 1, mean ± SD, mg/L | 28.39 ± 16.30 | 36.86 ± 12.86 | 0.002 |
| CRP day 3, mean ± SD, mg/L | 92.64 ± 53.16 | 114.06 ± 44.37 | 0.022 |
| CRP day 7, mean ± SD, mg/L | 41.14 ± 27.61 | 52.49 ± 29.70 | 0.043 |
| IL-6 day 0, mean ± SD, pg/mL | 6.14 ± 5.61 | 6.60 ± 7.26 | 0.715 |
| IL-6 day 1, mean ± SD, pg/mL | 8.09 ± 6.97 | 11.78 ± 9.18 | 0.024 |
| IL-6 day 3, mean ± SD, pg/mL | 7.65 ± 6.95 | 10.84 ± 8.42 | 0.037 |
| IL-6 day 7, mean ± SD, pg/mL | 6.58 ± 5.66 | 9.83 ± 8.26 | 0.024 |
| PCT day 0, mean ± SD, ng/mL | 0.45 ± 0.25 | 0.47 ± 0.27 | 0.722 |
| PCT 1 d, median (range), ng/mL | 0.97 (0.36-3.2) | 1.96 (0.54-10.08) | < 0.0001 |
| PCT 3 d, median (range), ng/mL | 0.795 (0.3-2.67) | 1.45 (0.6-4.88) | < 0.0001 |
| PCT 7 d, median (range), ng/mL | 0.5 (0.28-4.01) | 0.77 (0.38-5.87) | < 0.0001 |
LSD has become more frequently used at some institutions to treat portal hypertension and has been shown to be superior to OSD in reducing postoperative pain severity, time to first flatus, and the duration of hospital stay and convalescence[15,16]. We developed an even less invasive technique, MLSD, for portal hypertension, extending the advantages of LSD to patients likely to most benefit from it (namely cirrhotic patients with bleeding portal hypertension and hypersplenism). This study compared both subjective and objective parameters, including measures of surgical trauma, convalescence, and burden on the immune system, in patients undergoing MLSD and OSD. To our knowledge, this is the first retrospective clinical study comparing these techniques in patients with portal hypertension.
None of the patients included in this study had a liver transplant or TIPS. In China, there is a shortage of donor livers, whereas TIPS is usually used to treat portal hypertension, especially as a bridge to transplantation. Although TIPS can reduce portal pressure to prevent recurrent gastroesophageal variceal bleeding, it cannot be used to treat secondary hypersplenism. Furthermore, TIPS has been associated with portosystemic encephalopathy.
Traditional LSD utilizes several methods to remove massively enlarged spleens, including the creation of an enlarged incision to morcellate and remove the spleen enveloped in a cumbersome intracorporeal bag or hand-assisted laparoscopy to remove the spleen through a hand-assisted incision[15-21]. During MLSD, a massively enlarged spleen is removed through the existing 12 mm port using an electromechanical morcellator. Therefore, MLSD appears to involve less surgical trauma than LSD. Moreover, to avoid damage to organs or tissues when using the electromechanical morcellator to cut the spleen, the patient is placed in the reverse Trendelenburg position, the dissected spleen is placed in the left subphrenic space, and a splenic retractor or forceps is used to lift the upper pole of the spleen to maintain it in an ideal location.
Although the operation time of LSD was longer than that of OSD, LSD was associated with lower estimated blood loss and a lower volume of blood transfused[15]. We observed similar outcomes when comparing MLSD with OSD. Only one patient in the MLSD group required a blood transfusion. These advantages of MLSD and LSD are due to their good operational view and exposure, as well as the use of a LigaSure vessel-sealing device, which is efficient in dividing the splenogastric ligament and esophagogastric varices.
We also found that convalescence was more rapid after MLSD than after OSD. VAS pain score on the first postoperative day was significantly lower, and times to first oral intake, passage of flatus, and off-bed activity, as well as postoperative hospital stay, were significantly shorter in the MLSD than the OSD group. Similarly, LSD resulted in faster recovery than OSD, as shown by a reduced time to first flatus and postoperative hospital stay[15,16].
MLSD also showed other benefits, including fewer numbers of days of postoperative body temperature > 38.0 °C; a lower rate of no fever postoperatively; lower WBC counts on postoperative days 1, 3, and 7; and higher rates of normal WBC counts on postoperative days 1, 3, and 7. In contrast, a previous study found no significant differences in WBC count on postoperative day 7 between patients undergoing LSD and OSD[16]. These findings suggest that MLSD results in weaker inflammatory responses and better recovery than OSD.
The absence of incision complications in patients undergoing LSD may be due to the small sizes of the incisions, and earlier ambulation after surgery may result from decreased postoperative pain, but the postoperative rates of total and incision-associated complications did not differ significantly in patients undergoing LSD and OSD[16]. The incisions resulting from MLSD were less invasive than those resulting from LSD, with no incision complications after MLSD. However, we found that the postoperative rates of total and incision-associated complications differed in our MLSD and OSD groups. The difference between studies was likely due to the smaller sample size in the previous study, which included 24 patients in the LSD and 30 in the OSD group[16].
The earlier study found that ALT and AST concentrations on postoperative day 7 were significantly lower in the LSD than in the OSD group[16]. Our findings were similar, except that AST concentrations on postoperative day 3 were significantly lower in the MLSD than in the OSD group. We also found that BUN and CER concentrations on postoperative day 1 were lower in the MLSD group, providing further evidence for the benefits of MLSD. These findings also suggest a difference in the rate of recovery from surgical trauma to the liver and kidneys.
The immune response to surgical trauma involves a complex cascade of many types of mediators and immune cells. IL-6 is thought to play a pivotal role in the pathogenesis of surgical trauma, with increased IL-6 concentrations found to correlate with the magnitude of surgical trauma[22,23], as well as contributing to fever[24]. CRP is an acute phase molecule produced in the liver during tissue injury, whereas PCT is a pro-peptide of calcitonin produced by the thyroid gland and a highly specific marker of clinically relevant bacterial infections and sepsis[25,26].
Systemic stress responses are lower after laparoscopic than after conventional open surgery[4,27], with differences in cytokine concentrations and cell-mediated immune responses observed both in animal experiments and clinical trials[28-30]. Laparoscopic techniques have been shown to have an immunologic advantage over traditional open surgery[1,3,6], with lower concentrations of IL-6 and CRP induced during laparoscopic than during open general and urinary surgery[7-10,24]. These differences may be due to CO2 pneumoperitoneum; CO2 insufflation has a protective function during laparoscopic procedures, with CO2 acidification of the peritoneal cavity attenuating overall inflammatory response and suppressing peritoneal macrophages, which are cells that initiate inflammatory responses during surgery[31,32].
We observed similar findings, in that the concentrations of IL-6, CRP, and PCT on postoperative days 1, 3, and 7 were all lower after MLSD than after OSD. These objective measurements showed that MLSD resulted in improved immunologic function compared with OSD. Because liver function in these patients is usually very poor, postoperative immune dysfunction is an important aspect of treatment for patients with liver cirrhosis, bleeding portal hypertension, and hypersplenism. IL-6, CRP, and PCT may be better markers of immune recovery than WBC count in patients with liver cirrhosis, since patients with leukocytopenia due to hypersplenism frequently have WBC counts below the lower limit of the normal range.
Despite longer operation times, MLSD resulted in better and faster recovery, less liver and renal dysfunction, less fever, and less stimulation of host immunity than OSD. These findings may be due to: (1) the smaller size of the incision used to remove the enlarged spleen; (2) the reduced estimated blood loss during surgery; (3) CO2 pneumoperitoneum; and/or (4) fewer postoperative complications.
In conclusion, this study indicated that MLSD had numerous advantages over OSD and is technically feasible and safe. MLSD consisted of a combination of LSD and a novel technique with an electromechanical morcellator. MLSD was associated with minimal postoperative pain and scarring, faster and better postoperative recovery, and lower inflammatory immune responses than OSD. MLSD can therefore extend the advantages of LSD to patients with portal hypertension. It represents a promising and minimally-invasive treatment that may become the gold standard of surgical procedures for liver cirrhosis patients with bleeding portal hypertension and hypersplenism.
The authors thank Yao Tang, biostatistician at the Clinical Medical College of Yangzhou University, for his assistance with statistical analyses.
Although many types of laparoscopic surgery have shown immunologic benefits, the effects of modified laparoscopic splenectomy and azygoportal disconnection (MLSD) for portal hypertension have not been investigated thoroughly.
Systemic stress responses are lower after laparoscopic than after conventional open surgery, with differences in cytokine concentrations and cell-mediated immune responses observed both in animal experiments and clinical trials. Laparoscopic techniques have been shown to have an immunologic advantage over traditional open surgery, with lower concentrations of interleukin-6 (IL-6) and C-reactive protein induced during laparoscopic than during open general and urinary surgery.
The authors developed an even less invasive technique, MLSD. During the procedure, an electromechanical morcellator allows for easy extraction of the entire massive splenic tissue through the existing 12 mm port without the cumbersome intracorporeal bag, enlarged incision, or hand-assisted incision used in traditional laparoscopic splenectomy and azygoportal disconnection (LSD). Therefore, MLSD appears to involve less surgical trauma than LSD.
MLSD is technically feasible, safe, and resulted in better and faster recovery, less liver and renal dysfunction, less fever, and less stimulation of host immunity than open splenectomy and azygoportal disconnection. MLSD represents a promising and minimally-invasive treatment for liver cirrhosis patients with bleeding portal hypertension and hypersplenism.
Surgical trauma, a process also called surgical stress, activates systemic immunologic and inflammatory responses, which are characterized by (1) release of the pro-inflammatory cytokines tumor necrosis factor α, interleukin (IL)-1, and IL-6; (2) neutrophil activation and microvascular adherence; and (3) uncontrolled polymorphonuclear and macrophage oxidative burst.
The authors investigated the perioperative advantages of modified laparoscopic vs open splenectomy and azygoportal disconnection. The paper and the results are interesting. The manuscript is original and may be useful to clinicians.
P- Reviewer: Papparella A S- Editor: Qi Y L- Editor: Rutherford A E- Editor: Liu XM
| 1. | Novitsky YW, Litwin DE, Callery MP. The net immunologic advantage of laparoscopic surgery. Surg Endosc. 2004;18:1411-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 175] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Biffl WL, Moore EE, Moore FA, Peterson VM. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann Surg. 1996;224:647-664. [PubMed] |
| 3. | Sylla P, Kirman I, Whelan RL. Immunological advantages of advanced laparoscopy. Surg Clin North Am. 2005;85:1-18, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Buunen M, Gholghesaei M, Veldkamp R, Meijer DW, Bonjer HJ, Bouvy ND. Stress response to laparoscopic surgery: a review. Surg Endosc. 2004;18:1022-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Jiang G, Qian J, Yao J, Wang X, Jin S, Bai D. A New Technique for Laparoscopic Splenectomy and Azygoportal Disconnection. Surg Innov. 2013;21:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Vittimberga FJ, Foley DP, Meyers WC, Callery MP. Laparoscopic surgery and the systemic immune response. Ann Surg. 1998;227:326-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 205] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Schwenk W, Jacobi C, Mansmann U, Böhm B, Müller JM. Inflammatory response after laparoscopic and conventional colorectal resections - results of a prospective randomized trial. Langenbecks Arch Surg. 2000;385:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 135] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Sietses C, Wiezer MJ, Eijsbouts QA, Beelen RH, van Leeuwen PA, von Blomberg BM, Meijer S, Cuesta MA. A prospective randomized study of the systemic immune response after laparoscopic and conventional Nissen fundoplication. Surgery. 1999;126:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Scheepers JJ, Sietses C, Bos DG, Boelens PG, Teunissen CM, Ligthart-Melis GC, Cuesta MA, van Leeuwen PA. Immunological consequences of laparoscopic versus open transhiatal resection for malignancies of the distal esophagus and gastroesophageal junction. Dig Surg. 2008;25:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Grande M, Tucci GF, Adorisio O, Barini A, Rulli F, Neri A, Franchi F, Farinon AM. Systemic acute-phase response after laparoscopic and open cholecystectomy. Surg Endosc. 2002;16:313-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Cruickshank AM, Fraser WD, Burns HJ, Van Damme J, Shenkin A. Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci (Lond). 1990;79:161-165. [PubMed] |
| 12. | Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE. Pain measurement: an overview. Pain. 1985;22:1-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 744] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 13. | Hoffmann NE, Bischof JC. The cryobiology of cryosurgical injury. Urology. 2002;60:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 356] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 14. | Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37:171-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 663] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 15. | Jiang XZ, Zhao SY, Luo H, Huang B, Wang CS, Chen L, Tao YJ. Laparoscopic and open splenectomy and azygoportal disconnection for portal hypertension. World J Gastroenterol. 2009;15:3421-3425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Zheng X, Liu Q, Yao Y. Laparoscopic splenectomy and esophagogastric devascularization is a safe, effective, minimally invasive alternative for the treatment of portal hypertension with refractory variceal bleeding. Surg Innov. 2013;20:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Helmy A, Abdelkader Salama I, Schwaitzberg SD. Laparoscopic esophagogastric devascularization in bleeding varices. Surg Endosc. 2003;17:1614-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Wang Y, Ji Y, Zhu Y, Xie Z, Zhan X. Laparoscopic splenectomy and azygoportal disconnection with intraoperative splenic blood salvage. Surg Endosc. 2012;26:2195-2201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Yamamoto J, Nagai M, Smith B, Tamaki S, Kubota T, Sasaki K, Ohmori T, Maeda K. Hand-assisted laparoscopic splenectomy and devascularization of the upper stomach in the management of gastric varices. World J Surg. 2006;30:1520-1525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Wang Y, Zhan X, Zhu Y, Xie Z, Zhu J, Ye Z. Laparoscopic splenectomy in portal hypertension: a single-surgeon 13-year experience. Surg Endosc. 2010;24:1164-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Xin Z, Qingguang L, Yingmin Y. Total laparoscopic versus open splenectomy and esophagogastric devascularization in the management of portal hypertension: a comparative study. Dig Surg. 2009;26:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Allendorf JD, Bessler M, Kayton ML, Oesterling SD, Treat MR, Nowygrod R, Whelan RL. Increased tumor establishment and growth after laparotomy vs laparoscopy in a murine model. Arch Surg. 1995;130:649-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 140] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Hildebrandt U, Kessler K, Plusczyk T, Pistorius G, Vollmar B, Menger MD. Comparison of surgical stress between laparoscopic and open colonic resections. Surg Endosc. 2003;17:242-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Aminsharifi A, Salehipoor M, Arasteh H. Systemic immunologic and inflammatory response after laparoscopic versus open nephrectomy: a prospective cohort trial. J Endourol. 2012;26:1231-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Takakura Y, Hinoi T, Egi H, Shimomura M, Adachi T, Saito Y, Tanimine N, Miguchi M, Ohdan H. Procalcitonin as a predictive marker for surgical site infection in elective colorectal cancer surgery. Langenbecks Arch Surg. 2013;398:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Ha YE, Kang CI, Wi YM, Chung DR, Kang ES, Lee NY, Song JH, Peck KR. Diagnostic usefulness of procalcitonin as a marker of bacteremia in patients with acute pyelonephritis. Scand J Clin Lab Invest. 2013;73:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Gupta A, Watson DI. Effect of laparoscopy on immune function. Br J Surg. 2001;88:1296-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Hajri A, Mutter D, Wack S, Bastien C, Gury JF, Marescaux J, Aprahamian M. Dual effect of laparoscopy on cell-mediated immunity. Eur Surg Res. 2000;32:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Holub Z. Impact of laparoscopic surgery on immune function. Clin Exp Obstet Gynecol. 2002;29:77-81. [PubMed] |
| 30. | Tang CL, Eu KW, Tai BC, Soh JG, MacHin D, Seow-Choen F. Randomized clinical trial of the effect of open versus laparoscopically assisted colectomy on systemic immunity in patients with colorectal cancer. Br J Surg. 2001;88:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 116] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Hanly EJ, Aurora AA, Shih SP, Fuentes JM, Marohn MR, De Maio A, Talamini MA. Peritoneal acidosis mediates immunoprotection in laparoscopic surgery. Surgery. 2007;142:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Shimotakahara A, Kuebler JF, Vieten G, Kos M, Metzelder ML, Ure BM. Carbon dioxide directly suppresses spontaneous migration, chemotaxis, and free radical production of human neutrophils. Surg Endosc. 2008;22:1813-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |