Published online Jul 21, 2014. doi: 10.3748/wjg.v20.i27.9121
Revised: March 12, 2014
Accepted: April 15, 2014
Published online: July 21, 2014
Processing time: 196 Days and 20.1 Hours
AIM: To investigate the risk factors and surgical outcomes for spontaneous rupture of Barcelona Clinic Liver Cancer (BCLC) stages A and B hepatocellular carcinoma (HCC).
METHODS: From April 2002 to November 2006, 92 consecutive patients with spontaneous rupture of BCLC stage A or B HCC undergoing hepatic resection were included in a case group. A control arm of 184 cases (1:2 ratio) was chosen by matching the age, sex, BCLC stage and time of admission among the 2904 consecutive patients with non-ruptured HCC undergoing hepatic resection. Histological confirmation of HCC was available for all patients and ruptured HCC was confirmed by focal discontinuity of the tumor with surrounding perihepatic hematoma observed intraoperatively. Patients with microvascular thrombus in the hepatic vein branches were excluded from the study. Clinical data and survival time were collected and analysed.
RESULTS: Sixteen patients were excluded from the study based on exclusion criteria, of whom 3 were in the case group and 13 in the control group. Compared with the control group, more patients in the case group had underlying diseases of hypertension (10.1% vs 3.5%, P = 0.030) and liver cirrhosis (82.0% vs 57.9%, P < 0.001). Tumors in 67 (75.3%) patients in the case group were located in segments II, III and VI, and the figure in the control group was also 67 (39.7%) (P < 0.001). On multivariate analysis, hypertension (HR = 7.38, 95%CI: 1.91-28.58, P = 0.004), liver cirrhosis (HR = 6.04, 95%CI: 2.83-12.88, P < 0.001) and tumor location in segments II, III and VI (HR = 5.03, 95%CI: 2.70-6.37, P < 0.001) were predictive for spontaneous rupture of HCC. In the case group, the median survival time and median disease-free survival time were 12 mo (range: 1-78 mo) and 4 mo (range: 0-78 mo), respectively. The 1-, 3- and 5-year overall survival rates and disease-free survival rates were 66.3%, 23.4% and 10.1%, and 57.0%, 16.8% and 4.5%, respectively. Only radical resection remained predictive for overall survival (HR = 0.32, 95%CI: 0.08-0.61, P = 0.015) and disease-free survival (HR = 0.12, 95%CI: 0.01-0.73, P = 0.002).
CONCLUSION: Tumor location, hypertension and liver cirrhosis are associated with spontaneous rupture of HCC. One-stage hepatectomy should be recommended to patients with BCLC stages A and B disease.
Core tip: There are few reports concerning the risk factors associated with spontaneous rupture of hepatocellular carcinoma (HCC) and the best approach in cases of ruptured HCC. This retrospective case-control study showed that three predictive factors including hypertension, liver cirrhosis and tumor location in segments II, III and VI were associated with spontaneous rupture of HCC. Especially, the relationship between tumor location and spontaneous rupture of HCC was identified for the first time.
- Citation: Li J, Huang L, Liu CF, Cao J, Yan JJ, Xu F, Wu MC, Yan YQ. Risk factors and surgical outcomes for spontaneous rupture of BCLC stages A and B hepatocellular carcinoma: A case-control study. World J Gastroenterol 2014; 20(27): 9121-9127
- URL: https://www.wjgnet.com/1007-9327/full/v20/i27/9121.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i27.9121
Hepatocellular carcinoma (HCC) is one of the most common neoplasms encountered clinically, and its incidence is increasing worldwide because of the increasing prevalence of hepatitis B and C virus infections[1,2]. One of the life-threatening complications of HCC is the spontaneous rupture of the tumor, with intra-peritoneal hemorrhage. Spontaneous rupture of HCC occurs in 3%-26% of all patients with HCC, and high mortality rates in the range of 32%-6.7% have been reported[3-7].
There were very few reports of risk factors associated with spontaneous rupture of HCC. Kim et al[5] reported that CT findings were associated with an increased risk of rupture, including a large tumor, a contour protrusion and portal vein thrombosis, which indicated advanced HCC. In addition, clinicians often feel helpless when facing these complicated situations, and previous studies have shown a very poor prognosis for HCC rupture, with a 30-d mortality rate in the range of 30%-70%[8-14].
There is still a debate concerning the best approach in cases of HCC rupture[9]. Many published studies have reported their various emergency treatments for freshly ruptured tumors and the outcomes afterwards[3,7-14]. The immediate management for ruptured HCC patients usually includes emergency hepatectomy, hepatic artery ligation, suture plication, packing hemostasis and transarterial embolization. However, at our hospital, which is a hepatobiliary specialty hospital without an emergency department, most HCC patients with ruptured tumors were admitted during the routine work in outpatient department. The ruptured tumors were usually indicated by a history of sudden abdominal pain, and confirmed by imaging diagnosis or even in operation. A large series of HCC patients with ruptured tumors were classified with stages A and B disease according to the Barcelona Clinic Liver Cancer (BCLC) classification[15], and most of them were suitable for hepatic resection. We believed that the ruptured BCLC stages A and B HCCs might differ from those at advanced stages in risk factors and clinical outcomes.
Recently, there was a patient with small HCC (3 cm in diameter, located in the left lateral lobe) admitted in our department. Surgery was prepared for him. To our surprise, his blood pressure dropped to 50/20 mmHg and his abdomen enlarged suddenly when he lay on the operating table. Tumor rupture was suspected and confirmed when his abdomen was opened. This case shocked us, and we hypothesized that the tumor location may be associated with spontaneous rupture of HCC. We therefore conducted this retrospective case-control study trying to investigate the risk factors associated with spontaneous rupture of BCLC stages A and B HCC, and to reveal the outcomes after primary hepatectomy during a 10-year period at a single center in China.
HCC was stratified according to the BCLC staging classification. The following categories were used. Stage A includes single tumors smaller than 5 cm in diameter or up to 3 tumors all smaller than 3 cm in diameter. Stage B includes up to 3 tumors (≥ 1 of which is > 3 cm in diameter) or more than 3 tumors of any size. Single tumors exceeding 5 cm in diameter are included in stage B as well based on the article by Bruix and Llovet[16].
From April 2002 to November 2006, a total of 200 consecutive patients with spontaneously ruptured HCC visited the Eastern Hepatobiliary Surgery Hospital. Among them, 92 were classified with stages A and B disease according to the BCLC classification and underwent hepatic resection. A control arm of 184 cases (1:2 ratio) was chosen by matching the age, sex, BCLC stage and time of admission among the 2904 consecutive patients seen in our hospital with non-ruptured HCC undergoing hepatic resection. Histological confirmation of HCC was available for all patients and ruptured HCC was confirmed by focal discontinuity of the tumor with surrounding perihepatic hematoma observed intraoperatively. The tumor tissue samples of all the 276 patients were reviewed by a pathologist, and those with microvascular thrombus in the hepatic vein branches were excluded from the study.
Clinical data of all patients were retrospectively collected, including underlying diseases, laboratory blood test results, intraoperative parameters and postoperative pathological findings. Overall survival time and disease-free survival time were obtained by follow-up. The liver function status was evaluated using the Child-Pugh score system. Tumor location was determined according to the Couinaud’s classification to segment the liver.
Continuous data are expressed as mean ± SD or median (range) where appropriate and compared using the independent sample t-test. Categorical variables were compared using the χ2 test with Yates correction or the Fisher exact test where appropriate. P < 0.05 was considered significant. Hazard ratios (HRs) and their corresponding 95%CIs were calculated using simple logistic-regression analysis. Baseline factors associated with a P value < 0.1 in the univariate analysis were sequentially entered into the multivariate logistic regression analysis to indicate the relatively independent risk factors.
Survival rates were obtained by the Kaplan-Meier method and were compared using the log-rank test. A Cox regression model was used to analyse the prognostic predictors for survival. Survival time and disease-free survival time started from the date of hepatic resection until death and the diagnosis of recurrence or the closing date. The closing date of this study was March 31, 2011.
Sixteen patients were excluded from the study based on the exclusion criteria, of whom 3 were in the case group and 13 in the control group. Among the 89 patients with spontaneous rupture of HCC (case group), mean age was 48.5 ± 11.2 years and 83 (93.3%) were males. The corresponding figures for the 171 patients without rupture (control group) were 50.5 ± 11.4 years and a male prevalence of 88.3%. The most common initial symptoms of spontaneous rupture of HCC were sudden onset of abdominal pain (61 patients, 68.5%). There were 12 (13.5%) patients who developed hypovolemic shock at admission, while 16 (18.0%) patients had no symptoms. Emergency hepatic resection was performed in 12 (13.5%) patients, while the remaining 77 patients underwent limited one-stage operation.
The clinical data for the case group and control group are presented in Table 1. Compared with the control group, more patients in the case group had underlying diseases of hypertension (10.1% vs 3.5%, P = 0.030) and liver cirrhosis (82.0% vs 57.9%, P < 0.001). Tumors in 67 (75.3%) patients in the case group were located in segments II, III and VI, and the figure in the control group was also 67 (39.7%) (P < 0.001). No significant differences were observed between the case group and control group with respect to baseline levels of laboratory tests. Radical resection, defined as a negative margin and no residual tumor in the liver, was achieved in 80 (89.9%) patients in the case group, compared with 163 (95.3%) in the control group (P = 0.011). The 30-d mortality was 1.1% (1/89) in the case group and 0.5% (1/171) in the control group, and both patients died of liver failure.
| Variable | Case group (n = 89) | Control group (n = 171) | P value |
| Age (yr) | 48.5 ± 11.2 | 50.3 ± 11.5 | 0.215 |
| Sex (M/F) | 83/6 | 151/20 | 0.206 |
| Diabetes | 0.626 | ||
| Yes | 3 | 167 | |
| No | 86 | 4 | |
| Hypertension | 0.030 | ||
| Yes | 9 | 6 | |
| No | 80 | 165 | |
| HBsAg status | 0.195 | ||
| Positive | 82 | 151 | |
| Negative | 7 | 20 | |
| Liver cirrhosis | < 0.001 | ||
| Yes | 73 | 99 | |
| No | 16 | 72 | |
| Child-Pugh classification | 0.148 | ||
| A | 84 | 159 | |
| B | 5 | 12 | |
| Tumor BCLC classification | 0.653 | ||
| A | 31 | 68 | |
| B | 58 | 103 | |
| Tumor size (cm) | 8.1 ± 3.1 | 6.5 ± 4.2 | 0.001 |
| Tumor location1 | < 0.001 | ||
| Segment I | 2 | 1 | |
| Segments IIand III | 20 | 16 | |
| Segment VI | 47 | 52 | |
| Segments IV,V,VII and VIII | 20 | 102 | |
| Tumor protrudes from liver surface | 0.138 | ||
| Yes | 45 | 70 | |
| No | 44 | 101 | |
| Satellite nodule(s) | 0.288 | ||
| Yes | 29 | 45 | |
| No | 60 | 126 | |
| AFP (ng/mL) | 138.5 (1.8, 20181.0) | 73.8 (2.4, 224492.0) | 0.102 |
| WBC (× 109/L) | 5.7 ± 2.5 | 5.9 ± 1.8 | 0.381 |
| PLT (× 109/L) | 154.7 ± 71.8 | 147.0 ± 61.3 | 0.112 |
| Total bilirubin (umol/L) | 21.67 ± 3.25 | 13.57 ± 4.33 | 0.104 |
| Albumin (g/L) | 39.18 ± 3.16 | 40.52 ± 3.45 | 0.624 |
| ALT (IU/L) | 55.5 ± 49.0 | 60.5 ± 51.0 | 0.447 |
| PT (s) | 13.0 ± 1.5 | 13.2 ± 1.5 | 0.286 |
| BUN (mmol/L) | 6.01 ± 0.39 | 5.93 ± 1.01 | 0.342 |
| Cr (umol/L) | 71.65 ± 12.51 | 74.72 ± 19.46 | 0.179 |
| Types of liver resection | 0.199 | ||
| Minor ≤ 3 liver segments | 70 | 120 | |
| Major ≥ 4 liver segments | 19 | 51 | |
| Radical resection | 0.011 | ||
| Yes | 80 | 163 | |
| No | 9 | 8 | |
| Inflow blood occlusion time (min) | 15.4 ± 6.9 | 15.4 ± 7.0 | 0.993 |
| Blood loss (mL) | 550 (300, 3200) | 200 (50, 2000) | 0.011 |
| Blood transfusion (yes/no) | 38/51 | 29/142 | < 0.001 |
| Tumor grade (Iand II/III and IV)2 | 3/86 | 20/151 | 0.025 |
| 30-d mortality | 1 (1.1) | 1 (0.6) | 0.079 |
Four variables including hypertension, liver cirrhosis, tumor size ≥ 10 cm, and tumor location in segments II, III and VI were selected on multivariate analysis to determine the independent risk factors associated with HCC rupture. The results showed that hypertension (HR = 7.38, 95%CI: 1.91-28.58, P = 0.004), liver cirrhosis (HR = 6.04, 95%CI: 2.83-12.88, P < 0.001) and tumor location in segments II, III and VI (HR = 5.03, 95%CI: 2.70-6.37, P < 0.001) were predictive for spontaneous rupture of HCC (Table 2).
| Variable | HR | 95%CI for HR | P value |
| Hypertension | 0.004 | ||
| Yes | 7.38 | 1.91-28.58 | |
| No | 1 | ||
| Liver cirrhosis | < 0.001 | ||
| Yes | 6.04 | 2.83-12.88 | |
| No | 1 | ||
| Tumor size | 0.184 | ||
| ≥ 10 cm | 1.62 | 0.80-3.31 | |
| < 10 cm | 1 | ||
| Tumor locaction1 | < 0.001 | ||
| Segments II, III and VI | 5.03 | 2.70-6.37 | |
| Other segments | 1 |
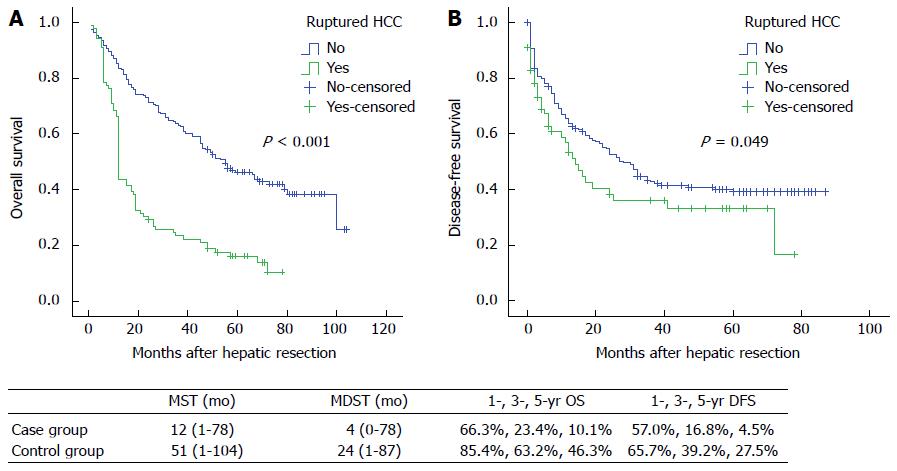
At the closing date of the study, 80 (89.9%) patients in the case group and 119 (69.6%) in the control group died. The median survival time (MST) and median disease-free survival time (MDST) of patients in the case group were 12 mo (range: 1-78 mo) and 4 mo (range: 0-78 mo), respectively, while the figures in the control group were 51 mo (range: 1-104 mo) and 24 mo (range: 1-87 mo), respectively. The 1-, 3- and 5-year overall survival rates were 66.3%, 23.4% and 10.1% in the case group, compared with 85.4%, 63.2% and 46.3% in the control group (P < 0.001). In addition, the 1-, 3-, and 5-year disease-free survival rates in the case group were 57.0%, 16.8% and 4.5%, respectively, while they were 65.7%, 39.2% and 27.5% in the control group (P = 0.049) (Figure 1).
Nine variables were selected on multivariate analysis to determine the prognostic predictors of survival in patients with spontaneously ruptured HCC (Table 3). Only radical resection remained statistically predictive for overall survival (HR = 0.32, 95%CI: 0.08-0.61, P = 0.015), as well as for disease-free survival (HR = 0.12, 95%CI: 0.01-0.73, P = 0.002).
| Variable | Overall survival | Disease-free survival | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age | 1.01 | 0.99-1.03 | 0.348 | 1.02 | 0.99-1.05 | 0.097 |
| Liver cirrhosis (yes vs no) | 1.05 | 0.55-2.03 | 0.878 | 1.29 | 0.54-3.09 | 0.566 |
| BCLC stage (stage A vs stage B) | 1.35 | 0.60-3.06 | 0.470 | 1.13 | 0.41-3.07 | 0.817 |
| Tumor size | 0.96 | 0.88-1.06 | 0.456 | 0.92 | 0.81-1.05 | 0.235 |
| Tumor location | 1.02 | 0.59-1.76 | 0.936 | 1.24 | 0.64-2.42 | 0.524 |
| Satellite nodule(s) (yes vs no) | 0.77 | 0.46-1.27 | 0.299 | 0.76 | 0.39-1.47 | 0.413 |
| Blood loss | 1.00 | 1.00-1.00 | 0.085 | 1.00 | 1.00-1.00 | 0.940 |
| Blood transfusion (yes vs no) | 1.00 | 0.51-1.98 | 0.994 | 1.12 | 0.49-2.59 | 0.788 |
| Radical resection (yes vs no) | 0.32 | 0.08-0.61 | 0.015 | 0.12 | 0.01-0.73 | 0.002 |
Spontaneous rupture of HCC is one of the most common and life-threatening emergencies in liver surgery. However, there were very few studies investigating the risk factors associated with this dreaded complication of HCC. A commonly accepted mechanism of rupture described by Zhu et al[17] is that it is initiated by invasion and occlusion of the hepatic veins by tumor cells, which results in increased pressure within the tumor mass. The venous congestion in combination with various factors, such as central tumor necrosis, trauma and coagulopathy, leads to hemorrhage within the tumor. This further increases the pressure in the tumor and results in splitting of the overlying liver parenchyma and rupture at the surface. We think that this hypothesised mechanism may account for spontaneous rupture of advanced HCC when the hepatic vein is invaded by the tumor. Moreover, rupture of advanced HCC may cause heavy bleeding, and thus the patients are more likely to seek emergency care. In the present study, we included patients with BCLC stages A and B HCC which had no vascular invasion, and the results showed some other risk factors associated with spontaneous rupture of HCC.
In our series, we noticed that most ruptured tumors were located in the left lateral lobe (segments II and III, n = 20) and the right posterior inferior lobe (segment VI, n = 47), resulting in a proportion of 75.3%, and tumor location in segments II, III and VI were predictive for spontaneous rupture of HCC. According to gross anatomy, the shape of the liver is similar to a wedge. Compared with the other parts of the liver, either the left lateral lobe (segments II and III) or the right posterior inferior lobe (segment VI) is a “small room” restricted by the capsule of the liver. Therefore, when the tumor there becomes larger than the “room”, the inner pressure of the tumor can break the local capsule. Sometimes a strike from outside may lead to the rise of the pressure on the basis of “small room and big guest”, and cause rupture of the tumor as well. We also found that there was almost no overlying liver parenchyma on the ruptured tumor, neither was it showed by imaging examination nor found in operation. Especially, we did not find any “tunnel”-like tumor tissues at the most possible spot of rupture. Chen et al[8] also reported that left-lobe tumors presented a higher risk of rupture. So we doubt that hepatic vein occlusion is a main factor to initiate the rupture of HCC. Instead, we think that the differences in mechanics due to tumor location may play a major role in the pathogenesis of tumor rupture.
Previous studies have reported that the maximum tumor size > 5 cm was one of the risk factors predicting rupture of HCC[5,8]. Interestingly, in our study, tumor size ≥ 10 cm was not a predictive factor for HCC rupture, while the underlying diseases of hypertension and liver cirrhosis were predictive for spontaneous rupture of HCC. Moreover, some tumors as small as 2 cm have been found to rupture, which was consistent with a report by Tanaka et al[18]. It is difficult to explain how a small HCC located in the periphery would rupture via the above mechanism. More recent studies suggested that underlying vascular dysfunction may play a role[10]. The vessels in the ruptured HCC tend to be more friable due to increased collagenase expression and increased collagen IV degradation[19]. Our results are consistent with this proposed mechanism, and we think the reasons may be as follows. A long history of hypertension always causes injury of the blood vessels, making them more friable. In addition, patients with liver cirrhosis always have underlying coagulopathy. Both of them would lead to hemorrhage within the tumor and then initiate tumor rupture.
Spontaneous rupture of HCC can be a fatal complication of HCC. Previous studies have shown a very poor prognosis, with 30-d mortality rates in the range of 30%-70%[8-14,20,21]. The present study included patients with BCLC stages A and B HCC, and an overall 30-d mortality rate of 1.1% (one patient) was observed, which was much lower than those in previous reports.
Several studies have demonstrated that emergency hepatic resection for ruptured HCC may achieve long-term survival[20,21]. In another series, the 1- and 3-year survival rates for patients who underwent emergency resection were only 60% and 42%, respectively[22]. The MST of patients with ruptured HCC who had been treated by hepatectomy in the range of 1.2-25.7 mo has been reported[4,7]. In two case series of delayed resection for ruptured HCC from Japan, no in-hospital mortality was observed, and 1- and 3-year survival rates of 71%-77% and 48%-54%, respectively, were achieved[23,24]. It seems that hepatic resection was the most valuable treatment for patients with ruptured HCC, irrespective of emergency or delayed operation. However, there is still a disparity concerning the prognosis for these patients undergoing hepatic resection, because the surgical outcomes may be complicated by the stage of HCC and the inclusion of transarterial embolization in the treatment algorithm.
In our series, all the patients with ruptured HCC underwent one-stage hepatic resection without initial treatments. The MST and MDST were 12 mo and 4 mo, respectively, and the 1-, 3- and 5-year overall survival rates were 66.3%, 23.4% and 10.1%, respectively. The results were much worse than those patients with non-ruptured HCC undergoing hepatic resection, although recent studies have stated that the long-term survival for ruptured HCC may be equivalent to non-ruptured HCC[20]. We think the reason may be as follows. The ruptured HCC always stays in the “small room”, the increased inner pressure of the tumor not only causes tumor rupture but also causes intrahepatic metastasis. Considering that patients with ruptured HCC harbor advanced disease at presentation, the incidence of coexisting cirrhosis is high, and peritoneal seeding may occur at the time of rupture. The current tumor-node-metastasis staging system classifies ruptured HCC as T4 and as stage IV[25], which indicated advanced stage. Therefore, we think the results attained from our series are acceptable and one-stage hepatic resection should be recommended to patients with resectable ruptured HCC at BCLC stages A and B.
Furthermore, this study identified that radical resection remained predictive for surgical outcomes in patients with ruptured HCC. Inversely, some other acknowledged prognostic factors like BCLC stage, tumor size and satellite nodule(s) in non-ruptured HCC were not demonstrated to be statistically significant in ruptured HCC, suggesting that ruptured HCC may differ from non-rupture HCC in biological behaviour and pathological characteristics.
In summary, this retrospective case-control study showed that three predictive factors including hypertension, liver cirrhosis and tumor location in segments II, III and VI were associated with spontaneous rupture of HCC. Especially, the relationship between tumor location and spontaneous rupture of HCC was identified for the first time, which challenges the well-known concept that the congestion of hepatic venous vein is a main factor to initiate the rupture of HCC. Once the risk factors were determined, the next step we want to do is to determine whether we can adopt some measures to prevent tumor rupture in these patients. Although the surgical outcomes in patients with ruptured HCC was much worse than those without, we still think one-stage hepatic resection should be recommended to patients with ruptured HCC at BCLC stages A and B.
Spontaneous rupture of hepatocellular carcinoma (HCC) occurs in 3%-26% of all patients with HCC, and high mortality rates in the range of 32%-6.7% have been reported.
There is still a debate concerning the best approach in cases of HCC rupture. Many published studies have reported their various emergency treatments for freshly ruptured tumors and the outcomes afterwards.
This retrospective case-control study tried to investigate the risk factors associated with spontaneous rupture of barcelona clinic liver cancer stages A and B HCC, and to reveal the outcomes after primary hepatectomy during a 10-year period at a single center in China.
This study identified that radical resection remained predictive for surgical outcomes in patients with ruptured HCC.
A good series of a single institutional experience of patients with localised spontaneously ruptured HCC treated by surgical resection. It explores biologically plausible risk factors for rupture with appropriate statistical analysis. Important message is that outcomes are not necessarily grim as some 5-year survivors are possible following resection with a low surgical mortality.
P- Reviewers: Cheng A, Olah A, Yip D S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15:223-243, vii-x. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 376] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 2. | Kobayashi M, Ikeda K, Hosaka T, Sezaki H, Someya T, Akuta N, Suzuki F, Suzuki Y, Saitoh S, Arase Y. Natural history of compensated cirrhosis in the Child-Pugh class A compared between 490 patients with hepatitis C and 167 with B virus infections. J Med Virol. 2006;78:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Miyoshi A, Kitahara K, Kohya N, Noshiro H, Miyazahi K. Outcomes of patients with spontaneous rupture of hepatocellular carcinoma. Hepatogastroenterology. 2011;58:99-102. [PubMed] |
| 4. | Liu CL, Fan ST, Lo CM, Tso WK, Poon RT, Lam CM, Wong J. Management of spontaneous rupture of hepatocellular carcinoma: single-center experience. J Clin Oncol. 2001;19:3725-3732. [PubMed] |
| 5. | Kim HC, Yang DM, Jin W, Park SJ. The various manifestations of ruptured hepatocellular carcinoma: CT imaging findings. Abdom Imaging. 2008;33:633-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Tan FL, Tan YM, Chung AY, Cheow PC, Chow PK, Ooi LL. Factors affecting early mortality in spontaneous rupture of hepatocellular carcinoma. ANZ J Surg. 2006;76:448-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Al-Mashat FM, Sibiany AM, Kashgari RH, Maimani AA, Al-Radi AO, Balawy IA, Ahmad JE. Spontaneous rupture of hepatocellular carcinoma. Saudi Med J. 2002;23:866-870. [PubMed] |
| 8. | Chen CY, Lin XZ, Shin JS, Lin CY, Leow TC, Chen CY, Chang TT. Spontaneous rupture of hepatocellular carcinoma. A review of 141 Taiwanese cases and comparison with nonrupture cases. J Clin Gastroenterol. 1995;21:238-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Rossetto A, Adani GL, Risaliti A, Baccarani U, Bresadola V, Lorenzin D, Terrosu G. Combined approach for spontaneous rupture of hepatocellular carcinoma. World J Hepatol. 2010;2:49-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Zhu LX, Geng XP, Fan ST. Spontaneous rupture of hepatocellular carcinoma and vascular injury. Arch Surg. 2001;136:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Tarantino L, Sordelli I, Calise F, Ripa C, Perrotta M, Sperlongano P. Prognosis of patients with spontaneous rupture of hepatocellular carcinoma in cirrhosis. Updates Surg. 2011;63:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Yoshida H, Mamada Y, Taniai N, Mizuguchi Y, Kakinuma D, Ishikawa Y, Kanda T, Matsumoto S, Bando K, Akimaru K. Long-term results of elective hepatectomy for the treatment of ruptured hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2008;15:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Buczkowski AK, Kim PT, Ho SG, Schaeffer DF, Lee SI, Owen DA, Weiss AH, Chung SW, Scudamore CH. Multidisciplinary management of ruptured hepatocellular carcinoma. J Gastrointest Surg. 2006;10:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Hai L, Yong-Hong P, Yong F, Ren-Feng L. One-stage liver resection for spontaneous rupture of hepatocellular carcinoma. World J Surg. 2005;29:1316-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Marrero JA. Staging systems for hepatocellular carcinoma: should we all use the BCLC system? J Hepatol. 2006;44:630-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 851] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 17. | Zhu LX, Wang GS, Fan ST. Spontaneous rupture of hepatocellular carcinoma. Br J Surg. 1996;83:602-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Tanaka A, Takeda R, Mukaihara S, Hayakawa K, Shibata T, Itoh K, Nishida N, Nakao K, Fukuda Y, Chiba T. Treatment of ruptured hepatocellular carcinoma. Int J Clin Oncol. 2001;6:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Zhu LX, Liu Y, Fan ST. Ultrastructural study of the vascular endothelium of patients with spontaneous rupture of hepatocellular carcinoma. Asian J Surg. 2002;25:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Yeh CN, Lee WC, Jeng LB, Chen MF, Yu MC. Spontaneous tumour rupture and prognosis in patients with hepatocellular carcinoma. Br J Surg. 2002;89:1125-1129. [PubMed] |
| 21. | Chiappa A, Zbar A, Audisio RA, Paties C, Bertani E, Staudacher C. Emergency liver resection for ruptured hepatocellular carcinoma complicating cirrhosis. Hepatogastroenterology. 1999;46:1145-1150. [PubMed] |
| 22. | Chen MF, Hwang TL, Jeng LB, Jan YY, Wang CS. Clinical experience with hepatic resection for ruptured hepatocellular carcinoma. Hepatogastroenterology. 1995;42:166-168. [PubMed] |
| 23. | Shimada R, Imamura H, Makuuchi M, Soeda J, Kobayashi A, Noike T, Miyagawa S, Kawasaki S. Staged hepatectomy after emergency transcatheter arterial embolization for ruptured hepatocellular carcinoma. Surgery. 1998;124:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Shuto T, Hirohashi K, Kubo S, Tanaka H, Hamba H, Kubota D, Kinoshita H. Delayed hepatic resection for ruptured hepatocellular carcinoma. Surgery. 1998;124:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Collaborative Staging Task Force of the American Committee on Cancer. Collaborative Staging Manual and Coding Instructions, version 1.0. Published jointly by the American Joint Committee on Cancer (Chicago, IL) and the U.S. Department of Health and Human Services (Bethesda, Maryland). Bethesda: NIH Publication 2004; No. 04-5496. |